Abstract
Celastrol, a quinine methide triterpene extracted from the perennial vine Tripterygium wilfordii, has been identified as a neuroprotective agent in various models of neurodegenerative disorders. We have reported earlier that systemic and intravitreal administration of celastrol stimulate the survival of retinal ganglion cells (RGCs) injured by optic nerve crush (ONC) and that mechanisms underlying celastrol’s RGC protection may be associated with inhibition of TNF-alpha-mediated cell death. The present study evaluates the effect of celastrol on the survival of RGCs injured by ocular hypertension. Intraocular pressure (IOP) elevation resulted in approximately 23% of RGCs loss. Reduction in RGC numbers was observed in all four retinal quadrants: 30% in superior, 17% in inferior, 11% in nasal and 35% in temporal regions. Celastrol (1 mg/kg) or vehicle (DMSO) was administered three times per week by intraperitoneal injection, starting on the day of laser photocoagulation of the TM and continued for the entire duration of the experiment (5 weeks). Celastrol treatment stimulated RGC survival by an average of 24% in the entire retina compared to the vehicle-treated group. RGC numbers were increased in all four quadrants: approximately 40%, 17%, 15% and 30% more RGCs were counted in the superior, inferior, nasal and temporal regions, respectively. The average RGC numbers for the entire retinas of the celastrol/IOP group were only ∼5% and 10% lower than that in vehicle- or celastrol-injected animals with normal IOP, respectively. Our data indicate a significant celastrol-mediated neuroprotection against elevated IOP-induced injury.
Keywords: Celastrol, Retina, Ganglion cells, Optic nerve, Glaucoma, Neuroprotection
1. Introduction
Glaucomatous neuropathy affects millions of people worldwide and is often only diagnosed after patients have already suffered irreversible damage to their vision. If left untreated, glaucoma could lead to debilitating visual impairments. Vision loss in this disease is due to damage to retinal ganglion cells (RGCs) and their axons in the optic nerve. The current glaucoma treatment–lowering intraocular pressure (IOP)–has been proven to be effective in slowing the progression of the disease in most patients, however it is common for visual function to deteriorate despite IOP reduction. This dictates the necessity of developing new therapies for this disease that may be used as complementary or alternative treatment. Since the mechanisms responsible for glaucomatous damage are not well defined, multiple neuroprotective strategies that target different pathways potentially involved in this process were proposed and tested [1]. Yet, despite extensive studies in this area over the past two decades and the accumulation of a wealth of information about RGC degeneration and neuroprotection in animal models, there has been no substantial progress in developing new clinically relevant therapeutic approaches. RGCs’ susceptibility to damage in glaucoma is almost certainly associated with more than one risk factor. Risk factors sufficient for or contributing to the development of the disease may vary by glaucoma type and determine not only the likelihood of developing the disease, but also its severity and rate of progression. Some of the cellular factors that are commonly associated with the pathogenesis of glaucoma include biomechanical stress associated with IOP, oxidative stress, axonal transport failure, insufficient nutrient supply, autoimmunity, protein misfolding/aggregation and glial cell dysfunction [2–4]. In our effort to simultaneously target multiple pathways that are known to be associated with RGC damage during glaucomatous neurodegeneration, we have earlier evaluated the effect of celastrol, a quinine methide triterpene extracted from the perennial vine Tripterygium wilfordii, in the optic nerve crush (ONC) model of RGC degeneration. Celastrol has been used in traditional oriental medicine for centuries as a natural remedy for inflammation and a variety of autoimmune diseases. Its neuroprotective effect has been identified in a comprehensive drug screen against various neurode-generative diseases and furthermore its cell protective properties were demonstrated in animal models of Parkinson’s, Huntington’s, Alzheimer’s diseases, and amyotrophic lateral sclerosis [5–10]. The mechanisms underlying celastrol’s neuroprotective effects are primarily associated with the induction of the heat shock response, activation of the cellular antioxidant defense system, attenuation of microglial activation, inhibition of tumor necrosis factor (TNF)-alpha, and nitric oxide synthase production [10–13]. Considering that the pathogenesis of glaucoma is thought to be associated with oxidative damage, protein misfolding/aggregation, microglial activation, and TNF alpha, celastrol could represent a therapeutic drug that targets the multifactorial nature of this disease [14–18].
Recently, we analyzed the effect of celastrol on the survival of RGCs injured by ONC [19]. Both intraperitoneal (i.p.) and intravitreal injection of celastrol stimulated the survival of injured RGCs. The average density of RGCs in animals treated with celastrol (1 mg/kg; daily i.p. injection) was increased by approximately 250%. The RGC survival was also increased by approximately 80% in ONC animals treated with a single intravitreal injection of 1 mg/kg or 5 mg/kg of celastrol. In-travitreal injection of 0.2 mg/kg of celastrol had no effect on cell survival in ONC-injured RGCs compared to DMSO-injected controls. Furthermore, to explore the mechanisms of potential celastrol-mediated RGC protection, we analyzed the expression of proteins that have been associated with celastrol-induced heat shock and antioxidant responses as well as the TNF alpha. Expression of heat shock protein 70 (Hsp70), heat shock factor 1 (Hsf1), Hsf2, and heme oxigenase 1 (HO-1) was not affected in celastrol-treated animals, whereas the level of TNF-alpha was significantly reduced, suggesting that celastrol-mediated protection of ONC-injured RGCs involves cell death pathways associated with TNF-alpha.
The current study evaluates the effect of celastrol on the survival of RGCs in an ocular hypertensive animal model of RGC degeneration. Both ONC and elevated IOP models are characterized by progressive degeneration of RGCs, however the IOP model is traditionally considered to be more relevant to human glaucoma since the elevated IOP is a well recognized risk factor for the most common forms of the disease.
2. Materials and methods
2.1. Animals
Adult Brown Norway rats (3 month-old; 250–300 g) were used to generate experimental glaucoma models. The use of animals and the procedures involving animals were approved by the Animal Research Committee of the University of California at Los Angeles and were in compliance with the National Institutes of Health Guide for the Care and Use of Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were housed with standard food and water provided ad libitum in a room with the temperature set at 21 °C and illuminated with fluorescent lights (330 lx) automatically turned on at 03:00 am and off at 03:00 pm The animals were kept at least 1 week in this environment before IOP measurement or trabecular meshwork (TM) laser photocoagulation. Photocoagulation was performed on one eye of each rat while the contralateral eye served as an untreated control. Celastrol (1 mg/kg) or vehicle (DMSO) was administered three times per week by i.p. injection. Four groups of animals were used in this study to evaluate celastrol’s cell protective effect: Vehicle (untreated eyes of animals injected with vehicle; n = 10), Vehicle/IOP (laser-treated eyes of animals injected with vehicle; n = 10), Celastrol (untreated eyes of animals injected with celastrol; n = 14), and Celastrol/IOP (laser-treated eyes of animals injected with celastrol; n = 14).
2.2. Ocular hypertension model
Trabecular laser photocoagulation was used to increase IOP. After 1 week of accommodation, light and dark phase IOPs were measured in awake Brown Norway rats with Tonolab (TonoLab; Colonial Medical Supply, Franconia, NH) twice a week. Trabecular laser photocoagulation was performed as described previously (Kwong et al., 2011). Briefly, approximately 200 laser burns were delivered ab externo to the 360° TM at laser settings of 200 μm diameter, 100 mW power, and 50 m sec durations. Trabecular laser photocoagulation was repeated 2 weeks after the first treatment on eyes with no significant IOP elevation compared with contralateral control eyes. IOP elevation was sustained for 5 weeks.
2.3. Quantification of RGCs
Animals were deeply anesthetized with intramuscular injections of 80 mg/kg sodium pentobarbital. The eyes were enucleated, immersed in fixative for 0.5 h, and the lenses were removed. The eyecups were postfixed for another 0.5 h. Entire retinas were used for immunohistochemistry with a custom-made antibody against Rbpms according to a published protocol [20]. Briefly, the retinas were washed with PBS, incubated with 10% fetal bovine serum for 1 h to block nonspecific staining, followed by incubation with the primary antibody against Rbpms in PBS containing 1% triton, 0.5% BSA, and 0.9% sodium chloride (PBS-T-BSA) overnight at 4 °C. The retinas were washed in PBS-T-BSA and incubated with secondary Alexa Fluor 488 goat anti-rabbit IgG antibody (1/1000) overnight at 4 °C. Retinas were then washed with PBS, flat mounted with several radial cuts on a glass slide, air dried and covered with a cover-slip in an aqueous mount. Topographical analysis of RGC density was performed under a fluorescence microscope (LSM410; Carl Zeiss, Oberkochen, Germany). Three areas (0.32 × 0.24 mm each) per retinal quadrant (superior, temporal, inferior and nasal) at 1, 2, 3 and 4 mm from the optic disc were analyzed. RGCs were counted in a masked manner.
2.4. Statistical analysis
Data are presented as the mean ± standard deviation (SD). A repeated measures ANOVA was conducted to compare RGC density in Vehicle, Celastrol, Vehicle/IOP and Celastrol/IOP groups. All measurements at four distances and four quadrants from each animal were included in the overall ANOVA model comparing RGC density in Vehicle, Celastrol, Vehicle/IOP and Celastrol/IOP groups, and the effects of quadrants and distances were controlled by multi-factors ANOVA model. When comparisons of RGC density among groups were performed within each quadrant, all measurements at four distances from each animal were included in the ANOVA model and the distance effect was controlled by multi-factors ANOVA model. P < 0.05 was considered statistically significant.
3. Results and discussion
The rat experimental glaucoma model to investigate celastrol’s cell protective effect against ocular hypertension induced RGC damage was generated by laser photocoagulation of the TM. Mean IOPs for the light and dark phases of the circadian cycle for experimental and control eyes were calculated and representative IOP profiles of vehicle-treated rat and celastrol-treated rat are shown in Fig. 1A and B respectively. TM photocoagulation to elevate IOP was performed unilaterally, while the contralateral eye received no treatment. The average light phase IOP in the Vehicle/IOP and Celastrol/IOP groups were 23.81 ± 2.68 mmHg and 21.74 ± 3.13 mmHg, respectively compared to 18.91 ± 1.07 in Vehicle group and 17.81 ± 1.06 mmHg in Celastrol group. The dark phase average IOP values were 32.67 ± 0.99, 32.15 ± 1.56, 41.17 ± 4.63 and 39.52 ± 4.99 mmHg in Vehicle, Celastrol, Vehicle/ IOP and Celastrol/IOP groups, respectively. As expected, both the light and dark phase IOP in experimental eyes of vehicle-treated animals were significantly elevated after TM photocoagulation, when compared to contralateral eyes (light IOP: P = 0.0002; dark IOP P = 0.0002). Similarly, both the light and dark phase IOP in experimental eyes of celastrol-treated animals were significantly elevated after TM photo-coagulation, when compared to contralateral eyes (light IOP: P = 0.0004; dark IOP P = 0.0001). There was no statistical difference between the average light and dark IOP phases of experimental eyes from celastrol-treated group and vehicle-treated group (light IOP: P = 0.1; dark IOP: P = 0.41). Since a single laser TM photocoagulation was insufficient to maintain elevated IOP for the duration of the experiment, a second treatment was performed two weeks after the first procedure. Although the first and second TM photocoagulation both resulted in a significant response of the light phase IOP, elevation of the dark phase IOP in experimental eyes was more prominent after the second laser procedure.
Fig. 1.
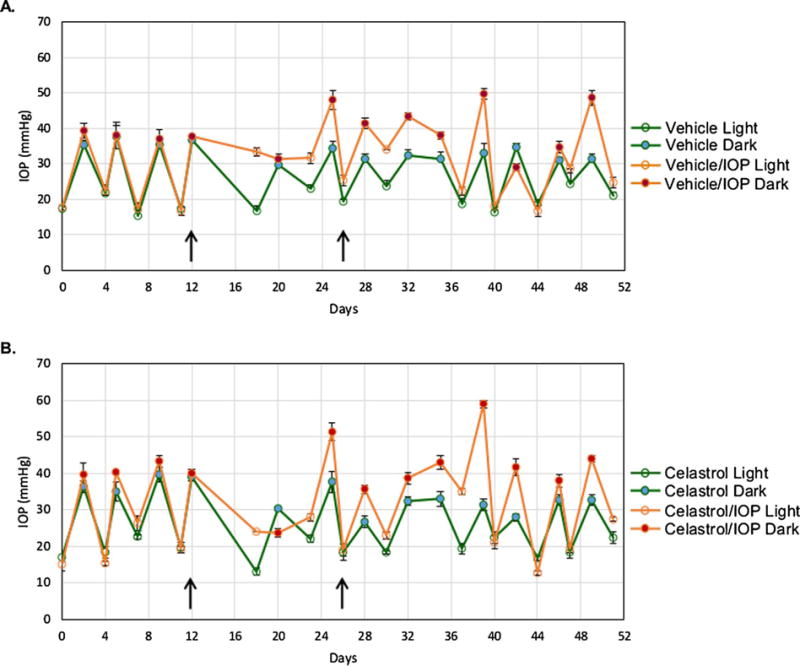
Representative IOP profiles for vehicle- (A) and celastrol-treated (B) animals. The light and dark phase IOP readings of an individual rat from the vehicle-treated group (Animal #2334) and celastrol-treated group (Animal #2325) are shown. One IOP reading was recorded by TonoLab after five successful measurements were made. Three IOP readings were used for IOP analysis in this experiment. Data are presented as mean ± SD. The laser trabecular meshwork photocoagulation indicated by arrows was performed unilaterally, twelve days after establishing the baseline IOP, and was repeated two weeks later to maintain elevated IOP during the course of experiment. The contralateral eyes received no treatment and served as controls.
To evaluate the cell protective effect of celastrol, animals were treated i.p. with 1 mg/kg of the drug three times per week starting on the day of laser photocoagulation of the TM and continued for the entire duration of the experiment (5 weeks). The control rats received the vehicle (DMSO). The frequency of the drug administration in this study was reduced from daily i.p. injections of 1 mg/kg of celastrol that was used in experiments with ONC animals to avoid its potential side effects. The regimen for celastrol treatment in experiments with the ONC model was based on earlier published studies on successful use of celastrol in rat and mouse models of various human diseases with no side effects [21–23]. However, we observed an approximately 2% body weight loss in the celastrol-injected animals, whereas animals in the control group injected with DMSO only registered a weight gain of 6% during a two-week period [19]. Consistent with our findings, the body weight decrease of animals treated with celastrol was also observed during i.p. treatment of six-week-old diabetic db/db and non-diabetic db/m mice with 1 mg/kg/day of celastrol for two weeks [23]. No side effects, including the effect on the weight of animals, from the celastrol regimen used in this study, were observed.
The effect of celastrol on RGC survival was determined by comparing the density of Rbpms-labeled RGCs in flat mounted retinas with and without induced ocular hypertension in inferior, nasal, superior and temporal retinal quadrants of control (Vehicle group) and experimental animals (Celastrol group). All measurements at four distances and four quadrants from each animal were included in the overall ANOVA model comparing RGC density in Vehicle, Celastrol, Vehicle/ IOP and Celastrol/IOP groups, and the effects of quadrants and distances were controlled by multi-factors ANOVA model. When comparisons of RGC density among groups were performed within each quadrant, all measurements at four distances from each animal were included in the ANOVA model and the distance effect was controlled by multi-factors ANOVA model. The average number of RGCs in the entire retinas of DMSO- and celastrol-treated animals was similar: 1836 ± 562 cells/mm2 in the Vehicle group and 1949 ± 594 cells/ mm2 in the Celastrol group. Vehicle-injected animals with ocular hypertension suffered a loss of approximately 23% of RGCs compared to vehicle injected animals with normal IOP [Veh vs Veh/IOP, P = 0.0078]. Reduction in RGC numbers was observed in all four retinal quadrants analyzed in this study: 30% in superior (P = 0.051), 17% in inferior (P = 0.001), 11% in nasal (P = 0.11) and 35% in temporal (P = 0.012) regions, although RGC loss in only inferior and temporal regions was statistically significant (Fig. 2, Table 1). Uneven RGC damage associated with IOP elevation in this animal model may be related to the well-characterized focal retinal neurodegeneration in human glaucoma. In animals treated with celastrol, the effect of IOP elevation was much less dramatic: celastrol-mediated support for RGC survival in the entire retina resulted in preservation of approximately 24% more cells compared to that of the vehicle-treated group (Cel/IOP vs Veh/IOP, P = 0.021; Figs. 2 and 3). RGC protection was observed in all four quadrants: ∼40% (P = 0.044) more RGCs were counted in the superior region, 17% more in inferior (P = 0.0041), 15% more in nasal (P = 0.038) and 30% more in temporal (P = 0.12; Fig. 2). The average number of RGC in the entire retinas of the celastrol/IOP group were about 5% and 10% lower compared to vehicle- and celastrol-treated animals with normal ocular tensions, respectively. With respect to potential underlying mechanisms in celastrol-medited RGC protection, earlier, we had shownthat celastrol’s protective effect on RGCs injured by ONC may be associated with the downregulation of TNF-alpha. Since TNF-alpha signaling has been implicated in glaucomatous neurode-generation [24], it is possible that the RGC protective effect of celastrol from IOP-induced injury also involves modulation of TNF-alpha expression.
Fig. 2.
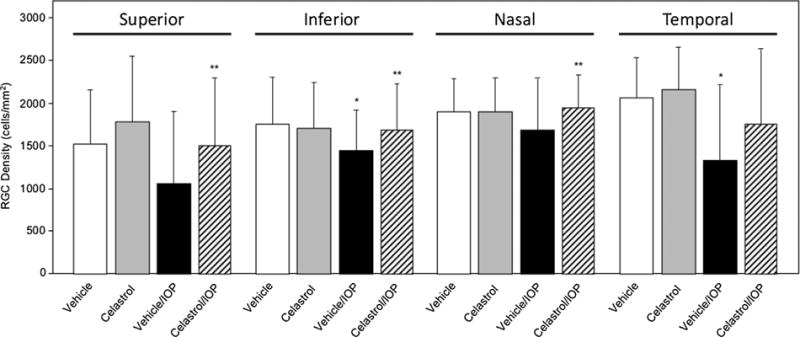
The effect of celastrol treatment on RGC numbers in superior, inferior, nasal and temporal retinal quadrants. A repeated measures ANOVA was conducted to compare RGC density in Vehicle, Celastrol, Vehicle/IOP, and Celastrol/IOP groups. A significant decrease in RGC density in Vehicle/IOP compared to Vehicle group was observed in the inferior (*P = 0.001) and temporal (*P = 0.012) quadrants. RGC density was significantly increased in the superior (**P = 0.044), inferior (**P = 0.0041) and nasal (**P = 0.038) retinal quadrants of Celastrol/IOP compared to Vehicle/IOP animals. The effect of distance (mm) from the optic nerve head was adjusted. Data are presented as mean ± SD.
Table 1.
Retinal ganglion cell density in superior, inferior, nasal and temporal retinal quadrants in animals treated with celastrol or vehicle. Data presented as mean ± SD.
| Animal Group |
Distance from ONH |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inferior |
Nasal |
Superior |
Temporal |
|||||||||||||
| 1 mm | 2 mm | 3 mm | 4 mm | 1 mm | 2 mm | 3 mm | 4 mm | 1 mm | 2 mm | 3 mm | 4 mm | 1 mm | 2 mm | 3 mm | 4 mm | |
| Vehicle | 2204.5 ± 369.4 | 2237.2 ± 281.3 | 1665.8 ± 174.5 | 1014.6 ± 145.1 | 2246.3 ± 311.5 | 2116.9 ± 324.3 | 1875.0 ± 216.6 | 1474.9± 233.7 | 2330.0 ± 276.1 | 1712.9 ± 335.8 | 1248.7 ± 450.0 | 889.1 ± 355.8 | 2247.6 ± 315.3 | 2387.6 ± 324.4 | 2314.3 ± 269.4 | 1410.8 ± 193.5 |
| Celastrol | 2246.2 ± 209.2 | 2076.2 ± 254.6 | 1606.4 ± 293.6 | 1006.8 ± 273.5 | 2353.6 ± 135.9 | 2282.6 ± 195.0 | 2065.0 ± 201.2 | 1509.3 ± 265.8 | 2595.4 ± 245.4 | 2341.4 ± 511.3 | 1367.3 ± 351.0 | 933.0 ± 284.8 | 2455.4 ± 287.3 | 2412.4 ± 281.7 | 2381.6 ± 304.3 | 1550.4 ± 390.0 |
| Vehicle/IOP | 1950.8 ± 166.2 | 1736.4 ± 239.2 | 1375.5 ± 244.8 | 826.4 ± 134.3 | 1987.4 ± 483.4 | 1965.2 ± 655.3 | 1701.1± 595.2 | 1216.0 ± 459.7 | 1354.6 ± 1021.4 | 1417.4 ± 949.3 | 1036.9 ± 745.7 | 538.7 ± 370.3 | 1400.4 ± 958.2 | 1503.7 ± 973.5 | 1480.1 ± 981.1 | 1070.9 ± 748.8 |
| Celastrol/IOP | 2195.7 ± 223.3 | 2017.3 ± 266.4 | 1631.6 ± 377.5 | 1027.3 ± 372.3 | 2209.7 ± 256.3 | 2190.1 ± 257.4 | 2036.9 ± 256.8 | 1448.6 ± 295.4 | 1786.6 ± 893.1 | 1978.1 ± 774.3 | 1451.4 ± 618.4 | 887.3 ± 496.3 | 1886.6 ± 934.2 | 2035.1 ± 968.3 | 1919.3 ± 894.4 | 1264.6 ± 668.9 |
Fig. 3.
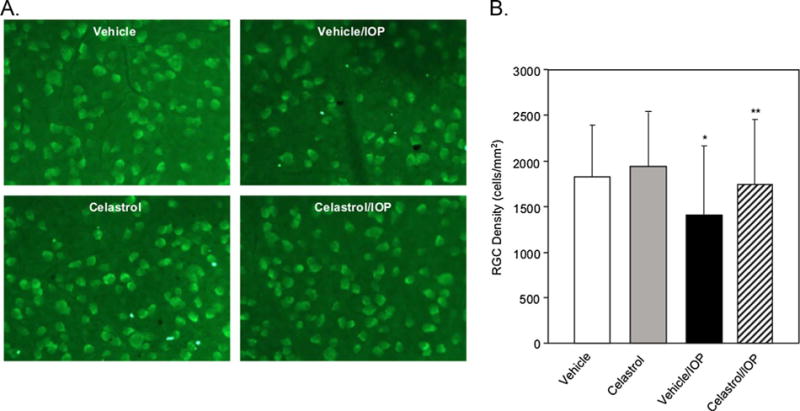
Celastrol supports RGC survival from ocular hypertension damage. A. Rbpms-positive RGCs in flat mounted retinas from vehicle- and celastrol-treated animals with normal and elevated IOP. Images represent temporal retinal quadrants. B. A repeated measures ANOVA was conducted to compare RGC density in vehicle-treated, celastrol-treated, vehicle-treated with IOP elevation, and celastrol-treated with IOP elevation groups after controlling for the quadrant and distance effects. A significant decrease of RGC density was observed in Vehicle/ IOP compared to Vehicle group (*P = 0.0078). There was a significant increase of RGC density in Celastrol/IOP compared to Vehicle/IOP group (**P = 0.021). Data are presented as mean ± SD.
In summary, the celastrol-mediated support for RGC survival in the animal model of ocular hypertension described in this paper, together with our earlier published data on RGC protective effect of this drug against optic nerve crush injury, provide support for the further evaluation of celastrol as potential treatment for glaucoma and other optic neuropathies.
Acknowledgments
Funding
This work was supported by The Research to Prevent Blindness (JC) and by the National Institutes of Health (NIH) Grant EY018644 (NP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doozandeh A, Yazdani S. Neuroprotection in glaucoma. J Ophthal Vis Res. 2016;11:209–220. doi: 10.4103/2008-322X.183923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Tezel G. The role of glia, mitochondria, and the immune system in glaucoma, Invest. Ophthalmol. Vis Sci. 2009;50:1001–1012. doi: 10.1167/iovs.08-2717. [DOI] [PubMed] [Google Scholar]
- 4.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochem Biophys Res Commun. 2004;322:778–786. doi: 10.1016/j.bbrc.2004.07.186. [DOI] [PubMed] [Google Scholar]
- 6.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- 7.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 8.Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a. [DOI] [PubMed] [Google Scholar]
- 9.Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- 10.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 11.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Gines S, Macdonald ME, Gusella JF. Reversal of a full-length mutant huntingtin neuronal phenotype by chemical inhibitors of polyglutamine-mediated aggregation. BMC Neurosci. 2005;6:1–12. doi: 10.1186/1471-2202-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trott A, West JD, Klaic L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist. 2003;9:485–495. doi: 10.1177/1073858403253460. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–1204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- 18.Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007;16:334–343. doi: 10.1097/01.ijg.0000243480.67532.1b. [DOI] [PubMed] [Google Scholar]
- 19.Kyung H, Kwong JM, Bekerman V, Gu L, Yadegari D, Caprioli J, Piri N. Celastrol supports survival of retinal ganglion cells injured by optic nerve crush. Brain Res. 1609;2015:21–30. doi: 10.1016/j.brainres.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong JM, Caprioli J, Piri N. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1052–1058. doi: 10.1167/iovs.09-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286:15138–15146. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang HL, Jin JZ, Wu D, Xu D, Lin GF, Yu H, Ma DY, Liang J. Celastrol exerts synergistic effects with PHA-665752 and inhibits tumor growth of c-Met-deficient hepatocellular carcinoma in vivo. Mol Biol Rep. 2013;40:4203–4209. doi: 10.1007/s11033-013-2501-y. [DOI] [PubMed] [Google Scholar]
- 23.Kim JE, Lee MH, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Han SY, Han KH, Han JY, Cha DR. Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One. 2013;28:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


