Abstract
Purpose
We investigated the effect on outcome of measurable or minimal residual disease (MRD) status after each induction course to evaluate the extent of its predictive value for acute myeloid leukemia (AML) risk groups, including NPM1 wild-type (wt) standard risk, when incorporated with other induction response criteria.
Methods
As part of the NCRI AML17 trial, 2,450 younger adult patients with AML or high-risk myelodysplastic syndrome had prospective multiparameter flow cytometric MRD (MFC-MRD) assessment. After course 1 (C1), responses were categorized as resistant disease (RD), partial remission (PR), and complete remission (CR) or complete remission with absolute neutrophil count < 1,000/µL or thrombocytopenia < 100,000/μL (CRi) by clinicians, with CR/CRi subdivided by MFC-MRD assay into MRD+ and MRD−. Patients without high-risk factors, including Flt3 internal tandem duplication wt/−NPM1-wt subgroup, received a second daunorubicin/cytosine arabinoside induction; course 2 (C2) was intensified for patients with high-risk factors.
Results
Survival outcomes from PR and MRD+ responses after C1 were similar, particularly for good- to standard-risk subgroups (5-year overall survival [OS], 27% RD v 46% PR v 51% MRD+ v 70% MRD−; P < .001). Adjusted analyses confirmed significant OS differences between C1 RD versus PR/MRD+ but not PR versus MRD+. CRi after C1 reduced OS in MRD+ (19% CRi v 45% CR; P = .001) patients, with a smaller effect after C2. The prognostic effect of C2 MFC-MRD status (relapse: hazard ratio [HR], 1.88 [95% CI, 1.50 to 2.36], P < .001; survival: HR, 1.77 [95% CI, 1.41 to 2.22], P < .001) remained significant when adjusting for C1 response. MRD positivity appeared less discriminatory in poor-risk patients by stratified analyses. For the NPM1-wt standard-risk subgroup, C2 MRD+ was significantly associated with poorer outcomes (OS, 33% v 63% MRD−, P = .003; relapse incidence, 89% when MRD+ ≥ 0.1%); transplant benefit was more apparent in patients with MRD+ (HR, 0.72; 95% CI, 0.31 to 1.69) than those with MRD− (HR, 1.68 [95% CI, 0.75 to 3.85]; P = .16 for interaction).
Conclusion
MFC-MRD can improve outcome stratification by extending the definition of partial response after first induction and may help predict NPM1-wt standard-risk patients with poor outcome who benefit from transplant in the first CR.
INTRODUCTION
In acute myeloid leukemia (AML), failure to achieve morphologic complete remission (CR) after a first cycle of induction in previously untreated patients is an established independent prognostic factor from earlier studies.1-3 Thus, morphologic response at this time point is often incorporated with genetic and pretreatment clinical parameters to guide further therapy,4 including second induction courses, choice of consolidation, and whether intensification from allogeneic stem-cell transplantation (SCT) may be appropriate in otherwise intermediate-risk patients. Despite morphologic response criteria being standard, a different approach for measuring response has been proposed5,6 owing to the independent prognostic value from measurable or minimal residual disease (MRD) assays when discrepant with morphology,7-9 or in CR10-12 and the equivalent poor outcomes between MRD positivity and active-disease premyeloablative SCT.13,14
Studies have shown the prognostic value of MRD monitoring by polymerase chain reaction (PCR) for patients with validated molecular targets, usually after two courses of chemotherapy.11,12,15 Multiparametric flow cytometry MRD (MFC-MRD) may identify, as early as after course 1 (C1), patients with a poorer response despite achieving CR and is an assay that can be applied across AML genetic subgroups.12,16-20 There are, however, insufficient data to ascertain the relative prognostic effect of MFC-MRD positivity in CR post-C1 compared with morphologic active disease; it is feasible that the outcomes of patients with detectable MRD resemble those of refractory patients who achieve the cytoreduction criteria for a morphologic partial remission (PR).21,22 Evaluating this will help refine which response categories are the most useful prognostic surrogate end points to assess effectiveness of the first induction course.
It is also uncertain for patients who complete a second chemotherapy course whether the quality of response after C1, with inclusion of MFC-MRD assessment, adds prognostic information to CR-MRD status after course 2 (C2). The value of MFC-MRD status to differentiate outcome at either time point is likely to be heterogeneous between established risk subgroups due to disease, treatment, and assay factors, but the extent of this has not been established.
Treatment decisions, including predicting the benefit of SCT, are particularly challenging for the standard-risk subgroup. MFC-MRD assays are most likely to influence therapeutic choices for NPM1-wild type (wt) patients of standard risk, following data indicating postinduction reverse transcriptase, quantitative PCR (RT-qPCR) of blood-mutated transcripts reliably predicts outcome for patients with NPM1 mutation.23,24 Thus, there is a specific need to define the usefulness of MFC-MRD for risk stratification in this subgroup.
In this study, we aimed to determine the prognostic effect of MFC-MRD measurement incorporated into response assessment after induction courses for the different risk subgroups, including NPM1-wt patients at standard risk, in a large cohort of younger patients with AML who had undergone intensive treatment in the National Cancer Research Institute (NCRI) AML17 trial.
METHODS
Patients
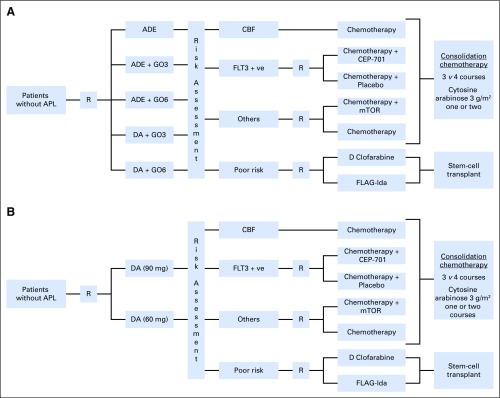
Patients were enrolled in the NCRI AML17 trial (ISRCTN Registry No. 55675535) from April 6, 2009, to December 31, 2014. A list of treatments is provided in Appendix Fig. A1 (online only).
The AML17 protocol was designed primarily for younger patients, generally age < 60 years. Patients with high-risk myelodysplastic syndrome, which was defined as > 10% marrow blasts at diagnosis, and secondary AML were eligible. Patients with acute promyelocytic leukemia were not included in this MRD study. After first induction, patients were defined by risk of relapse, using a validated score comprising cytogenetics, WBC count, age, secondary disease, morphologic response to C125,26 and FLT-3 internal tandem duplication(ITD)/NPM1 mutation status.
Morphologic-based response criteria were as follows: (1) CR, < 5% blasts in a cellular bone marrow with count recovery, CRi if 5% blasts but best response was with neutropenia < 1,000/μL or thrombocytopenia < 100,000/μL; (2) partial remission (PR), decrease of pretreatment bone marrow blast percentage by at least 50% to 5% to 15% in a cellular marrow (hematologic recovery not required)1; and (3) resistant disease (RD), > 15% marrow blasts (patients surviving at least 7 days after completion of treatment). Responses were classified by centers.
Patients designated as favorable or at standard risk received the second daunorubicin/cytosine arabinoside course and were then randomized to receive either 1 or 2 courses of high-dose cytosine arabinoside. High-risk patients were offered a randomization between FLAG-Ida or daunorubicin/clofarabine with the intention of eventually proceeding to allogeneic stem-cell transplantation (SCT). FLT3-ITD mutant patients were directed to the lestaurtinib randomization until 2012.
The trial was sponsored by Cardiff University, approved by Wales-REC3 and conducted in accordance with the Declaration of Helsinki.
Multiparameter Flow Cytometry Detection of MRD
Samples for multiparameter flow cytometry (MFC)-MRD were requested at baseline (bone marrow and/or blood) and following each course (bone marrow). A summary of sample logistics and processing is provided in the Data Supplement. MFC-MRD analysis was performed centrally, using standardized gating strategy that screened for “different-from-normal” leukemia-associated-immunophenotypes (LAIPs) on blasts pretreatment and tracked these (approximately 0.02% to 0.05% sensitivity thresholds) but also applied the different-to-normal approach in follow-up samples to detect changes in blast LAIPs (approximately 0.05% to 0.1% sensitivity threshold). In this study, only samples for which there were pretreatment LAIPs to monitor could be reported as MFC-MRD negative, whereas samples with any level of MRD detected above a diagnostic LAIP or different-from-normal follow-up LAIP threshold were reported as MFC-MRD positive. Clinicians were not informed of MFC-MRD results.
Statistical Analysis
All end points were based on the revised criteria of the International Working Group for Diagnosis.21 Survival percentages were calculated using the Kaplan-Meier method with cumulative incidence of relapse calculated using competing-risks methodology. Baseline characteristics were compared using χ2 or Mantel-Haenszel tests, with continuous variables compared using the Wilcoxon rank-sum test. Time-to-event outcomes were compared using log-rank tests and Cox regression. Outcomes are reported as effect sizes with 95% confidence intervals; significance was set at P < .05. Stratified analyses used stratified log-rank tests and are displayed as forest plots with tests for interaction using standard methodology.27 Comparison of transplantation versus not was analyzed using the method of Mantel and Byar to mitigate immortal time bias. Median follow-up for survival was 39.0 months (range, 1.0 to 80.5 months).
RESULTS
Induction Response by Morphology and MFC-MRD: Patient Characteristics
Between 2009 and 2014, 6,539 samples (bone marrow [BM] or peripheral blood at diagnosis, BM post-treatment courses) from 2,450 patients with non-acute promyelocytic leukemia recruited to AML17 were prospectively analyzed for MFC-MRD (Appendix Fig A2, online only). Among patients in CR post-C1, the presence of MRD data were associated with secondary AML, and the absence of an NPM1 mutation (reflecting the prioritizing of BM for RT-qPCR monitoring of NPM1 mutations23 during the second phase of the trial); survival at 5 years was 52% (with MRD data) versus 50% (without MRD data). In adjusted analyses, the presence of MRD data was not associated with survival (hazard ratio [HR], 0.99 [95% CI, 0.84 to 1.16]; P = .9).
Post-C1, 1,443 patients contributed data; 420 were refractory by morphology (n = 197 RD; n = 223 PR) and 1,023 (70.9%) achieved CR/CRi with MFC-MRD data (n = 446 MFC-MRD negative [MRD−]; n = 577 MFC-MRD positive [MRD+]). After C2, 806 patients were in CR/CRi with MFC-MRD data (n = 503 MRD−; n = 303 MRD+).
The clinical characteristics of patients according to response post-C1 and MRD status for patients in CR/CRi post-C1 or C2 are listed in Table 1. There was a significant association between responses post-C1 or C2 and cytogenetic group; however, count recovery post-C1 was not significantly associated with MRD after either course.
Table 1.
Characteristics of Study Population by Response
Outcome Comparison for Morphologic Response and MFC-MRD Status After C1
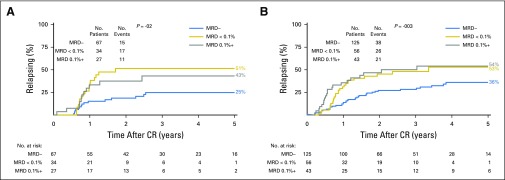
We evaluated overall survival (OS) by C1 response status. Five-year OS for all enrolled in AML17 excluding early deaths was 52% for those achieving CR/CRi versus 31% for refractory patients (P < .001). MRD status in CR/CRi versus PR or RD further differentiated 5-year survival outcomes (Fig 1A). A PR or MRD+ response gave intermediate survival at 5 years. Survival rates appeared equivalent between these two responses for the patients at good or standard risk; 5-year OS for MRD− versus MRD+ versus PR versus RD were 63% versus 44% versus 35% versus 24%, respectively, for all patients; 70% versus 51% versus 46% versus 27%, respectively, when patients at poor risk were excluded (Fig 1B); and 66% versus 49% versus 46% versus 30%, respectively, for standard risk alone (P < .001 for all analyses; Fig 1C). Similar results were observed for survival censored at SCT (Fig 1D; Fig A3A, online only) and also for NPM1-wt patients at standard risk (Fig A3B and A3C).
Fig 1.
Overall survival (OS) according to response status after course 1. (A) All patients. (B) Patients at good and standard risk (patients known to be at poor risk excluded). (C) Patients at standard risk. (D) OS for patients at standard risk censored at allogeneic stem-cell transplantation. CR, complete remission; CRi, complete remission with absolute neutrophil count < 1,000/µL or thrombocytopenia < 100,000/μL; MRD, measurable residual disease; PR, partial remission; RD, resistant disease.
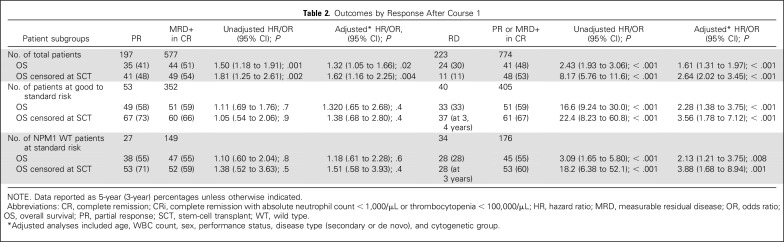
Adjusted analyses confirmed significant survival differences between RD and PR/MRD+ but not between PR and MRD+ for patients at good or standard risk (RD v PR/MRD+: OS HR, 2.28 [95% CI, 1.38 to 3.75]; P < .001; PR vs MRD+: HR, 1.32; P = .4) and for NPM1-wt patients at standard risk (RD v PR/MRD+: OS HR, 2.13 [95% CI, 1.21 to 3.75]; P = .008; PR vs MRD+: HR, 1.18, P = .6). Results were similar when censored at SCT (Table 2).
Table 2.
Outcomes by Response After Course 1
Thus, the prognostic effect from morphologic response criteria after first induction was restricted to RD in the good and standard-risk subgroups when MFC-MRD status was incorporated into response assessment.
Only 25 patients were refractory by morphology post-C1 but MRD− (n = 22 PR; n = 3 RD) with 61% 3-year and 49% 5-year OS. Seven of 577 MRD+ patients were in morphologic CR but had ≥ 5% aberrant blasts by MFC (range, 5.4% to 38%); six died within 2 years, with one patient alive at 58.6 months.
Relative Prognostic Effect of MFC-MRD After C1 and C2 by Genetic/Risk Score Subgroup
In AML17, patients received two courses of induction regardless of remission status after C1, but C2 differed for patients designated as poor risk by trial risk score. Analyses of survival and relapse by MFC-MRD status of patients with disease in CR/CRi for C1 (n = 1,010) and C2 (n = 803) were performed stratified by cytogenetic28 and trial risk subgroups (Fig 2; Appendix Fig A4, online only) to investigate the relative prognostic effect from clearance of blasts below MFC-MRD detection threshold at either of these response assessment time points. There was some evidence that the benefit from MFC-MRD negativity on OS was lower in patients at poor risk compared with other subgroups with the NCRI AML17 treatment schedule (P for test for trend = .01 for C1; P = .05 for C2). Overall, MFC-MRD status appeared more prognostic for relapse and OS at C2 (relapse: HR, 1.88 [95% CI, 1.50 to 2.36], P < .001; survival: HR, 1.77 [95% CI, 1.41 to 2.22], P < .001) than C1 (relapse: HR, 1.70 [95% CI, 1.40 to 2.06], P < .001; survival: HR, 1.50 [95% CI, 1.23 to 1.84], P < .001), although this difference diminished when C1 analysis was restricted to patients who received C2 and survived at least 30 days post-C2 (relapse: HR, 1.80 [95% CI, 1.49 to 2.18], P < .001; survival:, HR, 1.87 [95% CI, 1.52 to 2.29], P < .001).
Fig 2.
Forest plots for overall survival by multiparametric flow cytometry-MRD status for patients in complete remission. (A) After course 1. (B) After course 2, stratified by cytogenetic risk group and NCRI AML17 risk score group. CBF, core binding factor; HR, hazard ratio; MRD, measurable residual disease; NS, not significant; O-E, observed minus expected; Var, variance.
Outcomes of Combined C1 Response Status and C2 MFC-MRD Status
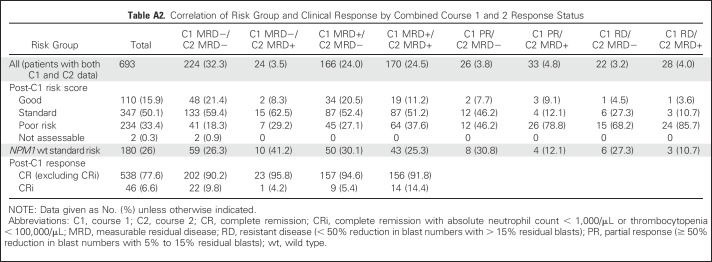
In patients with response/MFC-MRD data for both C1 and C2 time points (n = 693), C2 MFC-MRD positivity remained significant on OS and relapse when adjusting for C1 response (5-year survival: HR, 1.79 [95% CI, 1.38 to 2.32], P < .001; relapse: HR, 1.52 [95% CI, 1.18 to 1.96], P = .001; Fig 3). A total of 24 patients converted from C1 MRD− to C2 MRD+, with a particularly poor prognosis (n = 15 relapses; n = 13 deaths); one had adverse risk cytogenetics and five had Flt3-ITD mutations (Appendix Table A1). Patients who were MRD− at both C1 and C2 had the best outcome (n = 224; n = 76 relapses; n = 58 deaths); of these, 80.8% were at good or standard risk and 26.3% were NPM1-wt patients at standard risk (Appendix Table A2).
Fig 3.
Forest plots for (A) overall survival and (B) relapse by combined response data after courses 1 and 2. Effect of multiparametric flow cytometry-MRD status in CR after course 2 stratified by post-C1 response status. C1, course 1; CR, complete remission; HR, hazard ratio; MRD, measurable residual disease; NS, not significant; O-E, observed minus expected; PR, partial remission; RD, resistant disease; Var, variance.
MRD Status Combined With Peripheral Count Recovery
We examined the additional prognostic effect of combining MRD status with response by peripheral count recovery post-C1 and C2 (Appendix Table A3). The frequencies of CRi as best response in the total cohort were similar in MRD+ versus MRD− patients post-C1 (9.3% v 9.6%) and C2 (13.1% v 12.0%); CRi frequencies were not relatively increased in the NPM1-wt standard-risk subgroup. C1 CRi was associated with significantly decreased 5-year OS for total (39% v 53%; P = .002) and in MRD+ (19% v 45%; P = .001), but not for MRD− patients. MRD+ NPM1-wt patients at standard risk in CRi also had a lower OS at 5 years (25% v 48%; P = .4), although difference was not significant. The effect of CRi versus CR was smaller post-C2, although outcomes were still worse in CRi/MRD+ patients. The reduced survival associated with CRi was not due to increased relapse.
Outcome by MFC-MRD Status for NPM1-wt Patients at Standard Risk
Because it is possible that the most appropriate MFC-MRD cutoff level for discriminating outcome may differ among AML genetic subgroups, we compared the 5-year cumulative incidence of relapse for C1 MRD− versus MRD+ < 0.1% versus MRD+ ≥ 0.1% by our assay in core binding factor (CBF)-AML and NPM1-mutated as well as NPM1wt standard-risk patients. For patients with CBF-AML and NPM1 mutation, post-C1 MRD+ at any level (< 0.1% or ≥ 0.1%) significantly increased relapse (Appendix Fig A5, online only). However, in the NPM1-wt standard-risk subgroup, low-level MRD+ (< 0.1%) post-C1 did not alter relapse risk compared with MRD− but was associated with a higher cumulative incidence of relapse (CIR) when detected post-C2 (Fig 4A). MRD+ levels of ≥ 0.1% detected in 35% and 13% NPM1-wt patients at standard risk post-C1 and post-C2, respectively, predicted a high probability of relapse (C1 3-year CIR, 68%; C2 CIR, 89%). MRD status after second induction was also significantly prognostic for survival: 33% for any level of MRD positivity versus 63% for MRD− at 5 years (3 years, 47% v 69%; P = .003; Fig 4B).
Fig 4.
Standard-risk NPM1-wild type. (A) Cumulative incidence of relapse by MRD level. (MRD− v MRD+ < 0.1% v MRD+ ≥ 0.1%) after courses 1 and 2. (B) Overall survival (OS) according to MRD status after course 2 (MRD− v MRD+). (Not shown: MRD+ ≥ 0.1%, OS of 24%; MRD+ < 0.1%, OS of 39%). (C) Mantel-Byar analysis for survival according to first CR stem-cell transplant by MRD after course 2. CR, complete remission; CRi, complete remission with absolute neutrophil count < 1,000/µL or thrombocytopenia < 100,000/μL; HR, hazard ratio; MRD, measurable residual disease; NS, not significant; O-E, observed minus expected; Var, variance.
Of the 204 NPM1-wt patients at standard risk who had C2 MRD data, 83 had an allograft (n = 44 in first CR: n = 29 MRD− and n = 15 MRD+). When survival was censored at any SCT, rates of 5-year OS were 35% versus 88% (3 years, 47% v 88%; P < .001; Appendix Fig A6, online only).
We next investigated the effect of SCT in first CR according to C2 MRD status in Mantel-Byar analyses. Although numbers were small, results suggested that transplant might be considered in MRD+ (HR, 0.72; 5% CI, 0.31 to 1.69) but not MRD− patients (HR, 1.68 [95% CI, 0.75 to 3.85]; P for interaction = .16; Fig 4C).
DISCUSSION
Response to induction therapy is a powerful prognostic indicator in AML. There are, however, differing practices for the implementation of technologies that measure residual leukemia to assess response. Flow cytometry is often used to support the definition of CR by morphology; those centers with access to experienced laboratories, including some trial groups, have extended its use to define CR without MRD.5 It has been reported that outcomes after myeloablative SCT for patients with pretransplant MFC-MRD < 5% resemble those with at least 5% blasts by morphology.13 This and the similar event-free survival observed in approximately 80 pediatric patients with MRD positivity after first induction, whether < 5% or ≥ 5% blasts by morphology,7 suggest that dichotomizing patients by a 5% blast CR cutoff fails to capture some prognostic information. Our results confirm this. By incorporating MFC-MRD with established response criteria of PR and RD, distinct prognostic groups for 5-year survival emerge after the first course of standard induction. Importantly, the response subgroup with intermediate outcome comprises patients on either side of the current CR blast threshold, those with MRD positivity in CR and those who are refractory but clinically classified as a PR; both responses are associated with similar 5-year survival, particularly in patients otherwise allocated as belonging to good- or standard-risk subgroups. This is also the case when PR is defined by European LeukemiaNet criteria5,21(Appendix Fig A7, online only). From this, three post-C1 response categories could be proposed: RD, PR (MFC-MRD+ whether below or above 5% blast threshold), and CR/CRi without MRD. CRi was an independent risk factor to MRD in a study that included patients with relapsed or refractory AML and differing induction intensities.29,30 From our data, outcomes for patients newly diagnosed with AML achieving negative MRD are equivalent between CRi and CR after a single standard induction. However, the relatively few patients in our cohort (4.8%) with both CRi and MRD positivity after C1 had as poor survival (OS, 19% for all;, 25% for NPM1-wt patients at standard risk) as patients with RD.
For those completing a second induction with a CR/CRi, MRD status after C2 increased prognostic discrimination. Although sample attrition bias may limit analyses comparing time points, MRD negativity post-C2 improved outcome overall even when adjusting for slower blast clearance by C1 response. This differs from our previous results in older adults17 and might reflect the better treatment tolerance and mutation profiles of younger adults. However, after the second daunorubicin/cytosine arabinoside induction, approximately 33% of patients at standard risk and approximately 34% of NPM1-wt patients at standard risk in CR/CRi had persistent BM MRD by our assay. Whether detectable MFC-MRD after completion of conventional induction is a sufficiently specific prognostic surrogate to guide therapy has been debated. The postconsolidation time point was more informative in the GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto) study for a cohort of which approximately 70% had intermediate cytogenetics.31,32 This suggests that in a proportion of those with postinduction MRD positivity, consolidation may confer a favorable outcome by additional MFC-MRD clearance (although it is of note that for some younger adults in the GIMEMA trials, the induction/consolidation regimen comprised two courses in total). Genetic profile, treatment intensity, and the later effects of any transplant may also modify interpretation and utility of MFC-MRD to inform postremission therapy. Our data are consistent with this because the prognostic effect as well as best MFC-MRD cutoff level differed between AML risk groups; MRD status appeared less discriminatory in the patients at poor risk. Importantly, however, in the NPM1-wt standard-risk subgroup, detectable MFC-MRD at ≥ 0.1% early in treatment was associated with significantly higher relapse rates (89% after C2). The false-negative 50% CIR observed for postinduction MFC-MRD negative patients in the NPM1-wt, standard-risk subgroup could reflect MFC-MRD sensitivity limitations, although a similar CIR was observed for patients with DNTM3A/NPM1 mutations who were MRD negative by NPM1-mutated transcript RT-qPCR.23 Exploratory analyses could not identify any significant clinical parameters that predicted MRD− relapses. Longitudinal, broad molecular studies may disclose whether increased preleukemic instability reinitiating AML33,34 or persistence of pretreatment minor or major leukemic clones35,36 contributes to these false-negative relapse risks. Notwithstanding, NPM1-wt patients at standard risk who achieved MRD negativity post-C2 had significantly better survival rates. Because their survival rate increased to 88% when censored for transplant, there is the possibility that transplant in first remission could be avoided in this subset. The Mantel-Byar analysis supports this with some evidence of interaction, although this should be interpreted cautiously because of the small number of patients and the interaction was not significant.
Transplant decisions have mainly been arbitrary in this subgroup, with no accepted approach to distinguish those patients likely to be cured with chemotherapy alone (or those whose response is likely to be successful after salvage therapy if they do relapse) from those who benefit from transplantation in first remission or potentially experimental therapy. Our results suggest that allogeneic transplant in first remission could be directed to those who are MRD+ rather than MRD−. This is the first indication that MRD status might have utility in directing therapy for NPM1-wt patients at standard risk despite their molecular heterogeneity. Large patient data sets likely requiring collaborative efforts will determine whether integrating MFC-MRD status with genomic profiles37,38 further informs outcome prediction.
ACKNOWLEDGMENT
We thank the staff of the Haematology Trials Office, Cardiff Experimental Cancer Medicine Centre and reference laboratories for supporting the trial; members of the NCRI AML working group and to our other coinvestigators, research nurses, and participants who provided samples from the NCRI AML Trial centers (Data Supplement).
We gratefully acknowledge input from members of the NCRI AMLworking party and the clinicians, research nurses and laboratory scientists who provided samples for molecular analyses from NCRI AMLtrials centers, as detailed below:
NCRI AML Working Party
Dr S Ali, Prof D Bowen, Prof J Cavenagh, Prof R Clark, Dr D Culligan, Prof C Craddock, Prof M Copland, Dr M Dennis, Prof R Gale, Dr B Gibson, Dr A Hunter, Dr B Huntly, Dr G Jones, Dr H Kaur, Prof A Khwaja, Dr L Kjeldsen, Dr S Knapper, Dr P Kottaridis, Dr P Mehta, Prof O Ottman, Prof N Russell, Dr D Taussig, Prof P Vyas, Richard Castle, Sophie Betteridge, Amanda Gilkes, Marie Gilmour, Melissa Wright, Dr R Dillon, Dr Kavita Raj, Dr U Overgaard, Prof K Bowles, Dr Cahalin, Dr J Kell, Dr R Kelly, Prof M McMullin, Dr M Nikolousis, Dr D Richardson, Dr K Wheatley.
NCRI AML Trial Centers and Contributors
Aalborg Hospital: Maria Kallenbach, Anne-Merete Kirkeby Olsen; Aarhus University Hospital: Ingrid Elizabeth Gejel, Peter Hokland, Jan Maxwell Nørgaard, Hans Beier Ommen, Charlotte Nyvold; Aberdeen Royal Infirmary: Dominic Culligan, Hazel Forbes, Benedict Milner; Arrowe Park Hospital: Ranjit Dasgupta, Barbara Hammer; Ayr Hospital/ Crosshouse: Phil Cannon, Paul Eynaud, Julie Gillies, Peter Maclean; Barnet General Hospital: Anita Amadi, Virginia Jennings, Andres Virchis; Barts and the London NHS Trust: David Taussig, Jamie Cavenagh, John Gribben, Simon Hallam, Sameena Iqbal, Sarah Knight, Heather Oakervee, Tobi Sogbanmu, Matthew Smith; Basingstoke and North Hampshire Foundation NHS Trust: Alison Milne, Ashok Roy, Nigel Sargant, Sylwia Simpson; Beatson West of Scotland Cancer Centre: Andrew Clark, Mhairi Copland, Donna Kelly, Mike Leach, Anne Parker, Mark Drummond, Pam McKay, Richard Soutar; Belfast City Hospital: Claire Arnold, Mark Catherwood, Robert Cuthbert, Caroline Kerr, Damian Finnegan, Lorraine McKenna, Mary Frances McMullin, Ken Mills, Victoria Pechey; Birmingham Heartlands Hospital: Donald Milligan, Manos Nikolousis, Neil Smith, Sundip Sohanpal; Blackpool Victoria Hospital NHS Foundation Trust: Paul Cahalin, Joyce Jones, Seye Kolade; Borders General Hospital: Ashok Okhandiar, Melanie Tolson, John Tucker; Bradford Royal Infirmary: Sam Ackroyd, Victoria Drew, Vickie Hawkins, Anita Hill, Lisa Newton, Adrian Williams; Bristol Haematology and Oncology Centre: Roger Evely, Lucy Henderson, Jonathan Heywood, David Marks, Priyanka Mehta, Rachel Protheroe, Graham Standen; Cambridge University Hospitals NHS Foundation Trust: Anthony Bench, Jenny Craig, Charles Crawley, George Follows, Linda Gidman, Brian Huntly, Pramila Krishnamurthy, Kuldip Singh, George Vassilou; Chesterfield Royal Hospital: Rod Collin, Sian Edwards, Lynne Hardy, Lesley Stevenson, Mark Wodzinski; Children's Hospital for Wales: Philip Connor, Meriel Jenney, Indu Thakur; Christchurch Hospital: Geraldine Duncan, Peter Ganly, Vickie Hanrahan, Joanne Sanders, Ruth Spearing; Christie Hospital NHS Trust: Safia Barber, Michelle Davies, Mike Dennis,Steven Heald, Samar Kulkarni, Simeon Mitton, Tim Somervaille, Jo Tomlins; Countess of Chester Hospital: Edwin Lee, Arvind Pillai, Janet Spriggs, Lesley Stevens, Salaheddin Tueger; Darent Valley Hospital: Anil Kamat, Lesley Knott, Tariq Shafi, Jaquie Smith-Hedges; Derby Hospitals NHS Foundation Trust: Juanah Addada, Julie Dockree, Joanna Grenyer, Christopher Millar; Derriford Hospital: Joanna Farrugia, Hannah Hunter, Patrick Medd, Tim Nokes, Wayne Thomas; Doncaster Royal Infirmary: Robert Cutting, Joe Joseph, Stuti Kaul, Youssef Sorour; Dorset County Hospital NHS Foundation Trust: Sally Love, Akeel Moosa, Amy Publicover; East Kent Hospitals University NHS Foundation Trust: Lavinia Davey, Marie Evans, Jindriska Lindsay, Chris Pocock, Vijay Ratnayake, Kamiran Saied; East Sussex Hospitals NHS Trust: Judy Beard, Kay Jones-Skipper, Satyajit Sahu; Epsom and St Helier University NHS Trust: Nikki Evans, Jane Mercieca; Falkirk and District Royal Infirmary: Christopher Brammer, Marie Hughes; Glan Clwyd Hospital: Margaret Goodrick, Earnest Heartin, Christine Hoyle, Fiona Redmond; Gloucestershire Royal Hospital: Eve Blundell, Chris Ford, Rebecca Frewin, Richard Lush, Adam Rye; Great Western Hospital: Norbert Blesing, Nicola Cowling, Jan Dodge, Atherton Gray, Sarah Green, Chanelle Meyer, Alex Sternberg; Guy’s and St Thomas' Foundation Trust: Beverley Hunt, Jennie Lok, Donal McLornan, Yvonne Morgan, Michael Neat, Kavita Raj; Hairmyres Hospital: Iain Singer; Hammersmith Hospital: Mary Rose Nazaret, Jiri Pavlu; Heatherwood and Wexham Park NHS Foundation Trust: Nicky Barnes, Nicola Bienz, Peter Mackie, Simon Moule, Mark Offer, Nicola Philpott; Hereford County Hospital: Lisa Robinson; Herlev Hospital: Morten Krogh Jensen, Ulrik Overgaard; Hillingdon Hospital: Richard Kaczmarski, Gail Poonawala; Hull Royal Infirmary: Sahra Ali, Andrew Fletcher, Simone Greene, Judith Hogg; Ipswich Hospital NHS Trust: Debo Ademokun, Isobel Chalmers, Andrew Hodson, Mary Selvaraj; James Cook University Hospital: Raymond Dang, Jamie Maddox, Pam McLinn, Dianne Plews, Angela Wood; James Paget University Hospital: Rachel Conway, Cesar Gomez, Manzoor Mangi, Shalal Sadullah; John Radcliffe Hospital: Angela Hamblin, Shirley Henderson, Anna Schuh, Adele Timbs, Paresh Vyas; Kettering General Hospital: Isaac Wilson- Morkeh, Mark Kwan, Matthew Lyttelton, Margaret Turns, Joanne Walsh; Leicester Royal Infirmary: Ann Hunter, Murray Martin; Lincoln County Hospital: Caroline Harvey, Rhiannan Pegg, Kandeepan Saravanamuttu; Maidstone Hospital: Evangelia Dimitriadou, Richard Gale, Donald Gillett, Saad Rassam; Manchester Royal Infirmary: Pippa Bulger, Vicki Conroy, Fiona Dignan, Patricia Sparham, Eleni Tholouli; Medway Maritime Hospital: Maadh Aldouri, Vivienne Andrews, Kay Jones, Nicola Southwell; Milton Keynes Hospital NHS Foundation Trust: Moez Dungarwalla, Subir Mitra, Denise White; Monklands Hospital: Linda Callachan, Janet Duncan, Lisa Ferguson, Lindsay Mitchell, John Murphy, Pamela Paterson, Alaeddin Raafat, Charlotte Thomas; New Cross Hospital: Supratik Basu, Claire Beardsmore, Sunil Hada, Alan MacWhannell, Julie Walsh; Ninewells Hospital and Medical Centre: Keith Gelly, Duncan Gowans, Ann Hyslop, Norene Keenan, David Meiklejohn, Sudhir Tauro; Norfolk and Norwich University Hospital NHS Foundation Trust: Matthew Lawes; North Middlesex University Hospital: Neil Rabin; Northampton General Hospital: Angela Bowen, Andrea Jones, Suchitra Krishnamurthy, Jan Miles, Jane Parker; Northwick Park Hospital: Robert Ayto, Louise Enfield, Shelley Harvey, Nicki Panoskaltsis; Nottingham University Hospital (Paediatrics): Kate Forman, Simone Stokley; Nottingham University Hospitals NHS Trust: Jenny Byrne, Ian Carter, Emma Das-Gupta, Julie Kenny, Laurence Pearce, Nigel Russell, Melissa Shaw; Odense University Hospital: Lone Friis, Claus Marcher, Birgitte Wolf Lundholm; Peterborough District Hospital: Susan George, Catherine Hoggarth, Sateesh Nagumantry, Kanchan Rege, Hannah Sims, Muthuswamy Sivakumaran; Pinderfields General Hospital: Mary Chapple, Victoria Hawkins, Paul Moreton, Charlotte Mountain, Louise Parker, Kavita Patil, David Wright; Poole General Hospital: Anita Immanuel, Fergus Jack, Monika Kozlowska, Rebecca Maddams, Kate Mutendera; Queen Alexandra Hospital: Robert Corser, Tanya Cranfield, Helen Dignum, Mary Ganczakowski, Christopher Jones, Shanqin Liu, Yvonne Silber; Queen Elizabeth Hospital Birmingham: Charles Craddock, Sally Jeffries, Jim Murray, Sandeep Nagra, Manoj Raghavan; Queen Elizabeth Hospital Woolwich: Betty Cheung, Suzanne Chukundah, Bridget Kabagambe, Nic Ketley, Duran Maestre, Ana Theodorah Nago; Queen Elizabeth Hospital, Kings Lynn: Jane Keidan, Annette Miles; Queens Hospital: Pamela Benson, Claire Hemmaway; Raigmore Hospital NHS Highland: Seonaid Arnott, Peter Forsyth, Chris Lush, Georgina Simpson; Rigshospitalet: Mette Klarskov Andersen, Morten Tolstrup Andersen, Ole Wei Bjerrum, Rikke Duus, Kirsten Grønbæk, Peter Kampmann, Lars Kjeldsen, Ove Juul Nielsen, Carsten Niemann; Royal Berkshire Hospital: Juliette Dye, Anna Gillham, Henri Grech, Asif Khan, Stuart Mucklow, Rebecca Sampson; Royal Bournemouth Hospital: Joseph Chacko, Rachel Hall, Helen McCarthy, Nicola Naraine; Royal Cornwall Hospital: Desmond Creagh Richard Noble, Bryson Pottinger; Royal Devon and Exeter Hospital: Emily Collyer, Malcolm Hamilton, Lydia Hill, Paul Kerr, Jackie Ruell, Mary Tamplin, Anthony Todd; Royal Free Hospital: Panos Kottaridis; Royal Hallamshire Hospital: Harpreet Kaur, John Snowden, Gill Wilson; Royal Manchester Children's Hospital: Denise Bonney; Royal Oldham: Allameddine Allameddine, David Osborne; Royal Surrey County Hospital: Johannes De Vos, Elisabeth Grey-Davies, Louise Hendry; Royal United Hospital: Christine Cox, Josephine Crowe, Christopher Knechtli; Russells Hall Hospital: Savio Fernandes, Steve Jenkins, Jeff Neilson, Angela Watts, Claire Watts; Salford Royal Hospital: John Houghton, Simon Jowitt, Anne-Marie Lydon, Sonya Ravenscroft, Rowena Thomas-Dewing, Sonya Zaman; Salisbury Hospital NHS Foundation: Nick Cross, Jonathan Cullis, Tamara Everington, Effie Grand, Claire Smith; Sandwell Hospital Trust: Richard Murrin, Igor Novitzky-Basso, Lisa Smith, Farooq Wandoo; Singleton Hospital: Helen Cheley, Saad Ismail, Unmesh Mohite, Kez Richards; South Devon Healthcare NHS Foundation Trust: Patrick Roberts, Nichola Rymes, Steve Smith, Deborah Turner; Southampton General Hospital (Paediatrics): Mary Morgan; Southampton University Hospital NHS Trust: Kate Hill, Matthew Jenner, Kim Orchard, Deborah Richardson; Southern General Hospital: Alistair Hart, Anne Morrison, Ian Macdonald, Emma Moody, Claudia Turley; St Helens and Knowsley NHS Trust: Toby Nicholson; St James University Hospital: Karen Benn, David Bowen, Gordon Cook, Paul Evans, Maria Gilleece, Richard Kelly, Suzanne Liebersbach, Mike Short, Fatima Umama; St Richards Hospital: Phillip Bevan, Sarah Janes; Stafford Hospital: Michelle Forrester-Amoako, Carol Harvey, Kamaraj Karunanithi, Neil Phillips, Paul Revell, Andrew Stewart; Taunton and Somerset Foundation Trust: Sarah Allford, Simon Bolam, Angela Locke; The Newcastle upon Tyne NHS Foundation Trust: Matt Collin, Catherine Cox, Graham Jackson, Gail Jones, Anne Lennard, Smeera Nair; University College London Hospitals: Kirit Ardeshna, Ben Carpenter, Sharon Edleston, Victoria Grandage, Rachael Hough, Asim Khwaja, Wong Wai Keong, Nishal Patel, Andres Virchis, Jamie Wilson, Kwee Yong; University Hospital Aintree: Barbara Hammer, Walid Sadik, Vikram Singh, Jeffery Smith, Barrie Woodcock, Lynny Yung; University Hospital Coventry and Warwickshire NHS Trust: Yvonne Beadle, Anton Borg, Beth Harrison, Nicholas Jackson, Peter Rose, Syed Bokhari; University Hospital Lewisham: Katrina Armour, Abel Jalloh, Tullie Jeghen, Naheed Mir; University Hospital of North Staffordshire NHS Trust: Andrew Stewart, Deepak Chandra, Kamaraj Karunanithi, Srinivas Pillai; University Hospital of North Tees and Hartlepool: Philip Mounter, Simon Sinclair; University Hospital of Wales: Caroline Alvares, Steve Austin, Joanne Gill, Wendy Ingram, Jonathan Kell, Steve Knapper, Sian Meyrick, Katja Williams; University of Liverpool and Royal Liverpool University Hospital: Richard Clark, Elizabeth Dale, Rak Salim, Usira Vithanarachchi, Sarah Watmough ; Victoria Hospital NHS Fife: Kerri Davidson, Maureen Devaney, Stephen Rogers, Peter Williamson; Wishaw General Hospital: Annielle Hung, Gila Helenglass; Worcestershire Royal Hospital: Fiona Clark, Elizabeth Maughan, Juliet Mills, Gaynor Pemberton, Nicholas Pemberton, Salim Shafeek; Worthing Hospital: Aisling O’Driscoll, Santosh Narat, Sarah Thompson; York Hospital: Lee Bond, Laura Munro, Nora Youngs; Ysbyty Gwynedd: Kathryn Chester, James Seale, Alice Thomas.
Appendix
Supplementary Information
Multiparameter flow cytometry detection of measurable residual disease.
Patients were allocated to one of three reference multiparameter flow cytometry–measurable residual disease (MFC-MRD) laboratories, their samples were sent by overnight mail to the allocated laboratory. Following ammonium chloride lysis, bone marrow/peripheral blood nucleated cells were labelled with the consensus antibody panel below.
AML17 MFC- MRD Antibody Panel.
Presentation samples were screened for leukemic-aberrant-immunophenotypes (LAIPs) and then at least tubes 1-2 from the panel were selected for MRD analysis of follow-up samples. Bone marrow aspirates to assess remission status were performed at 18-21 days after the end of chemotherapy. If the marrow was hypoplastic and assessment of status not possible, a repeat marrow was performed if possible.
Each MFC-MRD laboratory used the same sample processing protocol with cell acquisition performed on a FACSCanto (BD Biosciences, Franklin Lakes, NJ) flow cytometer (BD Biosciences). Acquisition was set for 500,000 to 1 million cells or as many cell events as possible for follow-up samples. Data review was performed regularly to ensure interlaboratory standardization and included periodically updated reference control bone marrow profiles. Postacquisition analyses of the flow cytometry data from the reference flow cytometric laboratories was performed centrally (blinded to clinical data) using FlowJo software (Treestar, Ashland, OR). LAIPs were screened for in blast populations of presentation samples, initially CD117+ and CD34+ blasts (gated by FSC/SSC/CD45/ CD117 or CD34) with preset ‘different from normal’ regions that were also applied as a ‘different from normal’ approach in follow-up samples. LAIPs were also screened for by overlaying CD117+ and/or CD34+ leukemic blasts with reference controls (‘normal’ CD117+ and/or CD34+ blasts). In presentation samples where blasts were mainly or all negative for CD117 and CD34, blasts were gated by CD45/SSC or FSC/SSC then CD45 intermediate and other markers (such as HLADR, CD56, CD33, CD13) followed by overlaying with reference controls to identify LAIPs for which sensitivity threshold was at least 0.05% of leukocytes (i.e. less than 0.05% of leukocytes from the control BMs fell within the defined LAIP gate). LAIPs for monitoring in follow-up samples were selected as blast subpopulations that deviated from the normal antigen profiles with sufficient detection sensitivity, usually comprised > 10% of leukemic blasts and from previous data (Freeman SD et al: J Clin Oncol 31:4123-31, 2013; Bradbury C et al: Leukemia 29:988-91, 2015) were known to be stable at follow-up (∼0.02-0.05% sensitivity thresholds). LAIP percentages were reported as percentage of nucleated cells expressing the identified LAIP. In some patients minor or major immunophenotypic changes from baseline LAIPs were detected by ‘different from normal’ LAIP regions. These were considered as MRD if new LAIPs fulfilled criteria for detection sensitivity with less than 0.05% of TNCs from the control BMs fell within the newly defined LAIP gate. If no adequate presentation sample was available for a patient the “different-from normal” LAIP approach applied to blasts was used to detect MFC-MRD positivity. In this study only samples for which there were pre-treatment LAIPs to monitor could be reported as MRD negative whilst samples with any level of MRD detected above a diagnostic or different-from-normal FU LAIP threshold were reported as MRD positive. Inadequate follow-up samples (defined by < 0.1% blasts and/or < 100 cell events within the total blast (gated by CD45/SSC plus CD34+ and/or CD117+) gate) were excluded from data analysis unless there was detectable MRD from a clear cluster of at least 20 LAIP cell events detected. Any level of MFC-MRD detected above the sensitivity threshold was considered MRD-positive.
No LAIP was identified in pretreatment samples of 102 patients (5% of adequate pretreatment samples). Adequate post course 1 samples were received in 71 of these patients, 14 had detectable MFC-MRD by different from normal approach at this timepoint (including 4 with > 5% blasts) and were included in the analysis.
Patients were designated in CR but without MRD data post course 1 or course 2 if there was 1) no / inadequate diagnostic sample or 2) adequate diagnostic sample but no LAIP identified (unless different-from-normal LAIP identified post course 1 / 2) or 3) no / inadequate samples post course 1 / 2.
Reasons for missed samples included prioritizing of bone marrow for RT-qPCR monitoring of NPM1 mutations in the second part of the AML17 trial.
Statistical analysis.
Survival endpoints are defined as per Cheson, which indicates the time of origin for all endpoints. Survival is calculated from entry, relapse from date of remission with death in remission as a competing risk. Follow-up was completed March 2016. Multivariable analyses were adjusted for the known prognostic factors of age, wbc, sex, performance status, disease type (secondary or de novo) and cytogenetic group.
Fig A1.
Flowchart of treatments given to patients in the NCRI AML17 trial. (A) Pre-October 2011 (induction gemtuzumab ozogamicin randomization). (B) Post-October 2011 (daunorubicin dose randomization in induction). Note: patients who did not satisfy the hepatic entry criteria (liver function < 2 × ULN) in (A) were allocated ADE; until June, 2010 the consolidation randomization was MACE vs MACE/MidAC; the DA dose randomization was closed in October, 2013, and patients subsequently received DA (60 mg); the lestaurtinib (CEP-701) randomization closed in October 2012; the mTOR (everolimus) randomization closed in August, 2012; the high risk randomization in October, 2012. All core binding factor (CBF) leukemias were eligible for gemtuzumab ozogamicin and were given 3 mg/m2 with course 2 if they did not receive it by gemtuzumab ozogamicin randomization with course 1. From June, 2012 patients with informative real-time quantitative polymerase chain reaction (RT-qPCR) MRD markers could enter the ‘Monitor vs no Monitor’ randomization that investigates the impact of serial RT-qPCR monitoring post completion of treatment on outcome, quality of life and health economics. ADE, cytarabine, daunorubicin, and etoposide; APL, acute promyelocytic leukemia; CBF, core binding factor; CEP-701, lestaurtinib; DA, daunorubicin and cytarabine; GO, gemtuzumab ozogamicin (3 or 6 mg/m2); FLAG-Ida, fludarabine, cytarabine, GCSF, and idarubicin; FLT3, FMS-like tyrosine kinase-3; mTOR, everolimus; R, randomization.
Fig A2.
CONSORT diagram. Outline of patient sample flow for MRD study. (*) Includes patients for whom remission status could not be classified as exact timing of any remission was unavailable. CR, complete remission; C1, course 1, C2, course 2. LAIP, leukemia-associated–immunophenotype; MRD, measurable residual disease.
Fig A3.
OS according to response status after course 1. (A) All patients. OS censored at allogeneic SCT. (B) NPM1–wild-type patients at standard risk. (C) NPM1–wild-type patients at standard risk, censored at allogeneic SCT. CR, complete remission; MRD, measurable residual disease; OS, overall survival; PR, partial remission; RD, resistant disease; SCT, stem-cell transplantation.
Fig A4.
Forest plots for relapse by multiparametric flow cytometry-MRD status for patients in CR (A) after course 1 and (B) after course 2 stratified by cytogenetic risk group and NCRI AML 17 risk score group. CR, complete remission; MRD, measurable residual disease.
Fig A5.
Cumulative incidence of relapse by multiparametric flow cytometry -MRD level. (MRD− v MRD+ < 0.1% v MRD+ ≥ 0.1%) after course 1. (A) CBF AML. (B) Standard-risk NPM1 mutant. AML, acute myeloid leukemia; CBF, core binding factor; MRD, measurable residual disease; MRD < 0.1%, MRD+ < 0.1%; MRD 0.1%+, MRD+ ≥ 0.1%.
Fig A6.
Standard-risk NPM1-wild type. Overall survival (OS) according to multiparametric flow cytometry-MRD status after course 2, censored at any allogeneic stem-cell transplantation. CR, complete remission; MRD, measurable residual disease.
Fig A7.
OS according to response status after course 1, applying European LeukemiaNet (ELN)/Cheson criteria for PR and RD instead of MRC criteria (ELN criteria for PR: all hematologic criteria of CR; decrease of bone marrow blast percentage to 5% to 25% with decrease of pretreatment bone marrow blast percentage by ≥ 50%). (A) All patients. (B) Patients at good and standard risk (patients known to be at poor risk excluded). (C) Patients at standard risk. (D) Patients at standard risk, OS censored at allogeneic SCT. CR, complete remission; MRD, measurable residual disease; OS, overall survival; PR, partial remission; RD, resistant disease; SCT, stem-cell transplantation.
Table A1.
Genetic Characteristics of C1 MFC-MRD- / C2 MFC-MRD+ Patients
Table A2.
Correlation of Risk Group and Clinical Response by Combined Course 1 and 2 Response Status
Table A3.
Outcomes for Patients by Peripheral Count Recovery Response Combined With MRD Status
Footnotes
Supported by Cancer Research UK and National Institutes of Health Research, which contributed nonprofit funding for this manuscript; no commercial funding was received.
Deceased.
Written on behalf of the UK National Cancer Research Institute AML Working Group.
See accompanying Editorial on page 1463
AUTHOR CONTRIBUTIONS
Conception and design: Sylvie D. Freeman, Robert K. Hills, David Grimwade, Alan K. Burnett, Nigel H. Russell
Provision of study materials or patients: Asim Khwaja
Collection and assembly of data: Sylvie D. Freeman, Robert K. Hills, Paul Virgo, Naeem Khan, Steve Couzens, Richard Dillon, Amanda Gilkes, Laura Upton, Ove Juul Nielsen, James D. Cavenagh, Gail Jones, Asim Khwaja, Paul Cahalin, Ian Thomas
Data analysis and interpretation: Sylvie D. Freeman, Robert K. Hills, Alan K. Burnett, Nigel H. Russell
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sylvie D. Freeman
No relationship to disclose
Robert K. Hills
No relationship to disclose
Paul Virgo
No relationship to disclose
Naeem Khan
No relationship to disclose
Steve Couzens
No relationship to disclose
Richard Dillon
No relationship to disclose
Amanda Gilkes
No relationship to disclose
Laura Upton
No relationship to disclose
Ove Juul Nielsen
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Roche
James D. Cavenagh
No relationship to disclose
Gail Jones
No relationship to disclose
Asim Khwaja
Consulting or Advisory Role: Jazz Pharmaceuticals, Karus Therapeutics
Paul Cahalin
No relationship to disclose
Ian Thomas
No relationship to disclose
David Grimwade
No relationship to disclose
Alan K. Burnett
Employment: CTI Life Sciences
Consulting or Advisory Role: Jazz Pharmaceuticals
Nigel H. Russell
No relationship to disclose
REFERENCES
- 1.Wheatley K, Burnett AK, Goldstone AH, et al. : A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. Br J Haematol 107:69-79, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Schlenk RF, Benner A, Hartmann F, et al. : Risk-adapted postremission therapy in acute myeloid leukemia: Results of the German multicenter AML HD93 treatment trial. Leukemia 17:1521-1528, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kern W, Haferlach T, Schoch C, et al. : Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: Data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 101:64-70, 2003 [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell MR, Tallman MS, Abboud CN, et al. : Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:926-957, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Estey E, Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassan R: Using minimal residual disease to improve treatment response definitions and hematopoietic cell transplantation strategy in acute leukemia. J Clin Oncol 34:300-302, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Inaba H, Coustan-Smith E, Cao X, et al. : Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol 30:3625-3632, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loken MR, Alonzo TA, Pardo L, et al. : Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: A report from Children’s Oncology Group. Blood 120:1581-1588, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang J, Goswami M, Tang G, et al. : The clinical significance of negative flow cytometry immunophenotypic results in a morphologically scored positive bone marrow in patients following treatment for acute myeloid leukemia. Am J Hematol 90:504-510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccisano F, Maurillo L, Del Principe MI, et al. : Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood 119:332-341, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Grimwade D, Freeman SD: Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Hematology (Am Soc Hematol Educ Program) 2014:222-233, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Ossenkoppele G, Schuurhuis GJ: MRD in AML: Does it already guide therapy decision-making? Hematology (Am Soc Hematol Educ Program) 2016:356-365, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki D, Wood BL, Othus M, et al. : Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol 34:329-336, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hourigan CS, Goswami M, Battiwalla M, et al. : When the minimal becomes measurable. J Clin Oncol 34:2557-2558, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Hourigan CS, Gale RP, Gormley NJ, et al. : Measurable residual disease testing in acute myeloid leukaemia. Leukemia 31:1482-1490, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Buccisano F, Maurillo L, Spagnoli A, et al. : Cytogenetic and molecular diagnostic characterization combined to postconsolidation minimal residual disease assessment by flow cytometry improves risk stratification in adult acute myeloid leukemia. Blood 116:2295-2303, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Freeman SD, Virgo P, Couzens S, et al. : Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 31:4123-4131, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Othus M, Wood BL, Stirewalt DL, et al. : Effect of measurable (‘minimal’) residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia 30:2080-2083, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravandi F, Jorgensen J, Borthakur G, et al. : Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer 123:426-435, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terwijn M, van Putten WL, Kelder A, et al. : High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J Clin Oncol 31:3889-3897, 2013 [DOI] [PubMed] [Google Scholar]
- 21. doi: 10.1200/JCO.2003.04.036. Cheson BD, Bennett JM, Kopecky KJ, et al: Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21:4642-4649, 2003 [Erratum: J Clin Oncol 20041;22(3):576. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson P, Hills RK, Grech A, et al. : An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica 101:1351-1358, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivey A, Hills RK, Simpson MA, et al. : Assessment of minimal residual disease in standard-risk AML. N Engl J Med 374:422-433, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Balsat M, Renneville A, Thomas X, et al. : Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the acute Leukemia French Association Group. J Clin Oncol 35:185-193, 2017 [DOI] [PubMed] [Google Scholar]
- 25. Burnett AK, Hills RK, Wheatley K, et al: A sensitive risk score for directing treatment in younger patients with AML. Blood 108:18, 2006. [Google Scholar]
- 26.Ling V, Burnett AK, Bradstock K, et al. : Utility of a clinical risk score to identify high-risk patients with de novo acute myeloid leukaemia in first remission after high-dose cytarabine (HiDAC) based induction chemotherapy. Br J Haematol 160:861-863, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Early Breast Cancer Trialists’ Cooperative Group . Treatment of Early Breast Cancer. 1. Worldwide Evidence 1985-1990. Oxford, UK, Oxford University Press, 1990. [Google Scholar]
- 28.Grimwade D, Hills RK, Moorman AV, et al. : Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354-365, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Xie H, Estey EH: Reply to D. Przepiorka et al. J Clin Oncol 33:3676-3677, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Xie H, Wood BL, et al. : Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol 33:1258-1264, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Buccisano F, Maurillo L, Gattei V, et al. : The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia 20:1783-1789, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Maurillo L, Buccisano F, Del Principe MI, et al. : Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol 26:4944-4951, 2008 [DOI] [PubMed] [Google Scholar]
- 33.da Silva-Coelho P, Kroeze LI, Yoshida K, et al. : Clonal evolution in myelodysplastic syndromes. Nat Commun 8:15099, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makishima H, Yoshizato T, Yoshida K, et al. : Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 49:204-212, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkin B, Londoño-Joshi A, Kang Q, et al. : Ultrasensitive mutation detection identifies rare residual cells causing acute myelogenous leukemia relapse. J Clin Invest 127:3484-3495, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlush LI, Mitchell A, Heisler L, et al. : Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547:104-108, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Bullinger L, Döhner K, Döhner H: Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol 35:934-946, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Gerstung M, Papaemmanuil E, Martincorena I, et al. : Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat Genet 49:332-340, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]