ABSTRACT
Objective
Although catatonia can occur secondary to a general medical condition, catatonia itself has been known to lead to various medical compolications. Although case reports on the association of catatonia with subsequent medical complications have been documented, no comprehensive large-scale study has been performed. To investigate specific medical complications after catatonia, we conducted a retrospective cohort study of specific medical complications of schizophrenia patients with catatonia.
Methods
The 1719 schizophrenia inpatients in our study were categorized into two groups: the catatonia group, i.e., those who exhibited catatonic stupor while they were hospitalized, and the noncatatonia group, i.e., those who never exhibited catatonic stupor. Differences between the two groups in the occurrence of subsequent medical complications were examined using linear and logistic regression analyses, and models were adjusted for potentially confounding factors.
Results
The catatonia group had an increased risk for mortality (odds ratio = 4.8, 95% confidence interval = 2.0–10.6, p < .01) and certain specific medical complications, i.e., pneumonia, urinary tract infection, sepsis, disseminated intravascular coagulation, rhabdomyolysis, dehydration, deep venous thrombosis, pulmonary embolism, urinary retention, decubitus, arrhythmia, renal failure, neuroleptic malignant syndrome, hypernatremia, and liver dysfunction (all p values < .01, except for deep venous thrombosis, p = .04 in the multiple linear regression analysis).
Conclusions
Catatonic stupor in schizophrenia substantially raises the risk for specific medical complications and mortality. Hyperactivity of the sympathetic nervous system, dehydration, and immobility, which are frequently involved in catatonia, might contribute to these specific medical complications. In catatonia, meticulous care for both mental and medical conditions should be taken to reduce the risk of adverse medical consequences.
Key words/Abbreviation: catatonic stupor, dehydration, immobility, medical complications, schizophrenia, sympathetic nervous system, DIC = disseminated intravascular coagulation
INTRODUCTION
Although catatonia can occur secondary to a general medical condition, catatonia itself has been known to lead to various medical complications and even death. Calmeil (1832) (1) described a patient with catatonia who progressed to stuporous exhaustion and subsequently died with no remarkable findings upon autopsy. Kahlbaum (1874) (2) reported that malnutrition, severe anemia, and tuberculosis often occur after advanced catatonia. Before the advent of neuroleptic drugs, numerous investigators reported similar links between catatonia and various subsequent medical complications (3–6). Jacobi (1930) (4) proposed a condition termed “late catatonia,” in which women during their middle- and old-age periods exhibit catatonia and rapidly progress to death. Stauder (1934) (5) coined the term “lethal catatonia” for a condition involving catatonia with subsequent death. According to Mann et al. (1986) (7), the typical progression of these patients begins with signs of catatonia, followed by somatic disturbances, and ends in death. Because many symptoms of this condition overlap with neuroleptic neuroleptic malignant syndrome, many investigators have suggested that neuroleptic neuroleptic malignant syndrome represents a neuroleptic-induced toxic or iatrogenic organic form of lethal catatonia (7). Because the clinical aspects of neuroleptic neuroleptic malignant syndrome and severe catatonia are quite similar, Philbrick and Rummans (1994) (8) proposed the term “malignant catatonia,” which is applied to both neuroleptic- and nonneuroleptic-induced severe catatonia. In this condition of severe catatonia, patients exhibit autonomic instability or hyperthermia and can have life-threatening medical complications.
The medical complications resulting from catatonia were reported in previous case reports (7–12), including rhabdomyolysis, renal failure, disseminated intravascular coagulation (DIC), tachycardia, bradycardia, cardiovascular collapse, acute respiratory distress syndrome, respiratory arrest, myocardial infarction, sepsis, seizures, hypoglycemia, upper gastrointestinal tract bleeding, hepatocellular damage, intestinal pseudo-obstruction, deep venous thrombosis, and pulmonary embolism. However, despite the presence of these life-threatening conditions in some case reports of catatonia patients, studies concerning the specific medical complications that arise after catatonia have not been undertaken, and to our best knowledge, no large-scale study has been performed to identify them and the largest case series study covered only 13 catatonia cases (13). Moreover, the mechanisms underlying the development of these medical conditions in catatonia patients have yet to be elucidated. To address these issues, we performed a retrospective cohort study of the association between catatonia and specific medical complications with a large population involving 1719 psychiatry inpatients with schizophrenia.
MATERIAL AND METHODS
Participants
Ethical aspects of this study were reviewed and approved by the Human Research Ethics Committee at the Ashikaga Red Cross Hospital. For our retrospective cohort study, we included all 1719 schizophrenia inpatients who were hospitalized in the psychiatric unit in Ashikaga Red Cross Hospital during the period between October 1999 and March 2016. All patients provided a general consent for their clinical information to be used for research purposes. Because some patients were incapable of giving consent because of their severe psychiatric symptoms, this study was performed after obtaining informed consent from either the patient or his or her caregiver who had legal custody of the participant. Diagnosis was based on criteria in the ICD-10, and all patients with schizophrenic disorders (F2) were included, i.e., schizophrenia (F20), schizotypal disorder (F21), delusional disorders (F22), brief psychotic disorder (F23), shared psychotic disorder (F24), schizoaffective disorders (F25), other psychotic disorder not due to a substance or known physiological condition (F28), and unspecified psychosis not due to a substance or known physiological condition (F29). Because this study focused on patients with schizophrenic disorders, patients with other psychiatric diseases that are also associated with catatonia, such as mood disorders, neurodevelopmental disorders, and neurodegenerative disorders, were not included. Although catatonia was established as being independent of schizophrenic disorders in DSM-5, it is known to sometimes occur in schizophrenic disorders as it does in mood disorders. Because each psychiatric disease has its own age distribution and likely has specific medical complications associated with it, a study using a mixture of all these groups might not yield clear results. Moreover, medical complications in catatonia reported in previous case reports (7–13) might partly be explained as adverse effects of antipsychotics, e.g., rhabdomyolysis, renal failure, cardiovascular collapse, seizures, hepatocellular damage, deep venous thrombosis, or pulmonary embolism. Inclusion of other psychiatric diseases might thus substantially affect the results because antipsychotics are much less frequently used for other psychiatric diseases than for schizophrenic disorders. Indeed, our aim was to investigate the incidence of medical complications under the most similar possible conditions, i.e., the same disorder, similar age distribution, and similar status of antipsychotic medication use.
Medical complications that preceded the catatonia diagnosis were counted as catatonia because of other medical conditions, such as encephalitis, autoimmune disorders, or endocrine disorders, namely, organic or symptomatic mental disorders. To rule out these medical conditions completely, laboratory tests including thyroid hormone levels were routinely conducted upon admission and at the onset of catatonia. Likewise, brain imaging, i.e., computed tomography and/or magnetic resonance imaging, and lumbar puncture were routinely performed for each patient upon onset of catatonia. In addition, an electroencephalogram was frequently administered to differentiate between catatonic stupor and loss of consciousness after a general medical condition. These conditions included two patients with anti-N-methyl-d-aspartate receptor encephalitis, one patient with neuropsychiatric systemic lupus erythematosus, one case with Grave's disease, and one patient with hepatic encephalopathy. Because these catatonia patients were categorized into organic, including symptomatic, mental disorders (F0 in the ICD-10), they were not included in this study.
The patients with schizophrenia were categorized into two groups: the catatonia group, which included those who exhibited catatonic stupor, and the noncatatonia group, which included those who did not exhibit catatonic stupor while they were in the hospital. For catatonia assessment, we applied only catatonic stupor, which is one of the most common signs of catatonia along with catatonic excitement. Although it is sometimes difficult to assess whether excitement is induced by catatonia or by other psychiatric symptoms such as hallucinations and delusions, the sign of stupor is remarkably clear to clinicians, e.g., being unable to move and requiring assistance with all activities of daily living, often requiring infusion therapy or tube feeding. Thus, it has been standard practice in our hospital to routinely assess and record catatonic stupor. Our assessment followed Bush and Fink's definition of catatonic stupor (14,15) with partial modification, i.e., a behavioral syndrome marked by extreme hypoactivity, immobility, and minimal responsiveness to stimuli at least for several hours, which prevented the patients from eating at least one meal.
Our hospital is in the northern Kanto region of Japan, approximately 100 km (62 miles) north of Tokyo, and serves a population of almost 4 million. It is the only general hospital in this region that has both a critical care medical center and a psychiatric unit, which involves both voluntary and involuntary admissions. Our psychiatric unit admits schizophrenia patients with severe medical complications and treat them in this psychiatric unit with the help of specialists in each medical field. Accordingly, all staff members receive specialized emergency response training. Approximately 45% of inpatients assessed for this study had one or more medical conditions that required treatment on admission, which included previously known chronic medical complications, such as hypertension and diabetes, and subsequent medical complications, such as pneumonia and ileus, that arose during outpatient treatment or inpatient treatment at other mental hospitals. All medical information, such as laboratory data, radiological assessments, and physiological function tests, was available for all the study participants, and because the hospital employs specialists in each medical field who offer advanced treatments, diagnoses were likely more precise and treatment more intensive than those at other hospitals in this region. Accordingly, the proportion of psychiatry patients with severe medical complications who also needed psychiatric and physical rehabilitation after the acute phase is greater than those at any other psychiatric hospital in this region. This wide range of treatment is a characteristic for our psychiatric unit.
Definition of Medical Complications
We used the definition of each medical complication that was established by experts in each field in Japan, with the exception of sepsis and neuroleptic malignant syndrome. The definition of sepsis was restricted to cases with positive blood cultures because some symptoms of sepsis overlap with autonomic symptoms and neuropsychiatric changes that are also observed in catatonia. For neuroleptic malignant syndrome, we used the definition of Caroff and Mann (1993) (16), which requires five items, i.e., neuroleptics treatment, hyperthermia, muscle rigidity, 5 of 10 typical sings such as tachycardia and diaphoresis, and exclusion of other causes. For each medical complication, both confirmed cases and probable cases were included in this study. Injuries were divided into two categories, i.e., suicide-related injuries and suicide-unrelated injuries. To be registered among the medical complications in this study, each complication must have caused the patient to be admitted to the hospital or occurred during hospitalization. Therefore, previously known chronic medical conditions, e.g., hypertension and diabetes, that were not causes for admission were excluded to focus on the condition of catatonia rather than long-term ailments. Those chronic medical conditions were counted as the presence of chronic diseases, which is explained below hereinafter.
In this study, we only counted medical complications while the patient was hospitalized and no subsequent admissions were included as outcome. Likewise, postdischarge deaths were not included in the mortality rate.
As mentioned previously, medical complications that preceded the catatonia diagnosis were counted as catatonia because of other medical conditions and those patients were not included in this study. However, one schizophrenic patient who presented with rhabdomyolysis and another one who presented with urinary retention 1 day before occurrence of catatonia stupor were included in this study. There were two reasons behind this inclusion. One reason is that they had already exhibited a state of substupor when rhabdomyolysis and urinary retention occurred. (As previously mentioned, stupor was defined as a behavioral syndrome marked by hypoactivity, immobility, and minimal responsiveness to stimuli at least for several hours, which prevent patients to eat at least one meal. In this context, we defined substupor as the same syndrome, which was not severe enough to prevent them from eating). Another reason is that rhabdomyolysis and urinary retention have not been reported as a medical condition, which causes catatonia, suggesting that those cases of catatonia do not result from rhabdomyolysis or urinary retention.
Covariates
All medical complications that occurred in more than one case in the catatonia group were compared between the two groups. Also compared were mortality, age, sex, presence of a chronic disease(s), length of hospital stay, dose of antipsychotic upon admission, use of first-generation antipsychotics, and global assessment of functioning (17,18) upon admission. The presence of chronic diseases excluded previous medical issues that had already been resolved, such as infantile asthma, but it included ongoing diseases that require continuous care, e.g., hypertension, diabetes, chronic obstructive pulmonary disease, epilepsy, collagen diseases, or those requiring antithrombotic treatment. The doses of antipsychotics were expressed as the chlorpromazine equivalent dose (milligram), which has frequently been used in Japan (19,20), e.g., 1 mg of risperidone was converted to 100 mg of chlorpromazine, and 5 mg of olanzapine was converted to 200 mg of chlorpromazine.
Statistical Analysis
For statistical analysis, the incidence of each medical complication was compared using Fisher's exact test. The logistic regression was calculated to obtain an odds ratio and a 95% confidence interval for each medical complication. Regarding demographics, age was compared using the Student's t test, whereas sex and the existence of a chronic disease(s) were compared using the χ2 test. The length of hospital stay and dose of antipsychotics upon admission were compared using the Mann-Whitney U test. Global assessment of functioning upon admission was compared using the Student's t test.
To account for potential confounding factors, i.e., demographics (age, sex, length of hospital stay), global assessment of functioning upon admission, the presence of chronic diseases, dose of antipsychotics, and use of first-generation antipsychotics, multiple linear regression analysis was performed for the medical complications that were significantly different between the two groups. Each explanatory variable, i.e., age, sex, length of hospital stay, the presence of chronic diseases, and dose of antipsychotics as well as the presence of catatonic stupor, was subjected to multiple linear regression analysis for each medical complication. Excel 2010 with add-on Statcel 3 (OMS Ltd, Tokyo, Japan) and statistical software R were used for all statistical analyses. Two-tailed p values are reported, and p values of less than .05 were considered statistically significant.
RESULTS
Table 1 presents data for patient age and sex, the presence of chronic diseases, length of hospital stay, dose of antipsychotics, and global assessment of functioning upon admission. All 140 admissions with catatonic stupor (108 patients with 32 readmissions) were treated with benzodiazepines, but not with antipsychotics, and 21 (15.0%) had electroconvulsive therapy. The catatonic state itself improved completely except for ten patients who died from subsequent medical complications during the catatonic state. As shown in Table 1, the patient age and sex and the presence of chronic diseases were not significantly different between the two groups. Global assessment of functioning was worse in the catatonia group, suggesting that this group had more severe mental conditions upon admission. The hospital stay was also longer for the catatonia group than for the noncatatonia group. Patients with catatonia often needed not only psychiatric treatment but also medical treatment as well as physical therapy after those treatments. In contrast, the dose of antipsychotics upon admission and proportion of first-generation antipsychotics were lower for the catatonia group than for the noncatatonia group, reflecting the different pharmacotherapeutic treatments prescribed between the two groups. Of 140 patients with catatonic stupor, 61 (43.6%) were not taking antipsychotic medications when catatonic stupor developed.
TABLE 1.
Demographics, Presence of Chronic Diseases, Dose of Antipsychotics, and Global Assessment of Functioning in the Catatonia and Noncatatonia Groups
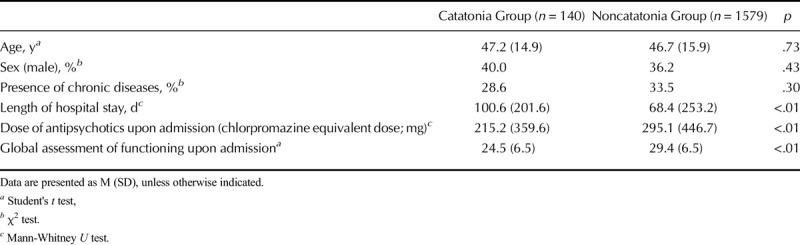
Table 2 presents data for the incidence of each medical complication for both groups. The incidence of many medical complications was higher in the catatonia group. Infections, i.e., pneumonia, urinary tract infection, and sepsis, were observed in the catatonia group more frequently than in the noncatatonia group. Deep venous thrombosis and pulmonary embolism also occurred more frequently in the catatonia group. Other medical complications that were found in the catatonia group more often than in the noncatatonia group were rhabdomyolysis, urinary retention, dehydration, DIC, decubitus, arrhythmia, renal failure, liver dysfunction, and hypernatremia. In particular, DIC, urinary retention, and arrhythmia had odds ratios of more than 10. In addition, the incidence of neuroleptic malignant syndrome was almost restricted to the catatonia group, suggesting that neuroleptic malignant syndrome and a severe form of catatonia, i.e., malignant catatonia (8), might represent two aspects of the same condition.
TABLE 2.
Medical Complications in the Catatonia and Noncatatonia Groups
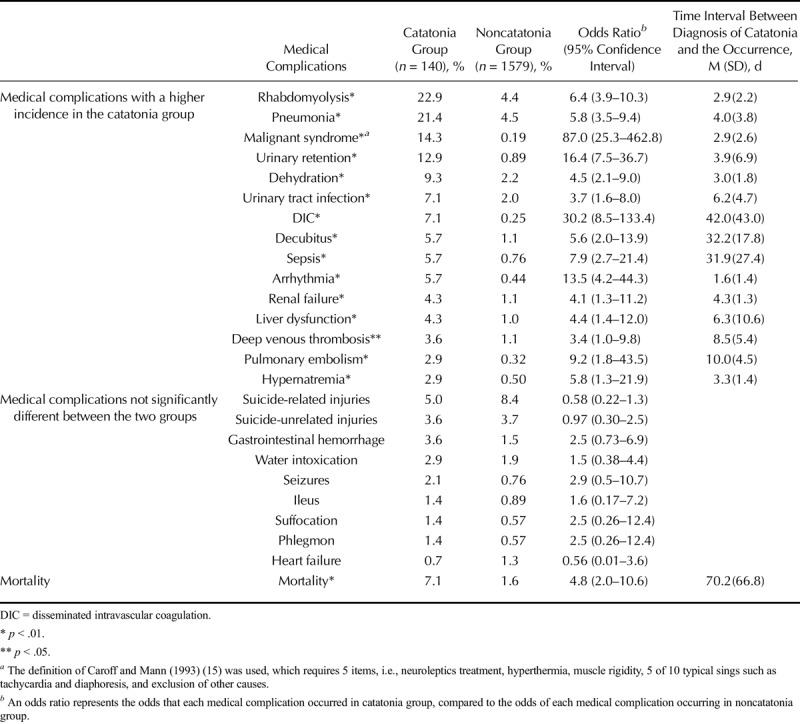
The medical complications with no significant difference in incidence between the two groups included injuries, gastrointestinal hemorrhage, water intoxication, seizure, suffocation, ileus, phlegmon, and heart failure. Although not statistically significant, there was a slightly lower incidence of suicide-related injuries in the catatonia group. Immobility in patients with catatonic stupor might have kept them from committing suicide. Interestingly, despite having a higher incidence of pneumonia, which often leads to heart failure in some patients (21), the catatonia group showed a relatively low incidence of heart failure. This result, together with the higher incidence of dehydration as well as hypernatremia and renal failure, which mostly result from dehydration (22–24), clearly reflects the established close relationship between dehydration and catatonia. There were no medical complications that occurred with significantly greater incidence in the noncatatonia group.
Mortality during hospitalization was higher in the catatonia group (7.1%) than the noncatatonia group (1.6%). Thus, 10 of 140 schizophrenia patients who exhibited catatonic stupor eventually died in our psychiatric unit despite intensive intervention. The cause of death for catatonia patients was pneumonia for three patients, tachyarrhythmia for two, DIC for one, septic shock for one, pulmonary embolism for one, respiratory failure for one, and an unknown cause for one. The unusually high mortality rates for both the catatonia and noncatatonia groups might reflect the policy of our psychiatric unit to admit schizophrenia patients with severe medical complications and treat them in this psychiatric unit with the help of specialists in each medical field.
Multiple Linear Regression Analysis
The multiple linear regression analysis indicated that the presence of catatonic stupor positively correlated with each medical complication for which the difference between the two groups was significantly significant after controlling for other variables (all p < .01, except for deep venous thrombosis, p = .012). Regarding other variables, older age positively correlated with pneumonia, dehydration, urinary tract infection, sepsis, decubitus, renal failure, deep venous thrombosis, hypernatremia, and mortality (all p < .01, except for renal failure, p < .05). Poor score on global assessment of functioning positively correlated with rhabdomyolysis, pneumonia, dehydration, urinary tract infection, sepsis, liver dysfunction, deep venous thrombosis, and hypernatremia (p < .01 for rhabdomyolysis, pneumonia, sepsis, and liver dysfunction, p < .05 for dehydration, urinary tract infection, deep venous thrombosis, and hypernatremia). Male sex positively correlated with rhabdomyolysis, pneumonia, and DIC (p < .01 for rhabdomyolysis, p < .05 for pneumonia and DIC). Length of hospital stay positively correlated with decubitus, sepsis, DIC, and mortality (p < .01 for sepsis, p < .05 for decubitus, DIC, and mortality). The presence of chronic diseases negatively correlated with deep venous thrombosis (p < .05). Use of typical antipsychotics correlated positively with sepsis (p < .05). In summary, catatonic stupor significantly affected the incidence of many medical complications much more so than any other variable, such as the dose of antipsychotics or the presence of chronic diseases. All results for these multiple linear regression models for each medical complication produced a p value of less than .01.
The close relationship between poor functioning and several medical complications might partly reflect a decline in function because of medical complications on admission, but not psychosocial functioning. The negative correlation between chronic diseases and deep venous thrombosis is difficult to explain. However, this might have resulted from continuous antithrombotic treatment among some patients with chronic diseases, which presumably prevented them from developing deep venous thrombosis. A higher incidence of pneumonia in males is compatible with recent studies on the sex disparity of community-acquired pneumonia (25,26), and a higher incidence of pneumonia might have led to development of DIC more frequently in males than females. A higher incidence of rhabdomyolysis in males might reflect the higher excitement or derangement with heavy physical exertion that might occur more often in male patients than female patients given that recent studies indicate that exercise or exertion causes rhabdomyolysis (27). Medical complications that correlated only with catatonia included malignant syndrome, urinary retention, arrhythmia, and pulmonary embolism, suggesting that these medical complications have especially a close relationship with catatonia.
DISCUSSION
Background of Medical Complications Associated With Catatonia
To our knowledge, this is the first comprehensive study of medical complications for catatonia. Our study extends the previous literature that supports a close relationship between catatonia and certain medical complications (7–13). To prevent these specific complications, it might be useful to consider what causes them.
We consider three background factors, i.e., hyperactivity of the sympathetic nervous system, dehydration, and immobility, that are closely related to the medical complications found in catatonic stupor. Dehydration and immobility have already indicated as conditions related to catatonia in a previous report on complications of catatonia (13). Hyperactivity of the sympathetic nervous system is one of the main characteristics of catatonia, e.g., hypertension, tachycardia, rapid respiratory rate, and diaphoresis (14,15). Consistent with this view, our study indicated that some medical complications closely related to the sympathetic nervous system, i.e., arrhythmia (28,29) and urinary retention (30,31), were found frequently in patients with catatonia. In particular, the incidence of each condition was only correlated with catatonia, but not other variables. Hyperactivity of the sympathetic nervous system was originally considered the hallmark of neuroleptic malignant syndrome. Delay et al. (32) defined neuroleptic malignant syndrome as the most severe form of autonomic symptoms, such as hyperthermia and diaphoresis, rather than the typical neurological symptom of rigidity. According to Dong et al. (33), the sympathetic nervous system affects blood coagulation, which facilitates the development of deep venous thrombosis, which we also found was associated with catatonia. Considering these views, the higher incidence of arrhythmia, urinary retention, deep venous thrombosis and its related pulmonary embolism, and neuroleptic malignant syndrome in catatonia patients may be partially attributed to hyperactivity of the sympathetic nervous system.
Second, dehydration has been associated with catatonia (13,14); our study also found this association. There are several potential factors that cause dehydration in catatonia, including diaphoresis, catatonic excitement, and extreme hypoactivity, which is often severe enough to prevent eating or drinking. Catatonic stupor usually involves the opposite symptom of catatonic excitement with extreme movements or exertion, which can lead to dehydration. Importantly, dehydration underlies many medical complications that were found to be associated with catatonia, i.e., renal failure (23,24), neuroleptic malignant syndrome (34), rhabdomyolysis (35–38), urinary tract infections (39), and deep venous thrombosis (40).
Finally, immobility and its related conditions resulting from catatonic stupor, i.e., bedridden status and catheter care for urination, are associated with many medical complications found in catatonia (13). Bedridden status increases the risk for pneumonia (41,42), decubitus (43), and deep venous thrombosis (44). Catheter-associated urinary tract infection is the most common nosocomial infection worldwide (45).
Sepsis and DIC are considered to be caused by infections, i.e., pneumonia and urinary tract infections. However, DIC might also be attributed to catatonia itself, although the causes of DIC are often multifactorial. Several articles have reported that DIC occurs in neuroleptic neuroleptic malignant syndrome (46–48), most cases of which overlap with malignant catatonia. Because DIC occurs in the course of a variety of severe diseases, e.g., sepsis, trauma, malignancy, obstetric complications, or heat stroke and hyperthermia (49), malignant catatonia might be included among the severe diseases that cause DIC. The observed higher incidence of liver dysfunction in catatonia is difficult to explain; it may be partly due to an adverse effect of medications that were used to treat medical complications associated with catatonia, e.g., antibiotics for infections.
In summary, these three background factors, i.e., hyperactivity of the sympathetic nervous system, dehydration, and immobility, can lead to a number of medical complications associated with catatonia, resulting, in the worst case, in death. In particular, deep venous thrombosis was related to all three factors, suggesting that catatonia poses a potentially high risk for pulmonary embolism and that implementation of preventive measures for deep venous thrombosis, in particular, is imperative (11).
How to Prevent These Medical Complications
To prevent these medical complications, improvement in these three background factors is highly recommended. Infusion therapy is often required to improve dehydration, although physical restriction for infusion therapy should be minimized because it poses further risk for immobility. Administration of benzodiazepines and electroconvulsive therapy can lower hyperactivity of the sympathetic nervous system and immobility because of catatonic stupor. Physical rehabilitation is also recommended to avoid becoming bedridden. Thus, in catatonia, meticulous care for both mental and medical conditions should be taken to reduce the risk of adverse medical consequences.
Limitations
Our study has several limitations that should be considered when interpreting the results. There are some selection biases in our present study. First, although we investigated and ruled out all potential medical conditions that might have caused catatonia, the possibility remains that catatonia in schizophrenia patients might have been exacerbated by what we thought were subsequent medical complications. To be precise, it might be difficult to distinguish medical complications that arose as a consequence of catatonic stupor from pre-existing medical conditions whose manifestations after catatonia might only represent the clinical evolution of an underlying condition. In this context, our study may have only found a close relationship between catatonic stupor and certain medical complications and did not establish that they were caused directly by catatonic stupor. However, as our case control study revealed, many medical complications occurred more frequently in the catatonia group than in the noncatatonia group, suggesting that these medical complications could be attributed to catatonic stupor—or at least that catatonic stupor exacerbated pre-existing medical conditions. In addition, regarding the subsequent medical complications that are described in Table 2, the incidence of catatonia caused by these medical complications was rarely reported, suggesting that most cases of catatonic stupor onset were independent of the medical diseases. Second, catatonia patients were limited to those with catatonic stupor, so some patients without stupor were excluded. However, signs of stupor are easy to identify even in a retrospective study, and it is sometimes rather difficult to differentiate catatonic excitement from excitement in general. According to Fink and Taylor (2003) (14), as many as 75% of schizophrenia patients with catatonia might present with catatonic stupor, although this estimate has not been proven. If we follow this assumption, one quarter of catatonia patients might have been left out of our present study. However, presumably, many medical conditions might be caused by immobility and the inability to eat or drink because of catatonic stupor rather than to the other symptoms associated with catatonia. Thus, most patients with medical complications in our study may have been included in the catatonia group, although those patients with injuries might have been left out, which might be closely related with catatonic excitement. Third, this study focused on patients with schizophrenia and those with other psychiatric and medical diseases were not included. Fourth, the generalizability of our results is limited because our study population was derived from a single hospital. Finally, because this study includes a retrospective analysis of hospital date, prospective studies are needed to confirm the findings.
CONCLUSIONS
Our study found that catatonic stupor in schizophrenia patients substantially raises the risk of certain medical complications as well as mortality. Hyperactivity of the sympathetic nervous system, dehydration, and immobility, which are frequently involved in catatonia, might contribute to these specific medical complications. In catatonia, meticulous care for both mental and medical conditions should be taken to reduce the risk of adverse medical consequences.
Acknowledgments
The authors thank the patients and their family members for allowing us to include patient medical and psychiatric information in this study.
Funayama acquired data, designed the study, and drafted the article. Takata, Koreki, and Ogino acquired data. Mimura supervised the research and drafted the article.
Source of Funding and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
REFERENCES
- 1.Calmeil LF. Dictionnaire de Médecine ou Répertoire Général des Sciences. Médicales sous le Rapport Théorique et Practique. 2nd ed. Paris: Bechet; 1832. [Google Scholar]
- 2.Kahlbaum KL. Die Katatonie oder das Spannungsirresein, Eine klinische Form psychischer Krankheit. Berlin: Verlag von August Hirschwald; 1874. [Google Scholar]
- 3.Bell LV. On a form of disease resembling some advanced stages of mania and fever. Am J Insanity 1849;6:97–127. [Google Scholar]
- 4.Jacobi E. Die Psychosen im Klimakterium und in der Involution. Arch Psychiat Nervenkr 1930;90:595–705. [Google Scholar]
- 5.Stauder KH. Die tödliche Katatonie. Arch Psychiatr Nervenkr 1934;102:614–34. [Google Scholar]
- 6.Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry 2014;55:1485–90. [DOI] [PubMed] [Google Scholar]
- 7.Mann SC, Caroff SN, Bleier HR, Welz WK, Kling MA, Hayashida M. Lethal catatonia. Am J Psychiatry 1986;143:1374–81. [DOI] [PubMed] [Google Scholar]
- 8.Philbrick KL, Rummans TA. Malignant catatonia. J Neuropsychiatry Clin Neurosci 1994;6:1–13. [DOI] [PubMed] [Google Scholar]
- 9.Regestein QR, Alpert JS, Reich P. Sudden catatonic stupor with disastrous outcome. JAMA 1977;238:618–20. [PubMed] [Google Scholar]
- 10.Singerman B, Raheja R. Malignant catatonia—a continuing reality. Ann Clin Psychiatry 1994;6:259–66. [DOI] [PubMed] [Google Scholar]
- 11.Morioka H, Nagatomo I, Yamada K, Horikiri Y, Okamura H, Takigawa M. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci 1997;51:323–6. [DOI] [PubMed] [Google Scholar]
- 12.Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry 2014;75:644–51. [DOI] [PubMed] [Google Scholar]
- 13.Jaimes-Albornoz W, Serra-Mestres J. Complications of catatonia in patients in a general hospital referred to a liaison psychiatry service. Eur Psychiatry 2015;30(Supp 1):28–31. [DOI] [PubMed] [Google Scholar]
- 14.Fink M, Tayler MA. Catatonia. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 15.Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129–36. [DOI] [PubMed] [Google Scholar]
- 16.Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185–202. [DOI] [PubMed] [Google Scholar]
- 17.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976;33:766–71. [DOI] [PubMed] [Google Scholar]
- 18.Aas IH. Guidelines for rating Global Assessment of Functioning (GAF). Ann Gen Psychiatry 2011;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki A, Inada T. Dose equivalence of psychotropic drugs [in Japanese]. Jap J Clin Psychopharmacol 2008;5:887–90. [Google Scholar]
- 20.Sukegawa T, Inagaki A, Yamanouchi Y, Inada T, Yoshio T, Yoshimura R, Iwata N. Study protocol: safety correction of high dose antipsychotic polypharmacy in Japan. BMC Psychiatry 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siniorakis EE, Arapi SM, Panta SG, Pyrgakis VN, Ntanos IT, Limberi SJ. Emergency department triage of acute heart failure triggered by pneumonia; when an intensive care unit is needed? Int J Cardiol 2016;220:479–82. [DOI] [PubMed] [Google Scholar]
- 22.Shah MK, Workeneh B, Taffet GE. Hypernatremia in the geriatric population. Clin Interv Aging 2014;9:1987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordillo-Paniagua G, Velasquez-Jones L. Acute renal failure. Pediatr Clin North Am 1976;23:817–28. [DOI] [PubMed] [Google Scholar]
- 24.Kunzendorf U, Haase M, Rölver L, Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs 2010;70:1099–114. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez F, Masiá M, Mirete C, Soldán B, Rodríguez JC, Padilla S, Hernández I, Royo G, Martin-Hidalgo A. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect 2006;53:166–74. [DOI] [PubMed] [Google Scholar]
- 26.Raeven VM, Spoorenberg SM, Boersma WG, van de Garde EM, Cannegieter SC, Voorn GP, Bos WJ, van Steenbergen JE. Alkmaar study group; Ovidius study group. Atypical aetiology in patients hospitalised with community-acquired pneumonia is associated with age, gender and season; a data-analysis on four Dutch cohorts. BMC Infect Dis 2016;16:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furman J. When exercise causes exertional rhabdomyolysis. JAAPA 2015;28:38–43. [DOI] [PubMed] [Google Scholar]
- 28.Podrid PJ, Fuchs T, Candinas R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation 1990;82(Suppl 2):I103–13. [PubMed] [Google Scholar]
- 29.Hemingway H, Malik M, Marmot M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur Heart J 2001;22:1082–101. [DOI] [PubMed] [Google Scholar]
- 30.Tammela T. Postoperative urinary retention—why the patient cannot void. Scand J Urol Nephrol Suppl 1995;175:75–7. [PubMed] [Google Scholar]
- 31.Cataldo PA, Senagore AJ. Does alpha sympathetic blockade prevent urinary retention following anorectal surgery? Dis Colon Rectum 1991;34:1113–6. [DOI] [PubMed] [Google Scholar]
- 32.Delay J, Pichot P, Lempérière J. Un neuroleptique majeur non phénothiazinique et non réserpinique, l'halopéridol, dans le traitement des psychoses. Ann Méd Psycholog 1960;118:145–52. [Google Scholar]
- 33.Dong T, Cheng YW, Yang F, Sun PW, Zhu CJ, Zhu L, Zhang GX. Chronic stress facilitates the development of deep venous thrombosis. Oxid Med Cell Longev 2015;2015:384535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurrera RJ. Diaphoresis and dehydration during neuroleptic malignant syndrome: preliminary findings. Psychiatry Res 1996;64:137–45. [DOI] [PubMed] [Google Scholar]
- 35.Kodama K, Ikeda K, Kawamura S, Oyama T, Fojita S, Kobayashi Y. A case of severe dehydration with marked rhabdomyolysis. Jpn J Med 1985;24:150–4. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Kim W, Park SK, Kang ES, Kang KP, Kang SK. A case of acute renal failure, rhabdomyolysis and disseminated intravascular coagulation associated with severe exercise-induced hypernatremic dehydration. Clin Nephrol 2004;62:401–3. [DOI] [PubMed] [Google Scholar]
- 37.Cleary M, Ruiz D, Eberman L, Mitchell I, Binkley H. Dehydration, cramping, and exertional rhabdomyolysis: a case report with suggestions for recovery. J Sport Rehabil 2007;16:244–59. [DOI] [PubMed] [Google Scholar]
- 38.George M, Delgaudio A, Salhanick SD. Exertional rhabdomyolysis--when should we start worrying? Case reports and literature review. Pediatr Emerg Care 2010;26:864–6. [DOI] [PubMed] [Google Scholar]
- 39.Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr 2003;57(Suppl 2):S52–8. [DOI] [PubMed] [Google Scholar]
- 40.Melamed AJ, Suarez J. Detection and prevention of deep venous thrombosis. Drug Intell Clin Pharm 1988;22:107–14. [DOI] [PubMed] [Google Scholar]
- 41.Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis 2000;31:1066–78. [DOI] [PubMed] [Google Scholar]
- 42.Marrie TJ. Pneumonia in the long-term-care facility. Infect Control Hosp Epidemiol 2002;23:159–64. [DOI] [PubMed] [Google Scholar]
- 43.Perdue RW, Wilson JL. Decubitus ulcers. J Am Board Fam Pract 1989;2:43–8. [PubMed] [Google Scholar]
- 44.Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology 2013;28(Suppl 1):29–33. [DOI] [PubMed] [Google Scholar]
- 45.Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis 2012;25:365–70. [DOI] [PubMed] [Google Scholar]
- 46.Eles GR, Songer JE, DiPette DJ. Neuroleptic malignant syndrome complicated by disseminated intravascular coagulation. Arch Intern Med 1984;144:1296–7. [PubMed] [Google Scholar]
- 47.Johnson MD, Newman JH, Baxter JW. Neuroleptic malignant syndrome presenting as adult respiratory distress syndrome and disseminated intravascular coagulation. South Med J 1988;81:543–5. [DOI] [PubMed] [Google Scholar]
- 48.Yamawaki Y, Ogawa N. Successful treatment of levodopa-induced neuroleptic malignant syndrome (NMS) and disseminated intravascular coagulation (DIC) in a patient with Parkinson's disease. Intern Med 1992;31:1298–302. [DOI] [PubMed] [Google Scholar]
- 49.Dalainas I. Pathogenesis, diagnosis, and management of disseminated intravascular coagulation: a literature review. Eur Rev Med Pharmacol Sci 2008;12:19–31. [PubMed] [Google Scholar]


