Supplemental Digital Content is available in the text.
Keywords: cholesterol, LDL; coronary artery disease; hydroxymethylglutaryl-CoA reductase inhibitors; long-term adverse effects; secondary prevention; stroke
Abstract
Background:
Current guidelines call for high-intensity statin therapy in patients with cardiovascular disease on the basis of several previous “more versus less statins” trials. However, no clear evidence for more versus less statins has been established in an Asian population.
Methods:
In this prospective, multicenter, randomized, open-label, blinded end point study, 13 054 Japanese patients with stable coronary artery disease who achieved low-density lipoprotein cholesterol (LDL-C) <120 mg/dL during a run-in period (pitavastatin 1 mg/d) were randomized in a 1-to-1 fashion to high-dose (pitavastatin 4 mg/d; n=6526) or low-dose (pitavastatin 1 mg/d; n=6528) statin therapy. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina requiring emergency hospitalization. The secondary composite end point was a composite of the primary end point and clinically indicated coronary revascularization excluding target-lesion revascularization at sites of prior percutaneous coronary intervention.
Results:
The mean age of the study population was 68 years, and 83% were male. The mean LDL-C level before enrollment was 93 mg/dL with 91% of patients taking statins. The baseline LDL-C level after the run-in period on pitavastatin 1 mg/d was 87.7 and 88.1 mg/dL in the high-dose and low-dose groups, respectively. During the entire course of follow-up, LDL-C in the high-dose group was lower by 14.7 mg/dL than in the low-dose group (P<0.001). With a median follow-up of 3.9 years, high-dose as compared with low-dose pitavastatin significantly reduced the risk of the primary end point (266 patients [4.3%] and 334 patients [5.4%]; hazard ratio, 0.81; 95% confidence interval, 0.69–0.95; P=0.01) and the risk of the secondary composite end point (489 patients [7.9%] and 600 patients [9.7%]; hazard ratio, 0.83; 95% confidence interval, 0.73–0.93; P=0.002). High-dose pitavastatin also significantly reduced the risks of several other secondary end points such as all-cause death, myocardial infarction, and clinically indicated coronary revascularization. The results for the primary and the secondary composite end points were consistent across several prespecified subgroups, including the low (<95 mg/dL) baseline LDL-C subgroup. Serious adverse event rates were low in both groups.
Conclusions:
High-dose (4 mg/d) compared with low-dose (1 mg/d) pitavastatin therapy significantly reduced cardiovascular events in Japanese patients with stable coronary artery disease.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01042730.
Editorials, see p 2010 and p 2013
Clinical Perspective.
What Is New?
REAL-CAD (Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy With Pitavastatin in Coronary Artery Disease) is currently the largest randomized trial to compare high-dose and low-dose statin therapy.
It was also the first such trial performed in Asia.
High-dose compared with low-dose pitavastatin significantly reduced the primary end point (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina requiring emergency hospitalization).
All-cause death, myocardial infarction, and clinically indicated coronary revascularization were also significantly reduced.
Rates of serious adverse events were similar in the 2 treatment groups.
What Are the Clinical Implications?
The results of the REAL-CAD study confirmed that high-dose compared with low-dose pitavastatin can safely improve the prevention of cardiovascular events in Japanese patients with coronary artery disease, who commonly receive low-intensity statin therapy.
REAL-CAD is a practice-changing trial, suggesting that the administration of maximum tolerable doses of statins, within the range of local approval, would be the preferred statin strategy in patients with established coronary artery disease regardless of baseline low-density lipoprotein cholesterol levels.
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for cardiovascular events,1 and lowering LDL-C with statins has proved effective for primary and secondary prevention of coronary artery disease (CAD).2–9 Several previous “more versus less statins” trials in patients with CAD demonstrated that high-intensity statin therapy significantly reduced cardiovascular events compared with moderate-intensity statin therapy.10–14 On the basis of these results, the current American College of Cardiology/American Heart Association guideline recommends high-intensity statin therapy in patients ≤75 years of age with clinical atherosclerotic cardiovascular disease,15 whereas the current European Society of Cardiology guideline recommends an LDL-C target of ≤70 mg/dL for patients with very high cardiovascular risk.16 However, high-intensity statin therapy is not widely implemented in daily clinical practice, particularly in Asia, at least partly because there has been no previous trials of more versus less statins in Asia.17–19 Therefore, we conducted a large outcome trial comparing the efficacy of high-dose versus low-dose statin therapy in patients with established stable CAD in Japan. Our goal was to determine whether higher-dose statin therapy would be beneficial and safe in Japanese patients.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
The REAL-CAD study (Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy With Pitavastatin in Coronary Artery Disease) is a prospective, multicenter, randomized, open-label, blinded end point, physician-initiated superiority trial to determine whether high-dose (4 mg/d) compared with low-dose (1 mg/d) pitavastatin therapy could reduce cardiovascular events in Japanese patients with stable CAD. Pitavastatin is a statin with potent LDL-C–lowering effects developed by Kowa Pharmaceutical Co Ltd (Tokyo, Japan). Pitavastatin doses of 1 and 4 mg were reported to reduce LDL-C by 33.6% and 47.2%, respectively, in Japanese patients.20 A similar magnitude of LDL-C reduction was also reported in white and East Asian patients.21–23 Pitavastatin 4 mg is the maximum approved dose in Japan and has demonstrated effects comparable to atorvastatin 20 mg in terms of both LDL-C reduction and coronary plaque regression assessed by intravascular ultrasound, whereas pitavastatin 1 mg has an LDL-C–lowering effect comparable to that of atorvastatin 5 mg.24,25
Eligible patients were men and women 20 to 80 years of age with stable CAD as defined by a history of acute coronary syndrome or coronary revascularization >3 months ago or a clinical diagnosis of CAD with angiographically documented coronary artery stenosis of at least 75% diameter narrowing according to the American Heart Association classification.26 We excluded those patients with LDL-C <100 mg/dL without statin therapy before enrollment because the label in the instructions for pitavastatin restricted use to patients with hypercholesterolemia. Detailed inclusion and exclusion criteria are provided in the online-only Data Supplement. Patients were enrolled on an outpatient basis through academic and general hospitals and clinics across Japan. Eligible patients who provided informed consent were enrolled and received pitavastatin 1 mg once daily orally for a run-in period of at least 1 month. Patients were evaluated for secondary eligibility, excluding those patients with LDL-C ≥120 mg/dL after the run-in period, onset of acute coronary syndrome and/or coronary revascularization within the past 3 months, poor medication adherence to pitavastatin, occurrence of primary end point events, or adverse events prohibiting study continuation during the run-in period.
Patients who met the secondary eligibility criteria were randomized in a 1-to-1 fashion to oral pitavastatin, either 4 mg/d (high-dose group) or 1 mg/d (low-dose group), with an electronic data capture system and dynamic allocation stratified by facility, age (<65 or ≥65 years), sex, diabetes mellitus, and statin use before enrollment. The assignment algorithm was determined by the study statistician. This is an open-label trial. However, the independent event committee adjudicated all the end point events while blinded to the assigned group (online-only Data Supplement).
During follow-up, the patients’ visits dictated by the protocol were at 6 and 12 months in the first year and every 12 months thereafter. Serum lipid levels such as LDL-C, total cholesterol, triglycerides, and high-density lipoprotein cholesterol, as well as other blood tests such as creatine kinase, alanine aminotransferase, aspartate aminotransferase, creatinine, and hemoglobin A1c, were to be measured at baseline, at 6 and 12 months, and yearly thereafter, whereas high-sensitivity C-reactive protein (hsCRP) was to be measured at baseline and at 6 months.
The site investigators reported follow-up information through the web-based electronic data capturing system. Data were monitored by the data center, and the logical inconsistencies were resolved by queries. Final clinical follow-up data were collected through January to March 2016. From 2012 to 2016, site audits were performed for 3914 patients from 28 centers, and the independent data monitoring committee regularly assessed the safety aspect of study conduct.
End Points
The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina requiring emergency hospitalization. Cardiovascular death consisted of cardiac death, including sudden death and cardiac procedure-related death, as well as noncardiac vascular death. Death without obvious noncardiovascular cause was regarded as cardiovascular death. Myocardial infarction was defined as described by the Academic Research Consortium (ARC).27 A secondary composite end point including coronary revascularization was defined as a composite of the primary end point event and clinically indicated coronary revascularization, excluding target-lesion revascularization for lesions treated at prior percutaneous coronary intervention. Target-lesion revascularization was not included in this secondary end point because it was unknown whether statins are effective in preventing restenosis and/or thrombosis of lesions treated at prior percutaneous coronary intervention. Other secondary end points and the details for the definitions of end points are described in the online-only Data Supplement.
The study also evaluated adverse events that developed after the start of the assigned treatment and for which a causal relationship to study drug administration could not be ruled out. Adverse events were assessed and reported by the site investigators.
Statistical Analysis
From the previous trials of more versus less statins, we hypothesized that the present study would show 16% relative risk reduction with the high-dose pitavastatin treatment.28 A total of 1033 events would be required to detect a 16% relative risk reduction with 80% statistical power and a 2-sided α of 5%.29 Assuming an annual event rate of 2.5% based on the previous Japanese studies30–32 and an estimated dropout rate of 10%, a total of 12 600 patients would be required to achieve 1033 events during the planned 3 years of enrollment and at least 3 years of follow-up.
The actual event rate was lower than anticipated. However, on October 27, 2015, the steering committee decided not to extend the study further despite the original event-driven trial design because a substantial number of centers were reluctant to extend the study further.
The cumulative incidence of clinical events was estimated by the Kaplan-Meier method and compared by the log-rank test. The effect of the high-dose pitavastatin relative to the low-dose pitavastatin was assessed by the Cox proportional hazard model and was expressed as hazard ratio with 95% confidence interval. Proportional hazard assumptions were assessed on the plots of log (time) versus log [−log (survival)], and the assumptions were verified. Adherence to the study drug was assessed by the time-to-event analysis in which nonadherence was regarded as the event. Nonadherence to the study drug included <50% intake of the study drug, discontinuation of the assigned treatment, and loss of the drug adherence data.
Safety analyses were conducted using the data from all enrolled patients who had received at least 1 dose of pitavastatin and for whom postdose data were available (safety analysis set). Efficacy analyses were conducted after the exclusion of those patients who were randomized but were found not to meet the eligibility criteria (full analysis set). We conducted a sensitivity analysis in the safety analysis set population without exclusion of those randomized patients who did not meet inclusion and exclusion criteria. Patients lost to follow-up were censored at the time when their final clinical follow-up information was available. Number needed to treat during the 5-year follow-up was estimated from the event rate at 4 years because the number of patients at risk decreased substantially at 5 years.
We performed subgroup analyses for the primary and secondary composite end points in several prespecified subgroups. The formal interaction test was performed between the subgroup factors and the effect of the high-dose pitavastatin relative to the low-dose pitavastatin. Time-varying measurements such as LDL-C were analyzed with the generalized estimating equation models with robust variance adjustment and compound symmetry structure used as the initial assumption. Triglycerides and hsCRP were analyzed after log transformation. For describing the time profile, the average value (least-squares means) including the baseline was estimated for each of the groups with time-group interaction terms as covariates in the generalized estimating equation model for accommodating missing values. Time variables were modeled as categorical (dummy) variables. Group difference (treatment effect) and time-group interaction after the intervention were estimated with time, group, time-interaction and the baseline value as covariates. The baseline value was included in the model for reducing bias and variability resulting from the regression to the mean. Missing values were not imputed in the analyses.
Dr Ohashi was responsible for the analysis results as the statistician for this trial. All statistical analyses were conducted with SAS System Release 9.4 software. All P values are 2 sided.
The Steering Committee (online-only Data Supplement) designed the trial. All authors agreed to submit the manuscript for publication and vouch for adherence to the study protocol and for the accuracy and completeness of the data. The Comprehensive Support Project for Clinical Research of Lifestyle-Related Disease of the Public Health Research Foundation funded this study. The company manufacturing the study drug (Kowa Pharmaceutical Co Ltd) provided financial support but was not involved in design, analysis, data interpretation, or manuscript preparation. Ethics approval was granted by the Public Health Research Foundation ethics review committee and by ethics committees at all participating sites. All participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Results
Study Patients
From January 31, 2010, to March 31, 2013, a total of 14 774 patients were enrolled from 733 academic and general hospitals and clinics across Japan. After completion of the run-in period, 13 054 patients were randomized to either high-dose (n=6526) or low-dose (n=6528) pitavastatin. The safety analysis population consisted of 12 818 patients (high-dose, n=6390; low-dose, n=6428) after the exclusion of those patients who withdrew consent or for whom written informed consent was missing at the time of the site audits. The full analysis population consisted of 12 413 patients (high-dose, n=6199; low-dose, n=6214) after the exclusion of those patients who were found not to meet the eligibility criteria. The median follow-up period for the survivors was similar for the high-dose and low-dose groups (3.9 [range, 0.0–5.8] years and 3.9 [range, 0.0–5.9] years; P=0.08). Follow-up at 1 year was completed in 5607 patients (97.0%) in the high-dose group and in 5809 patients (96.9%) in the low-dose group. Final follow-up data beyond January 2016 were available for 5171 patients (83.4%) and for 5169 patients (83.2%), respectively (Figure 1). The rate of adherence to the study drug was high in both groups, although it was slightly but significantly lower in the high-dose group than in the low-dose group (97.1% and 98.7% at 6 months, 74.8% and 76.8% at 4 years; P=0.02; Figure I in the online-only Data Supplement).
Figure 1.
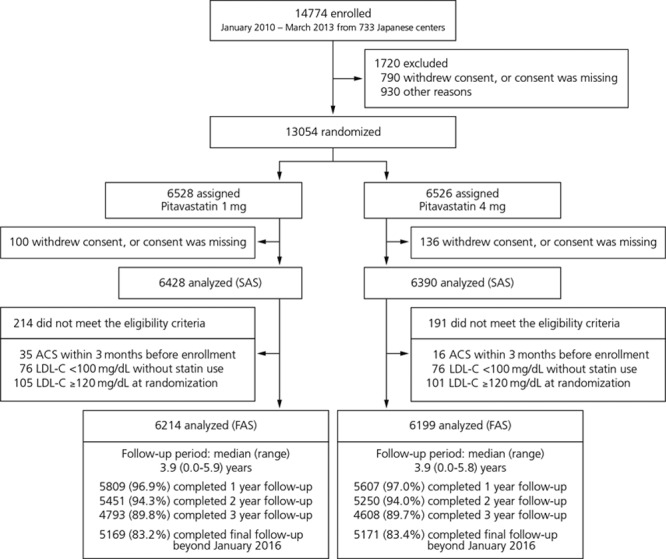
Disposition of patients. The reasons for not meeting the eligibility criteria were not mutually exclusive. ACS indicates acute coronary syndrome; FAS, full analysis set; LDL-C, low-density lipoprotein cholesterol; and SAS, safety analysis set.
The study population represented typical Japanese patients with stable CAD, with advanced age and a preponderance of male sex. Hypertension was present in 76% of patients and diabetes mellitus in 40%. A total of 51% had prior myocardial infarction, and 90% had prior coronary revascularization predominantly by percutaneous coronary intervention. For baseline medications, antiplatelet therapy, including dual therapy, was widely used, whereas the use of β-blockers was less prevalent. The baseline characteristics and medications were well balanced between the 2 groups (Table 1).
Table 1.
Baseline Characteristics

Lipid Parameters and hsCRP
The mean LDL-C before enrollment was 93 mg/dL with 91% of patients taking statins. The baseline LDL-C level after the run-in period was 87.7 and 88.1 mg/dL in the high-dose and low-dose groups, respectively. At 6 months, the LDL-C level was reduced by 16% (73.7 mg/dL) in the high-dose group and was unchanged (89.4 mg/dL) in the low-dose group (Figure 2). During the entire course of follow-up, LDL-C in the high-dose group was lower by 14.7 mg/dL than in the low-dose group. Total cholesterol and triglyceride levels were also significantly lower and high-density lipoprotein cholesterol level was significantly higher in the high-dose group than in the low-dose group (Figure 2).
Figure 2.

Changes in lipid parameters and high-sensitivity C-reactive protein (hsCRP) levels over time. A through C, Changes over time in low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. D, Change in hsCRP from baseline to 6 months. Values at baseline and at 6 months were basically derived from central laboratory measurements. If a value from central laboratory measurement was not available or not calculable, a value obtained from the measurement at each institution was used instead. If any value other than those centrally measured was missing, that value was not imputed from other data but was handled as a missing value and excluded from analysis. Central laboratory measurements were available for LDL cholesterol in 11 813 patients at baseline and in 11 319 patients at 6 months, whereas those for total cholesterol, triglycerides, and HDL cholesterol were available in 12 026 patients at baseline and in 11 320 patients at 6 months. Central laboratory measurements for hsCRP were available in 12 026 patients at baseline and in 11 319 patients at 6 months. Values at 1, 2, and 3 years were derived from measurements at each institution. P values were for the main therapeutic effect and for the interaction effect between therapeutic effect and time.
The level of hsCRP was similar and low in both the high-dose and low-dose groups (0.57 and 0.59 mg/L) at baseline but was significantly lower in the high-dose group than in the low-dose group at 6 months (0.49 and 0.59 mg/L; Figure 2). Blood pressure and hemoglobin A1c were well controlled and similar in both groups during follow-up (Figure II in the online-only Data Supplement).
Clinical Outcomes
High-dose compared with low-dose pitavastatin significantly reduced the primary end point. The primary end point occurred in 266 patients (4.3%) in the high-dose group and 334 patients (5.4%) in the low-dose group (hazard ratio, 0.81; 95% confidence interval, 0.69–0.95; P=0.01; Table 2). The cumulative 4-year incidence of the primary end point was significantly lower in the high-dose group than in the low-dose group (4.6% and 5.6%; P=0.01; Figure 3 and Table 2). The number needed to treat for the prevention of 1 primary end point event was 63 during the 5 years of follow-up. In the sensitivity analysis without exclusion of those randomized patients who did not meet inclusion and exclusion criteria, the magnitude of risk reduction by high-dose pitavastatin for the primary end point (hazard ratio, 0.81; 95% confidence interval, 0.69–0.95, P=0.01) was consistent with that in the main analysis.
Table 2.
Primary and Secondary End Points

Figure 3.

Kaplan-Meier curves for the primary end point and a secondary composite end point (primary end point plus coronary revascularization). The cumulative incidence was estimated by the Kaplan-Meier method. A and B, Kaplan-Meier curves for the primary end point (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina requiring emergency hospitalization) and for a secondary composite end point (a composite of primary end point or coronary revascularization based on clinical indication), respectively. Coronary revascularization as a component of the secondary composite end point excluded target-lesion revascularization for lesions treated at the time of prior percutaneous coronary intervention. CI indicates confidence interval; and HR, hazard ratio.
High-dose compared with low-dose pitavastatin also significantly reduced the secondary composite end point, including coronary revascularization, which occurred in 489 patients (7.9%) in the high-dose group and 600 patients (9.7%) in the low-dose group (hazard ratio, 0.83; 95% CI, 0.73–0.93; P=0.002; Table 2). The cumulative 4-year incidence of this secondary end point was also significantly lower in the high-dose group than in the low-dose group (8.5% and 10.4%; P=0.002) with a number needed to treat of 41 during the 5 years of follow-up (Figure 3 and Table 2).
High-dose pitavastatin also significantly reduced the risks of several other secondary end points such as all-cause death, myocardial infarction, and clinically indicated coronary revascularization. There was no significant difference in the risk of ischemic stroke, hemorrhagic stroke, or unstable angina requiring emergency hospitalization (Table 2).
The risk reduction for the primary end point and for the secondary composite end point, including coronary revascularization, by the high-dose pitavastatin was consistent across all the prespecified subgroups such as age (≥65 and <65 years), sex, diabetes mellitus, baseline LDL-C (≥95 and <95 mg/dL), high-density lipoprotein cholesterol (>40 and ≤40 mg/dL), triglycerides (≥150 and <150 mg/dL), and hsCRP levels (≥1 and <1 mg/L) and body mass index (≥25 and <25 kg/m2) without any significant interaction between the subgroup factors and the effect of high-dose pitavastatin (Figure 4). The magnitude of risk reduction by the high-dose pitavastatin in the low baseline LDL cholesterol subgroup was comparable to that in the high baseline LDL cholesterol subgroup.
Figure 4.

Subgroup analyses of the effects of high- vs low-dose pitavastatin for the primary end point and for a secondary composite end point (primary end point plus coronary revascularization) in the prespecified subgroups. A and B, Subgroup analysis for the primary end point and for a secondary composite end point, respectively. Numbers of patients with event were summarized per subgroup within each treatment. Hazard ratios (HRs) were calculated within each subgroup level for the treatment effect of pitavastatin 4 mg relative to pitavastatin 1 mg. The P value was derived from an interaction test between the subgroup factors and treatment effect of pitavastatin 4 mg relative to pitavastatin 1 mg. Horizontal bars indicate 95% confidence intervals (CIs). Coronary revascularization as a component of the secondary composite end point excluded target-lesion revascularization for lesions treated at the time of prior percutaneous coronary intervention. HDL indicates high-density lipoprotein; hsCRP, high sensitivity C-reactive protein; and LDL, low-density lipoprotein.
The rates of serious adverse events, including rhabdomyolysis, were low and did not differ between the 2 groups, although muscle complaints were reported more often in the high-dose group than in the low-dose group. However, the rate of creatine kinase elevation ≥5 the upper limit of normal did not differ between the 2 groups. There was no between-group difference in the new onset of diabetes mellitus (Table 3). Study drug discontinuation was slightly but significantly more frequent in the high-dose group than in the low-dose group (9.8% and 8.1%; P<0.001).
Table 3.
Adverse Events and Laboratory Test Abnormalities

Discussion
The main finding in the present study was that cardiovascular events were significantly reduced by high-dose (4 mg/d) compared with low-dose (1 mg/d) pitavastatin therapy in Japanese patients with stable CAD.
REAL-CAD is the largest-ever trial of more versus less statins, and the first trial of this type conducted in Asia. The results from the present trial were fully consistent with the results of the TNT trial (Treating to New Targets) comparing atorvastatin 80 mg with atorvastatin 10 mg in patients with stable CAD, which demonstrated that higher-dose statin therapy was associated with lower risk for cardiovascular events.11 The magnitude of relative risk reduction for the primary end point in the present study was comparable to that seen in European and North American trials of more versus less statins, suggesting that more intensive statins therapy could also be beneficial in Japanese patients.10–14 However, absolute risk reduction in the present study was substantially smaller than that observed in the TNT trial, reflecting the overall low event rate in Japanese patients. The very low level of hsCRP in this study is consistent with findings from previous Japanese studies33,34 and further reflective of the lower cardiovascular risk in Japanese patients with stable CAD.
REAL-CAD is a pragmatic physician-initiated trial exploring the optimal dose of statins for patients with established stable CAD within the range of approved doses in Japan. Despite current guidelines recommendations, rates of use of high-intensity statin therapy (atorvastatin 40/80 mg, rosuvastatin 20/40 mg) in patients with established CAD have been reported to be low in Asia (0%–25%).17–19 It is important to note that the statin dose in the high-dose group (pitavastatin 4 mg/d) in this study is equivalent to atorvastatin 20 mg/d in terms of LDL-C lowering, indicating that high-dose pitavastatin therapy in this study is what is generally considered moderate-intensity statin therapy in the international medical community. Most of the doses of high-intensity statin therapy defined in the American College of Cardiology/American Heart Association guideline are not approved in Japan. Furthermore, maximum approved doses of statins are prescribed very infrequently in Japan, even for secondary prevention. The mean LDL-C before the run-in period was 93 mg/dL with 91% of patients taking statins, which decreased to 88 mg/dL after the run-in period on pitavastatin 1 mg. This minimal decrease in LDL-C during the run-in period suggests that the standard of care in Japan was low-intensity statin therapy, highlighting the results of the present study as practice changing. The present study clearly demonstrated that, even in a dose range lower than the dose levels defined as high-intensity statin therapy, the higher statin dose was associated with greater protection from cardiovascular events than the lower statin dose. Furthermore, the favorable effect of high-dose pitavastatin was observed regardless of the baseline LDL-C level dichotomized as ≥95 and <95 mg/dL.
The present study also suggested the mortality benefit with high-dose relative to low-dose pitavastatin. We are conservative about placing too much emphasis on the observed mortality benefit because the present study did not have adequate power for evaluating the mortality difference and we cannot rule out the possibility of chance in this nonhierarchical multiple comparison for secondary end points. Furthermore, no single previous trials of more versus less statins has demonstrated mortality benefit. However, the present study is the largest-ever trial of more versus less statins, and its results appear to favor high-dose pitavastatin from the perspective of mortality. This study thus suggests that the administration of maximum tolerable doses of statins, within the range of local approval, should be the preferred statin strategy in patients with established CAD regardless of baseline LDL-C levels.
Our study has several important limitations. First, the present study was conducted as an open-label trial with its inherent limitations. However, to somewhat compensate for the open-label trial design, the primary end point was defined as not including coronary revascularization procedures because the decision for coronary revascularization is made by physicians who know the assigned treatment group. Second, the present study was terminated prematurely despite the original event-driven trial design, although we observed significant risk reduction for the primary end point. Third, final follow-up was not completed in a substantial proportion of patients, a potential limitation of physician-initiated studies that rely on voluntary efforts by the site investigators. However, the follow-up rates were comparable between the high- and low-dose groups, suggesting that the patients lost to follow-up would have affected the trial outcome in the same manner in both groups. Finally, the higher rate of study drug discontinuation and the lower rate of adherence to the study drug in the high-dose group might have nullified some of the effect of high-dose relative to low-dose therapy.
Conclusions
High-dose (4 mg/d) compared with low-dose (1 mg/d) pitavastatin therapy significantly reduced cardiovascular events in Japanese patients with stable CAD.
Acknowledgments
The authors thank all patients and investigators who participated in this study; Yoji Mitadera, Katsura Nakajima, and other members of the Public Health Research Foundation for their assistance as the administrative office; Teikyo Academic Research Center for its function as a data center; and Mieko Onuki, Yuna Yasuda, and other members of EDIT, Inc (Tokyo, Japan) for medical-writing support.
Sources of Funding
The Comprehensive Support Project for Clinical Research of Lifestyle-Related Disease of the Public Health Research Foundation funded this study. The company manufacturing the study drug (Kowa Pharmaceutical Co Ltd) was one of the entities providing financial support for Public Health Research Foundation projects but was not involved in design, analysis, data interpretation, or manuscript preparation.
Disclosures
Dr Taguchi received research grant from Chugai Pharmaceutical Co, Ltd, Sanwa Kagaku Kenkyusho Co, Ltd, JSPS KAKENHI Grant-in-Aid for Scientific Research, research grant, other research support, and honoraria from Public Health Research Foundation, Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Shionogi & Co, Ltd, Teijin Pharma Ltd, Astellas Pharma Inc, MSD KK, Mitsubishi Tanabe Pharma Corp, Sumitomo Dainippon Pharma Co, Ltd, AstraZeneca KK, Amgen Astellas BioPharma KK, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Mochida Pharmaceutical Co, Ltd, Pfizer Japan Inc. Dr Iimuro received research grant from Amgen Astellas BioPharma KK, research grant and honoraria from Public Health Research Foundation. Dr Iwata received other research support from Public Health Research Foundation, honoraria from Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, Takeda Pharmaceutical Co Ltd, MSD KK, Kowa Pharmaceutical Co Ltd, Mitsubishi Tanabe Pharma Corp. Dr Takashima received honoraria from Kowa Pharmaceutical Co Ltd, Sanofi KK, Daiichi Sankyo Co Ltd, Mitsubishi Tanabe Pharma Corp, AstraZeneca KK, Astellas Pharma Inc, MSD KK, Sumitomo Dainippon Pharma Co, Ltd, Amgen Astellas BioPharma KK, Otsuka Pharmaceutical Co, Ltd, Mochida Pharmaceutical Co, Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Abe received research grant, other research support, and honoraria from Public Health Research Foundation. Dr Amiya received research grant from Banyu Foundation Research Grant, honoraria from Otsuka Pharmaceutical Co, Ltd, Takeda Pharmaceutical Co Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr T. Ogawa received honoraria from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Teijin Pharma Ltd, Astellas Pharma Inc, Mitsubishi Tanabe Pharma Corp, AstraZeneca KK, Eisai Co, Ltd, Amgen Astellas BioPharma KK, Sanwa Kagaku Kenkyusho Co, Ltd, Otsuka Pharmaceutical Co, Ltd, other research support and honoraria from Public Health Research Foundation. Dr Ozaki received research grant and honoraria from Mochida Pharmaceutical Co, Ltd, Pfizer Japan Inc, Takeda Pharmaceutical Co Ltd, Sanofi KK, Shionogi & Co, Ltd, MSD KK, Mitsubishi Tanabe Pharma Corp, Otsuka Pharmaceutical Co, Ltd, Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Sakuma received honoraria from Bayer Yakuhin, Ltd, other research support and honoraria from Takeda Pharmaceutical Co Ltd, research grant and other research support and from Public Health Research Foundation. Dr Nakagawa received honoraria from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Shionogi & Co, Ltd, Astellas Pharma Inc, MSD KK, Mitsubishi Tanabe Pharma Corp, Sumitomo Dainippon Pharma Co, Ltd, AstraZeneca KK, Amgen Astellas BioPharma KK, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Pfizer Japan Inc, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Hibi received research grant from Public Health Research Foundation, Teijin Pharma Ltd, Daiichi Sankyo Co, Ltd, Sanofi KK. Dr Hiro received research grant from Otsuka Pharmaceutical Co, Ltd, Shionogi & Co, Ltd, Takeda Pharmaceutical Co Ltd, honoraria from Amgen Astellas BioPharma KK, Kowa Pharmaceutical Co Ltd, Kissei Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, research grant and honoraria from Astellas Pharma Inc, Eisai Co, Ltd, MSD KK, Sanofi KK, Daiichi Sankyo Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Mitsubishi Tanabe Pharma Corp, Bayer Yakuhin, Ltd, Pfizer Japan Inc, AstraZeneca KK, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Fukumoto received research grant from Sanofi KK, Shionogi & Co, Ltd, honoraria from Public Health Research Foundation, AstraZeneca KK, Eisai Co, Ltd, Kowa Pharmaceutical Co Ltd, research grant and honoraria from MSD KK, Otsuka Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Teijin Pharma Ltd, Bayer Yakuhin, Ltd, Mochida Pharmaceutical Co, Ltd, Astellas Pharma Inc, Sanwa Kagaku Kenkyusho Co, Ltd, Takeda Pharmaceutical Co Ltd, Mitsubishi Tanabe Pharma Corp, Pfizer Japan Inc. Dr Hokimoto received honoraria from Daiichi Sankyo Co, Ltd, AstraZeneca KK, Chugai Pharmaceutical Co, Ltd, Kowa Pharmaceutical Co Ltd, Pfizer Japan Inc, Takeda Pharmaceutical Co Ltd, research grant and other research support from Public Health Research Foundation. Dr Miyauchi received honoraria from Sanofi KK, Daiichi Sankyo Co, Ltd, AstraZeneca KK, Amgen Astellas BioPharma KK, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, MSD KK. Dr Yamazaki received honoraria from Takeda Pharmaceutical Co Ltd, Shionogi & Co, Ltd, Mitsubishi Tanabe Pharma Corp, Sumitomo Dainippon Pharma Co, Ltd, AstraZeneca KK. Dr Ito received research grant from Pfizer Japan Inc, research grant and honoraria from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Teijin Pharma Ltd, Astellas Pharma Inc, Mochida Pharmaceutical Co, Ltd. Dr Otsuji received research grant from Bayer Yakuhin, Ltd, Shionogi & Co, Ltd, Teijin Pharma Ltd, MSD KK, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Takeda Pharmaceutical Co Ltd, Daiichi Sankyo Co, Ltd, Astellas Pharma Inc, Mitsubishi Tanabe Pharma Corp, research grant, other research grant, and honoraria from Public Health Research Foundation. Dr K. Kimura received research grant from Public Health Research Foundation, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, MSD KK, Astellas Pharma Inc, Otsuka Pharmaceutical Co, Ltd, Pfizer Japan Inc. Dr Takahashi received research grant, other research support, and honoraria from Public Health Research Foundation. Dr Hirayama received research grant from Public Health Research Foundation, honoraria from Sanofi KK, MSD KK, Takeda Pharmaceutical Co Ltd, AstraZeneca KK, Pfizer Japan Inc, Amgen Astellas BioPharma KK, Astellas Pharma Inc, research grant and honoraria from Daiichi Sankyo Co, Ltd, Bayer Yakuhin, Ltd, Otsuka Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Corp. Dr Yokoi received honoraria from Astellas Pharma Inc, Shionogi & Co, Ltd, Sanofi KK, Takeda Pharmaceutical Co Ltd, MSD KK, AstraZeneca KK, research grant and honoraria from Daiichi Sankyo Co, Ltd. Dr Kitagawa received research grant from MSD KK, honoraria from Kowa Pharmaceutical Co Ltd, Shionogi & Co, Ltd, Mitsubishi Tanabe Pharma Corp, AstraZeneca KK, Amgen Astellas BioPharma KK, Eisai Co, Ltd, Pfizer Japan Inc, Sumitomo Dainippon Pharma Co, Ltd, research grant and honoraria from Takeda Pharmaceutical Co Ltd, Sanofi KK, Astellas Pharma Inc, Otsuka Pharmaceutical Co, Ltd, Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Urabe received research grant from Teijin Pharma Ltd, Astellas Pharma Inc, honoraria from Kowa Pharmaceutical Co Ltd, Sanofi KK, Shionogi & Co, Ltd, Pfizer Japan Inc, research grant and honoraria from Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, MSD KK, Mitsubishi Tanabe Pharma Corp, Sumitomo Dainippon Pharma Co, Ltd, AstraZeneca KK, Otsuka Pharmaceutical Co, Ltd, Eisai Co, Ltd, other research support and honoraria from Public Health Research Foundation. Dr Okada received honoraria from Public Health Research Foundation, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Astellas Pharma Inc, MSD KK, Mitsubishi Tanabe Pharma Corp, AstraZeneca KK, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Amgen Astellas BioPharma KK, Pfizer Japan Inc. Dr Terayama received research grant and honoraria from Public Health Research Foundation. Dr Toyoda received honoraria from Bayer Yakuhin, Ltd, research grant and honoraria from Daiichi Sankyo Co, Ltd. Dr Nagao received research grant from Takeda Pharmaceutical Co Ltd, Sanofi KK, honoraria from Pfizer Japan Inc, Mitsubishi Tanabe Pharma Corp, Sumitomo Dainippon Pharma Co, Ltd, Mochida Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, research grant and honoraria from Bayer Yakuhin, Ltd, Otsuka Pharmaceutical Co, Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Matsumoto received honoraria from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co, Ltd, Astellas Pharma Inc, Mochida Pharmaceutical Co, Ltd, AstraZeneca KK, Sumitomo Dainippon Pharma Co, Ltd, Amgen Astellas BioPharma KK, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Pfizer Japan Inc, other research grant and honoraria from Public Health Research Foundation. Dr Ohashi received honoraria from Public Health Research Foundation, Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Daiichi Sankyo Co, Ltd, Sanofi KK, Shionogi & Co, Ltd, Astellas Pharma, Chugai Pharmaceutical Co, Ltd, research grant and honoraria from Eisai Co, Ltd. Dr Kaneko received research grant from Public Health Research Foundation, Astellas Amgen BioPharma. Dr Fujita received research grant from Public Health Research Foundation. Mr Ohtsu received other research support and honoraria from Public Health Research Foundation. Dr H. Ogawa reports no conflicts. Dr Daida received research grant from Public Health Research Foundation, Eisai Co, Ltd, Pfizer Japan Inc, Otsuka Pharmaceutical Co, Ltd, Shionogi & Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Sanwa Kagaku Kenkyusho Co, Ltd, honoraria from AstraZeneca KK, Bayer Yakuhin, Ltd, Mochida Pharmaceutical Co, Ltd, Amgen Astellas BioPharma KK, research grant and honoraria from Sanofi KK, Takeda Pharmaceutical Co Ltd, Astellas Pharma Inc, MSD KK, Daiichi Sankyo Co, Ltd, other research support and honoraria from Kowa Pharmaceutical Co Ltd. Dr Shimokawa received research grant from Shionogi & Co, Ltd, Teijin Pharma Ltd, Astellas Pharma Inc, Otsuka Pharmaceutical Co, Ltd, honoraria from Kowa Pharmaceutical Co Ltd, Sanofi KK, AstraZeneca KK, Bayer Yakuhin, Ltd, research grant and honoraria from MSD KK, Mitsubishi Tanabe Pharma Corp, Daiichi Sankyo Co, Ltd, research grant, other research support, and honoraria from Public Health Research Foundation. Dr Saito received honoraria from Otsuka Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Corp, Mochida Pharmaceutical Co, Ltd, Kowa Pharmaceutical Co Ltd, other research support and honoraria from Public Health Research Foundation. Dr T. Kimura received research grant from Sumitomo Dainippon Pharma Co, Ltd, Astellas Pharma Inc, Otsuka Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Corp, Takeda Pharmaceutical Co Ltd, other research support and honoraria from Kowa Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, research grant, other research support, and honoraria from MSD KK, Sanofi KK, Mochida Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Public Health Research Foundation, Amgen Astellas BioPharma KK. Dr Inoue received research grant from Teijin Pharma Ltd, MSD KK, Eisai Co, Ltd, Pfizer Japan Inc, Kowa Pharmaceutical Co Ltd, Mitsubishi Tanabe Pharma Corp, other research support and honoraria from AstraZeneca KK, Amgen Astellas BioPharma KK, Mochida Pharmaceutical Co, Ltd, research grant, other research support, and honoraria from Bayer Yakuhin, Ltd, Sanofi KK, Shionogi & Co, Ltd, Astellas Pharma Inc, Sumitomo Dainippon Pharma Co, Ltd, Sanwa Kagaku Kenkyusho Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Public Health Research Foundation, Takeda Pharmaceutical Co Ltd, Daiichi Sankyo Co, Ltd. Dr Matsuzaki received honoraria from Mochida Pharmaceutical Co, Ltd. Dr Nagai received honoraria from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, Shionogi & Co, Ltd, MSD KK, Mitsubishi Tanabe Pharma Corp, Amgen Astellas BioPharma KK, Eisai Co, Ltd, Astellas Pharma Inc, Sumitomo Dainippon Pharma Co, Ltd, Mochida Pharmaceutical Co, Ltd, honoraria and expert witness from Public Health Research Foundation.
Supplementary Material
Footnotes
Drs Taguchi, Iimuro, and Iwata contributed equally.
The online-only Data Supplement, podcast, and transcript are available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.032615/-/DC1.
References
- 1.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383, 1389. doi: 10.1016/S0140-6736(94)90566-5. [PubMed] [Google Scholar]
- 3.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 4.West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation. 1998;97:1440–1445. doi: 10.1161/01.cir.97.15.1440. doi: 10.1161/01.CIR.97.15.1440. [DOI] [PubMed] [Google Scholar]
- 5.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 6.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [Google Scholar]
- 7.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 8.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 11.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. doi: 10.1016/S0140-6736(10)61350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 16.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 17.Setia S, Fung SS, Waters DD. Doctors’ knowledge, attitudes, and compliance with 2013 ACC/AHA guidelines for prevention of atherosclerotic cardiovascular disease in Singapore. Vasc Health Risk Manag. 2015;11:303–310. doi: 10.2147/VHRM.S82710. doi: 10.2147/VHRM.S82710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natsuaki M, Furukawa Y, Morimoto T, Nakagawa Y, Ono K, Kaburagi S, Inada T, Mitsuoka H, Taniguchi R, Nakano A, Kita T, Sakata R, Kimura T CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization: perspectives from the CREDO-Kyoto Registry Cohort-2. Circ J. 2012;76:1369–1379. doi: 10.1253/circj.cj-11-1356. doi: 10.1253/circj.CJ-11-1356. [DOI] [PubMed] [Google Scholar]
- 19.Wu NQ, Guo YL, Ye P, Chen H, Li YF, Hua Q, Zhu CG, Gao Y, Qing P, Li XL, Wang Y, Liu G, Dong Q, Li JJ. Statins usage and target achievement of LDL-C level in Chinese patients with coronary artery disease impacted by 2013 ACC/AHA cholesterol guideline. IJC Metabolic and Endocrine. 2017;14:33–37. doi: 10.1016/j.ijcme.2016.11.002. [Google Scholar]
- 20.Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, Mabuchi H, Tushima M, Sasaki J, Goto Y, Ogawa N. Clinical efficacy of pitavastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in patients with hyperlipidemia: dose-finding study using the double-blind, three-group parallel comparison. Arzneimittelforschung. 2002;52:251–255. doi: 10.1055/s-0031-1299888. doi: 10.1055/s-0031-1299888. [DOI] [PubMed] [Google Scholar]
- 21.Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20-40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13:1047–1055. doi: 10.1111/j.1463-1326.2011.01477.x. doi: 10.1111/j.1463-1326.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Chung N, Kwan J, Kim DI, Kim WH, Kim CJ, Kim HS, Park SH, Seo HS, Shin DG, Shin YW, Shim WJ, Ahn TH, Ho Yun K, Yoon MH, Cha KS, Choi SW, Han SW, Hyon MS. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: an 8-week, multicenter, randomized, open-label, dose-titration study in Korean patients with hypercholesterolemia. Clin Ther. 2007;29:2365–2373. doi: 10.1016/j.clinthera.2007.11.002. doi: 10.1016/j.clinthera.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu PY, Lin LY, Lin HJ, Hsia CH, Hung YR, Yeh HI, Wu TC, Chen JY, Chien KL, Chen JW. Pitavastatin and Atorvastatin double-blind randomized comPArative study among hiGh-risk patients, including thOse with Type 2 diabetes mellitus, in Taiwan (PAPAGO-T Study). PLoS One. 2013;8:e76298. doi: 10.1371/journal.pone.0076298. doi: 10.1371/journal.pone.0076298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi T, Yokote K, Saito Y, Iguchi A. Pitavastatin: efficacy and safety in intensive lipid lowering. Expert Opin Pharmacother. 2007;8:2315–2327. doi: 10.1517/14656566.8.14.2315. doi: 10.1517/14656566.8.14.2315. [DOI] [PubMed] [Google Scholar]
- 25.Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M JAPAN-ACS Investigators. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome] study). J Am Coll Cardiol. 2009;54:293–302. doi: 10.1016/j.jacc.2009.04.033. doi: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease: report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(suppl):5–40. doi: 10.1161/01.cir.51.4.5. doi: 10.1161/01.CIR.51.4.5. [DOI] [PubMed] [Google Scholar]
- 27.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 29.Schoenfeld DA. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68:316–319. doi: 10.2307/2335833. [Google Scholar]
- 30.Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H J-LIT Study Group. Japan Lipid Intervention Trial. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J. 2002;66:1096–1100. doi: 10.1253/circj.66.1096. doi: 10.1253/circj.66.1096. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA Lipid Intervention Study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 32.Japanese Coronary Artery Disease (JCAD) Study Investigators. Current status of the background of patients with coronary artery disease in Japan. Circ J. 2006;70:1256–1262. doi: 10.1253/circj.70.1256. doi: 10.1253/circj.70.1256. [DOI] [PubMed] [Google Scholar]
- 33.Momiyama Y, Kawaguchi A, Kajiwara I, Ohmori R, Okada K, Saito I, Konishi M, Nakamura M, Sato S, Kokubo Y, Mannami T, Adachi H, Kario K, Iso H, Ohsuzu F, Tsushima M. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: the Japan NCVC-Collaborative Inflammation Cohort (JNIC) Study. Atherosclerosis. 2009;207:272–276. doi: 10.1016/j.atherosclerosis.2009.04.015. doi: 10.1016/j.atherosclerosis.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28:1385–1391. doi: 10.1161/ATVBAHA.107.157164. doi: 10.1161/ATVBAHA.107.157164. [DOI] [PubMed] [Google Scholar]


