Supplemental Digital Content is available in the text.
Keywords: cardiovascular diseases; epinephrine; hypertension; sequence analysis, RNA; stellate ganglion
Abstract
Single or combinatorial administration of β-blockers is a mainstay treatment strategy for conditions caused by sympathetic overactivity. Conventional wisdom suggests that the main beneficial effect of β-blockers includes resensitization and restoration of β1-adrenergic signaling pathways in the myocardium, improvements in cardiomyocyte contractility, and reversal of ventricular sensitization. However, emerging evidence indicates that another beneficial effect of β-blockers in disease may reside in sympathetic neurons. We investigated whether β-adrenoceptors are present on postganglionic sympathetic neurons and facilitate neurotransmission in a feed-forward manner. Using a combination of immunocytochemistry, RNA sequencing, Förster resonance energy transfer, and intracellular Ca2+ imaging, we demonstrate the presence of β-adrenoceptors on presynaptic sympathetic neurons in both human and rat stellate ganglia. In diseased neurons from the prehypertensive rat, there was enhanced β-adrenoceptor–mediated signaling predominantly via β2-adrenoceptor activation. Moreover, in human and rat neurons, we identified the presence of the epinephrine-synthesizing enzyme PNMT (phenylethanolamine-N-methyltransferase). Using high-pressure liquid chromatography with electrochemical detection, we measured greater epinephrine content and evoked release from the prehypertensive rat cardiac-stellate ganglia. We conclude that neurotransmitter switching resulting in enhanced epinephrine release, may provide presynaptic positive feedback on β-adrenoceptors to promote further release, that leads to greater postsynaptic excitability in disease, before increases in arterial blood pressure. Targeting neuronal β-adrenoceptor downstream signaling could provide therapeutic opportunity to minimize end-organ damage caused by sympathetic overactivity.
The myocardial β-adrenergic receptor (βAR) signaling pathway plays a pivotal role in the pathogenesis of many cardiovascular diseases. Chronic cardiac adrenergic activation and impaired myocardial cyclic nucleotide (cN) signaling, resulting from enhanced catecholaminergic neurotransmission, are well-established contributors to ventricular hypertrophy, arrhythmia, and cardiomyocyte apoptosis.1–3 Sympathetic overactivity and vagal impairment (dysautonomia) are recurrent features in normotensive subjects with a familial predisposition for hypertension4,5 and in animal models of this disease.6–8 Moreover, patients with familial dysautonomia experience catecholaminergic supersensitivity, episodic hypertension, and have a high propensity for fatal cardiac events.9
β-Blockers are a mainstay treatment for many cardiovascular diseases and stress-related events.10 Chronic β-blocker therapy affords patients a wide-range of beneficial effects, including β1AR resensitization and restoration of intracellular cN signaling pathways, improvements in cardiac myocyte contractility, and reversal of ventricular remodeling.1,3 The precise mechanisms, however, that mediate and sustain the beneficial effects of β-blockers in disease remain unclear,11,12 although the presence of potentiating Gαs-coupled presynaptic βARs on presynaptic sympathetic terminals suggests a role for β-blockers in regulating cardiac-neuronal communication.13–22 The Adrenaline Hypothesis of hypertension argues that small incremental increases in plasma adrenaline (epinephrine) enhance sympathetic activity through sustained activation of presynaptic sympathetic βARs, leading to the development of hypertension.17,23,24 Whether epinephrine synthesis occurs before the onset of hypertension is not known, as there is limited cellular and molecular data within the sympathetic stellate ganglia to confirm this idea.
In this study, we investigated whether sympathetic βARs are present on human and rat sympathetic stellate ganglia (cervicothoracic ganglia, T1–T3) that preferentially innervate the heart.25–28 We aimed to establish whether intracellular second messenger signaling coupled to presynaptic βARs is impaired in prehypertensive states and contributes to altered Ca2+ and cN signaling before increases in arterial blood pressure. Finally, we aimed to assess which neurotransmitters are present within the cardiac-sympathetic ganglia to test the idea that epinephrine may act as the preferential sympathetic neurotransmitter, predisposing to disease.
Methods
Data Accessibility
Our RNA sequencing (RNAseq) raw FastQ files are deposited in the National Center for Biotechnology Information short reads archive under Short Reads Archive number SRP132271, and our quasi-mapped data will be available under Gene Expression Omnibus accession number (GSE110197).
Clinical Samples
For clinical samples, human stellate ganglia were kindly sent by Drs Ajijola, Ardell, and Shivkumar from University of California, Los Angeles, Cardiac Arrhythmia Center. Characteristics of human donors are included in the online-only Data Supplement (Table S1 in the online-only Data Supplement). The human study was approved by the University of California, Los Angeles, Institutional Review Board (approval no. 12-000701), and informed consent was obtained from all subjects.
Animals
Young male prehypertensive spontaneously hypertensive rats (pre-SHRs) of 3.5 to 5.5 week old, 16- to 20-week-old adult male normotensive Wistar rats, and age-matched spontaneously hypertensive rats (SHRs) were obtained from Envigo, United Kingdom. The SHR strain displays normal blood pressure at 4 weeks of age, where increases in arterial blood pressure develop progressively from 5 to 6 weeks of age.29–36 In this study, we used the Wistar rat strain as the normotensive control, given that Wistar rats are the progenitor strain from which the Wistar Kyoto was bred and the 2 strains display similar hemodynamic profiles at all ages.31,32,36–41 In addition, neither strain display a sympathetic Ca2+ phenotype (Figure S1A), making the Wistar a suitable control in this study. All rats were housed in standard plastic cages, and artificial lighting was fixed to a natural 12-hour light/dark cycle. Food and water were available ad libitum. All experiments were performed in accordance with the UK Home Office Animal Scientific Procedures Act 1986 and approved by the University of Oxford (PPL 30/3131; David J. Paterson). An expanded Materials and Methods section is available in the online-only Data Supplement for neuronal culture methodology, immunocytochemistry, RNAseq, Förster resonance energy transfer (FRET), Ca2+ imaging, and high-pressure liquid chromatography coupled to electrochemical detection protocols.
Results
Rat and Human Sympathetic Stellate Ganglia Express β1 and β2 Adrenoceptors
We sequenced the transcriptome of the sympathetic stellate ganglia from 16-week-old male Wistar rats (n=4) and SHR (n=4). At 16 weeks, it is well-established that SHR display hypertension and sympathetic hyperactivity.6,29–31,33,35,36,42 Using quasi-mapping RNAseq43 and quantitative real-time (qRT)-polymerase chain reaction (PCR), we identified the presence of β1AR (Adrb1) and β2AR (Adrb2) mRNA transcripts, in addition to α2AAR (Adra2a) and tyrosine hydroxylase (Th) mRNA transcripts, markers of presynaptic sympathetic neurons, respectively (Figure 1A; Figure S1). We selected the α2AAR isoform as an indicator of presynaptic neuronal phenotype based on reports that the α2AAR primarily regulates presynaptic sympathetic activity.44 The α2CAR isoform plays a secondary role in regulating presynaptic norepinephrine release,44 whereas the α2BAR isoform has a preferential role within the vasculature.44 The mRNA expression for α2AAR was also found to be significantly higher than α2CAR expression identified by RNAseq (data not shown). Using RNAseq, we found that Adrb2 mRNA expression was significantly lower in SHR ganglia compared with Wistar (Figure 1A; Figure S1C; P.adj=0.00945). Data points represent mean raw counts±SEM (Figure 1A).
Figure 1.

Rat sympathetic stellate ganglia express β1- and β2-adrenergic receptors (ARs). Using RNA sequencing, we identified the presence of β1AR (Adrb1), β2AR (Adrb2), and α2AAR (Adra2a) mRNA transcripts (A) from 16-wk-old male Wistar rats (n=4) and SHR (n=4). Adrb2 expression was significantly lower in SHR ganglia compared with Wistar (P.adj=0.00945; Salmon-DESeq2 method). There was no significant difference in the levels of mRNA for Adrb1 or Adra2a between strains or between age groups. Data points represent raw counts±SEM for each transcript (A). ELISAs confirmed protein expression of β1AR and β2ARs in 3.5- to 5-wk-old rat neurons (32 stellates, 16 animals/group) and 20-wk-old neurons (20 stellates, 10 animals/group); however, no statistical tests were conducted as stellates were pooled into a single sample to obtain adequate protein concentrations for the ELISA assays. In young rat stellates (B), the concentration of β1AR protein was calculated as 869.1±50.6 pg/mL (Wistar) and 114.2±23.7 pg/mL (pre-SHR). β2AR protein expression was calculated as 363.5±43.6 pg/mL (Wistar) and 82.2±20.0 pg/mL (pre-SHR). In adult rat stellates (C), β1AR expression was calculated as 674.1±44.6 pg/mL (Wistar) and 489.4±26.3 pg/mL (SHR). β2AR protein expression quantified as 353.3±11.2 pg/mL (Wistar) and 147.4±20.7 pg/mL (SHR). Data points depict mean±SEM of 3 to 4 technical replicates. β1AR (516±99.17 pg/mL) and β2AR (340±104.3 pg/mL) expression was also detected in stellate ganglia from human donors. Data points represent mean±SEM (6 replicates), from 3 pooled stellates obtained from 2 patients (D). Immunocytochemistry depicts β1AR (E) and β2AR (F) expression on TH (tyrosine hydroxylase)-positive neurons from 4-wk control rats. White arrows demonstrate the localization of β2AR on synaptic terminals.
The presence of Adrb1, Adrb2, Adra2a, and Th mRNA transcripts was identified and quantified by quantitative real time PCR (qRT-PCR) using RNA extracted from 4-week pre-SHR and Wistar rats (n=3 rats/group, unpooled; Figure S1D) and 16-week SHR and Wistar rats (n=4 rats/group, unpooled; Figure S1D). qRT-PCR data were analyzed using the ΔΔCT method, where raw counts in both strains were first normalized to a control housekeeping gene B2m, and the difference in counts between SHR and Wistar was calculated.45 Data points represent log2 (fold change)±SEM. There was no significant difference in the levels of mRNA for Adrb1, Adrb2, Adra2a, or Th between strains or between age groups by qRT-PCR although the trend for a reduction in Adrb2 expression remained.
Sandwich ELISAs were used to quantify the relative protein expression of β1AR and β2ARs in postganglionic sympathetic neurons obtained from 3- to 5-week-old normotensive pre-SHR and Wistar rats (Figure 1B; 32 stellates, 16 rats/group, pooled) or 19- to 20-week-old SHR and age-matched Wistar rats (Figure 1C; 20 stellates, 10 rats/group, pooled). The ELISA assays were biologically powered where 20 to 32 stellates were used per sample; however, the stellates tissue was pooled to obtain an adequate protein concentration for the ELISA assays, therefore no statistical comparisons were made. Data points indicate mean±SEM (of 3–4 technical replicates).
In 4 stellate ganglia samples obtained from 3 human donors (2 left stellates, 2 right stellates, unpooled), qRT-PCR confirmed the presence of mRNA transcripts encoding β1AR (Adrb1) and β2AR (Adrb2; S1E). Ganglia were α2aAR (Adra2a) positive, confirming a presynaptic phenotype. Samples were normalized to a control housekeeping gene B2m (3 replicates)using the ΔCT method.45 Data points represent normalized counts±SEM. ELISAs confirmed the expression of β1AR (516±99.17 pg/mL) and β2AR (340±104.3 pg/mL) in 3 human stellates obtained from 2 patients (Figure 1D; pooled, 6 replicates). Immunocytochemistry confirmed the expression of β1AR and β2AR on TH-positive neurons from 3- to 5-week-old control rats (Figure 1E and 1F; respectively) and SHR (data not shown).
βAR-Evoked cAMP Generation, PKA Activity, and [Ca2+]i Are Enhanced in Pre-SHR Neurons
To determine whether the presence of presynaptic βARs on sympathetic ganglia plays a functional role in modulating intracellular cN signaling pathways, we used FRET to quantify the relative levels of cAMP and PKA (protein kinase A) activity in response to a relatively nonselective βAR agonist, isoprenaline. To assess whether βAR-mediated cAMP generation facilitated signaling via the canonical cAMP–PKA–Ca2+ pathway, we used the loss-of-FRET sensor Epacs1-H187 (EpacH187)46 to measure changes in intracellular cAMP. Isoprenaline administration at 10 nmol/L led to significantly greater cAMP generation in pre-SHR (55.6%±16.8%) compared with that measured in Wistar neurons (7.1%±1.4%; 2-way ANOVA; P<0.0001) that was also observed at higher concentrations of isoprenaline (Figure 2A and 2B). PKA activity was measured using the gain-of-FRET sensor AKAR4.47 Using the same concentration of isoprenaline (10 nmol/L), we found that PKA activity was significantly higher in pre-SHR (26.1%±5.4%) versus Wistar neurons (4.5%±1.1%; 2-way ANOVA; P<0.0001), which was also observed at 100 nmol/L isoprenaline (Figure 2C and 2D). Raw YFP (yellow fluorescent protein) and CFP (cyan fluorescent protein) fluorescence traces as emitted from the cytosolic loss-of FRET sensor EpacH187 and the gain-of-FRET sensor AKAR4 in response to isoprenaline (10–100 nmol/L) are presented in the online-only Data Supplement (Figure S2A and S2B).
Figure 2.
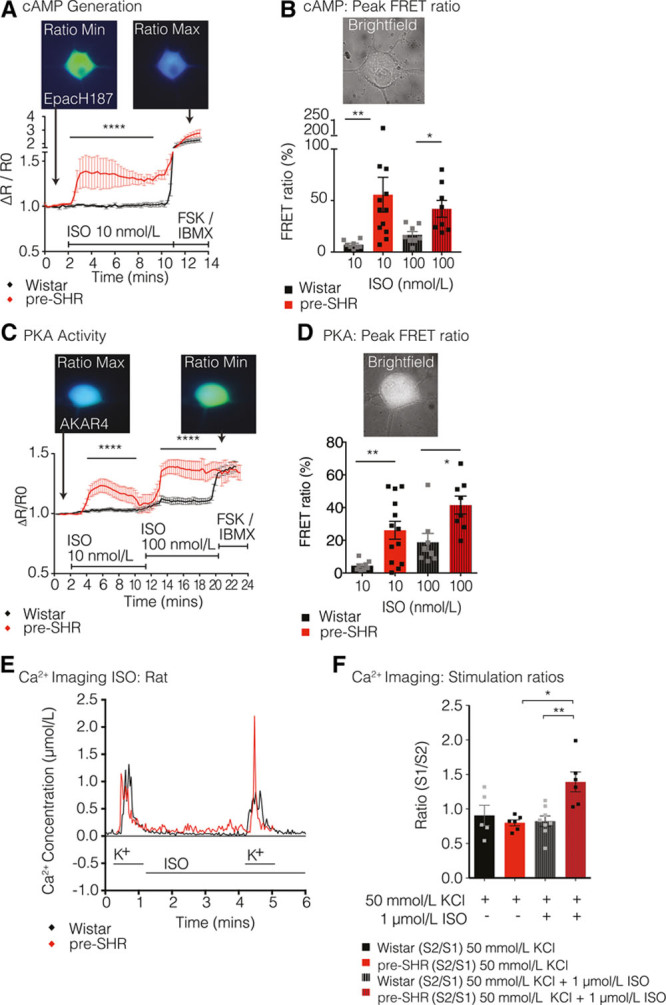
β-Adrenergic receptor (βAR) stimulation increases cAMP–PKA–Ca2+ signaling in pre-SHR neurons. Isoprenaline (ISO) generated significantly higher levels of cAMP at 10 nmol/L (A) in pre-SHR (55.6%±16.8%; n=12) compared with Wistar neurons (7.1%±1.4%; n=8; 2-way ANOVA; P<0.0001) and at 100 nmol/L (B) in pre-SHR (42.0%±8.2%; n=8) vs Wistar (16.8%±3.2%; n=8; 2-way ANOVA; P<0.0001). ISO increased PKA activity to a significantly greater extent at 10 nmol/L (C) in pre-SHR (26.1%±5.5%; n=13) vs Wistar neurons (4.5%±1.1%; n=8; 2-way ANOVA; P<0.0001) and at 100 nmol/L (D) in pre-SHR (41.5%±5.5%; n=8) vs Wistar (18.8%±5.4%; n=8; 2-way ANOVA; P<0.0001). Cells that did not respond appropriately to forskolin (FSK, 25 μmol/L) and IBMX (3-isobutyl-1-methylxanthine; 100 μmol/L) were excluded from the final analysis. Ca2+ imaging was conducted on neurons obtained from 4-wk rats using Indo-1AM (E and F). Wistar and pre-SHR neurons (n=8, 6, respectively) were exposed to 2 KCl challenges (50 mmol/L; stimulations 1 and 2) where stimulation 2 was conducted in the presence of ISO (1 μmol/L). Time-controlled experiments were performed in the absence of ISO (Wistar, n=5; and pre-SHR n=6). There was significantly higher [Ca2+]i evoked in the presence of ISO in pre-SHR neurons compared with Wistar neurons (unpaired Student t test; P=0.0027). Bar charts represent mean±SEM. FRET indicates Förster resonance energy transfer.
To assess whether isoprenaline-dependent βAR activation enhances intracellular Ca2+ ([Ca2+]i), we measured responses to KCl in the absence or presence of isoprenaline. Ca2+ recordings were obtained using Indo-1AM labeled sympathetic neurons from 4-week pre-SHR and Wistar rats. In pre-SHR stellate neurons, KCl stimulation in the presence of isoprenaline led to significantly higher [Ca2+]i than KCl stimulations alone (Figure 2E and 2F; P=0.0272). There was significantly higher KCl-evoked [Ca2+]i in the presence of isoprenaline in pre-SHR neurons compared with that recorded in control neurons (Figure 2E and 2F; P=0.0027). A time-controlled example trace is shown in the online-only Data Supplement (Figure S2C).
Relative Contribution of β1AR and β2AR Signaling in Neuronal cAMP Generation
To ascertain whether the observed increases in isoprenaline-evoked cAMP occurs predominantly through either β1AR or β2AR activation, cells were challenged with either a β1AR agonist (dobutamine, 50 μmol/L) after β2AR blockade with ICI-118,551 (ICI, 10 nmol/L) or in alternative experiments, administration of a β2AR agonist (salbutamol, 10 μmol/L) after β1AR antagonism (metoprolol, 100 nmol/L). Administration of the β1AR agonist dobutamine led to significantly greater cAMP generation in pre-SHR (15.82%±2.8%) compared with Wistar neurons (−0.31%±2.4%; 2-way ANOVA; P<0.0001; Figure 3A and 3C).
Figure 3.
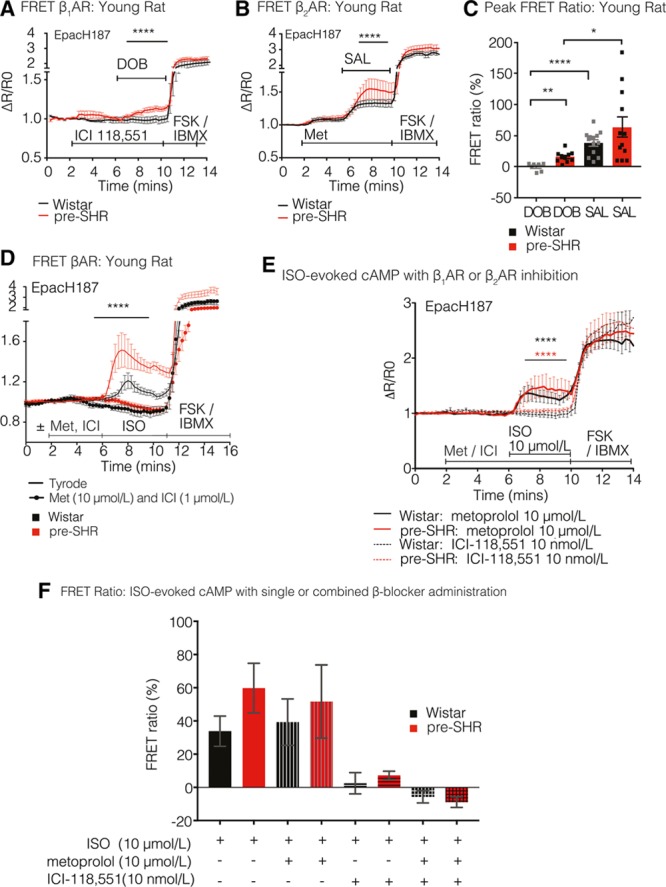
Relative contribution of presynaptic β1-adrenergic receptor (AR) and β2AR in neuron cAMP generation. For measurements of cAMP generation, 4-wk control and age-matched pre-SHR neurons were transduced with the EpacH187 FRET biosensor. Cells were stimulated with a β1AR agonist, dobutamine (DOB, 50 μmol/L) after β2AR inhibition with a selective antagonist, ICI-118,551 (ICI, 10 nmol/L) that led to a significantly greater increase in cAMP generation in pre-SHR (15.82%±2.8%; n=5) compared with Wistar neurons (−0.31%±2.4%; n=10; 2-way ANOVA; P<0.0001). In Wistar neurons, administration of DOB did not increase cAMP from baseline (A). In alterative experiments, neurons were stimulated with a β2AR agonist salbutamol (SAL, 10 μmol/L) after β1AR inhibition with a selective antagonist, metoprolol (MET, 100 nmol/L). SAL administration led to a greater increase in cAMP generation in pre-SHR (63.8%±16.6%; n=12) compared with Wistar neurons (38.6%±5.2%; n=13; 2-way ANOVA; P<0.0001; B). Peak FRET ratios (%) evoked by DOB or SAL were calculated (C). SAL generated significantly higher cAMP levels, than that evoked by DOB in Wistar (P<0.0001, Mann–Whitney) and in pre-SHR PGSNs (P=0.0173, unpaired 2-tailed Student t test). To confirm that ISO-evoked cAMP was acting downstream of βAR activation, we tested ISO-evoked cAMP in the absence and presence of a combination of β1AR and β2AR antagonists (ISO, 10 μmol/L; MET, 10 μmol/L, ICI, 1 μmol/L, respectively) or selective blockade of either β1AR (MET, 10 μmol/L) or β2AR (ICI, 10 nmol/L). The dual combination of β-blockers abolished cAMP generation in both pre-SHR (n=6) and Wistar neurons (n=6), demonstrating that ISO-dependent cAMP generation is dependent on βAR activation (D). There was significantly greater inhibition of cAMP after β2AR compared with β1AR inhibition in Wistar (P<0.0001; 2-way repeated measures ANOVA) and pre-SHR neurons (P<0.0001; 2-way repeated measures ANOVA), suggesting that β2AR plays a predominant role in cAMP generation, regardless of strain (E). Peak FRET responses are depicted (F).
We observed that in Wistar neurons, administration of dobutamine did not increase cAMP from baseline. Administration of the β2AR agonist salbutamol also led to a significantly greater cAMP generation in pre-SHR (63.8%±16.6%) compared with Wistar neurons (38.6%±5.2%; 2-way ANOVA; P<0.0001; Figure 3B and 3C). There was significantly higher peak salbutamol-evoked cAMP compared with dobutamine-evoked cAMP in Wistar (P<0.0001; Mann–Whitney) and in pre-SHR neurons (P=0.0173, unpaired 2-tailed Student t test), highlighting a greater contribution of β2AR versus β1AR in generating cAMP, regardless of strain (C).
To confirm that isoprenaline-evoked cAMP is acting through β1AR and β2ARs rather than inducing off-target effects, we tested isoprenaline-evoked cAMP in the absence and presence of a combination of β1AR and β2AR antagonists (isoprenaline, 10 μmol/L; metoprolol, 10 μmol/L; ICI, 1 μmol/L, respectively). The combination of β1AR and β2AR antagonists abolished cAMP generation entirely in response to a high concentration of isoprenaline in both pre-SHR (n=6) and Wistar neurons (n=6), demonstrating that isoprenaline-dependent cAMP generation is dependent on selective β1AR and β2AR activation (Figure 3D). To support these observations, we also selectively inhibited β1AR (metoprolol, 10 μmol/L) or β2AR (ICI, 10 nmol/L) and measured the resulting cAMP generation in response to isoprenaline (10 μmol/L). β2AR blockade reduced isoprenaline-evoked cAMP generation to a greater extent than β1AR blockade in both Wistar and pre-SHR neurons, confirming our previous observations for a preferential effect of β2AR versus β1AR mediated signaling in postganglionic sympathetic neurons (Figure 3E and 3F). We measured a slight but significantly greater cAMP generation in pre-SHR versus Wistar neurons in the presence of either metoprolol (P=0.0472) or ICI (P<0.001) using 2-way repeated measure ANOVAs; however, the peak FRET responses themselves were not different significantly between strains (Figure 3F). The selectivity and specificity of the selected β1AR and β2AR agonists (dobutamine and salbutamol, respectively) and the β1AR and β2AR antagonists (metoprolol and ICI) have been previously reported.48–51
Epinephrine-Synthesizing Enzyme PNMT Is Present in Rat and Human Stellate Ganglia
RNAseq was performed to obtain an overview of the transcriptome in stellate ganglia obtained from 16-week-old male SHR (n=4) and Wistar rats (n=4). We identified the presence of mRNA transcripts-encoding enzymes required for norepinephrine synthesis (Figure 4A): phenylalanine hydroxylase (Pah), Th, L-DOPA decarboxylase (Ddc), dopamine β-hydroxylase (Dbh). Furthermore, RNAseq identified the presence of the mRNA transcript encoding phenylethanolamine-N-methyltransferase (Pnmt), the enzyme required for the conversion of norepinephrine to epinephrine in both Wistar and SHR stellate ganglia. In the RNAseq data set, Pah and Ddc mRNA transcript expression were also shown to be significantly lower in SHR neurons (Figure 4B; Figure S3A; P.adj=0.0719; 6.64×10–15, Pah, Ddc, respectively). These findings were validated in 4-week Wistar and pre-SHR by qRT-PCR, and a significant ≈4-fold reduction in Pah expression was observed in pre-SHR ganglia (Figure 4C; P=0.0098). Data were normalized to a control housekeeping gene (B2m), and SHR gene counts were subsequently normalized to Wistar using the ΔΔCT method. Data are presented as Log2(fold change).45 Using the same method, we also confirmed the presence of Pnmt and Th by qRT-PCR in neurons from 16-week Wistar and SHR (Figure S3B). There was no significant difference in mRNA expression of either Th or Pnmt between age groups or between phenotypes. ELISA assays confirmed protein expression of TH (Figure 4D) and PNMT (Figure 4E) in stellate ganglia from 4-week pre-SHR, 20-week-old SHR, and age-matched Wistar rats. The ELISA assays were high-powered biologically, where 20 to 32 stellates were used per sample; however, stellates were pooled to obtain adequate protein concentrations for the ELISA assays, therefore no statistical comparisons were made. Data points indicate mean±SEM (of 2–3 technical replicates).
Figure 4.
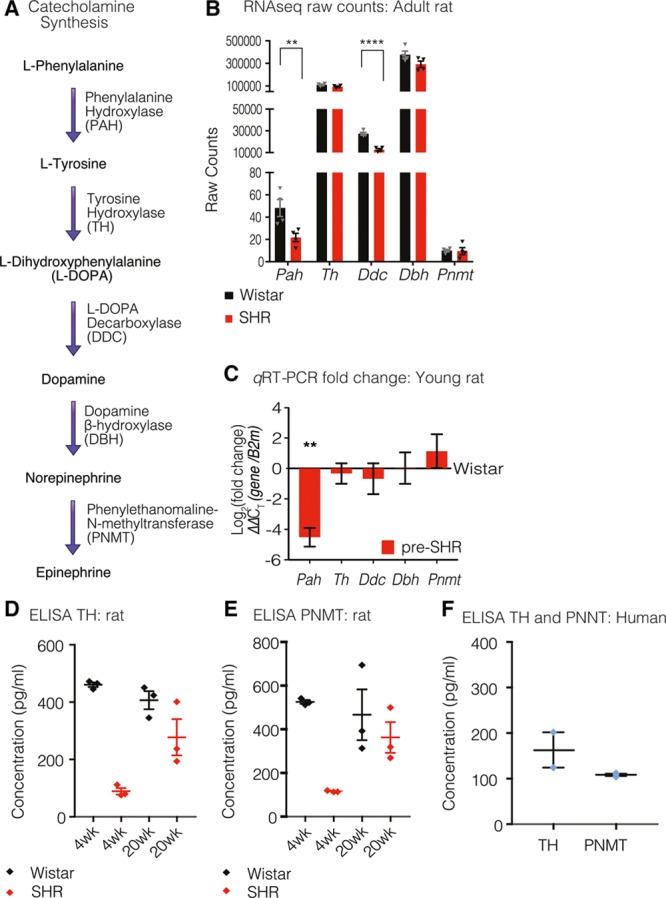
The epinephrine-synthesizing enzyme PNMT (phenylethanolamine-N-methyltransferase) is present in rat and human stellate ganglia. The catecholamine synthesis pathway is outlined (A). RNA sequencing (RNAseq) revealed mRNA transcripts that encode the enzymes required for norepinephrine (NE) synthesis: phenylalanine hydroxylase (Pah), tyrosine hydroxylase (Th), L-DOPA decarboxylase (Ddc), dopamine β-hydroxylase (Dbh; B). We also identified the transcript that encodes Pnmt required for the conversion of NE to epinephrine (Epi). Pah and Ddc mRNA expressions as determined by RNAseq were significantly lower in SHR neurons (P.adj=0.0719, Pah; 6.64×10–15, Ddc; Salmon-DESeq2). Data depicts raw counts±SEM (B). Transcript expression was validated via quantitative real-time polymerase chain reaction (qRT-PCR) using RNA extracted from 4-wk Wistar (n=4) and pre-SHR (n=4) ganglia (C). For qRT-PCR analyses, genes were normalized to the housekeeping gene (B2m), and SHR counts were normalized to Wistar using the ΔΔCT method. There was a significant (4-fold) decrease in Pah in pre-SHR neurons (C; P=0.0098, unpaired 2-tailed Student t test). SHR (red bars) are depicted relative to number of counts calculated from Wistar samples (x axis). The protein concentration for TH (D) was quantified in 4-wk Wistar (460.9±7.979 pg/mL), pre-SHR ganglia (89.12±11.37 pg/mL), 20-wk Wistar (406.6±31.57 pg/mL), and SHR ganglia (277.5±63.03 pg/mL). PNMT protein expression was also quantified (E) in 4-wk Wistar (525.7±8.69 pg/mL), pre-SHR ganglia (117±3.73 pg/mL), 20-wk Wistar (466.7±116.2 pg/mL), and SHR ganglia (362.7±70.08 pg/mL). Data represent mean±SEM (2–3 technical replicates). We confirmed the protein expression of TH (163±38.83 pg/mL) and PNMT (108.4±2.386 pg/mL) in human stellates (F). Data points represent mean±SEM (2–3 replicates) from 3 pooled stellates obtained from 2 patients. Where stellates were pooled to obtain adequate protein concentrations, no statistical tests were conducted.
To assess whether the presence of PNMT in sympathetic stellate ganglia is conserved in higher species, we obtained stellate ganglia from male human donors. qRT-PCR demonstrated the presence of both Th and Pnmt mRNA transcripts in human sympathetic stellate ganglia (Figure S3C). Data were normalized to a control housekeeping gene B2m using the ΔCT method45 and expressed as normalized count values (3 patients, 4 stellates). We also used ELISAs to confirm protein expression of both TH and PNMT in human stellate samples (Figure 4F). Data points represent mean±SEM (2–3 replicates) from 3 pooled stellates obtained from 2 patients.
Epinephrine Is Released From Pre-SHR but Not Wistar Whole-Stellate Ganglia
After the identification of PNMT, we investigated whether epinephrine is released from the whole rat stellate ganglia under basal conditions or with electric field stimulation. We measured significantly greater total norepinephrine content in homogenized Wistar stellates (43.3±2.173 pg; n=8) compared with pre-SHR stellate ganglia (29.82±6.366 pg; n=4; P=0.0294). In the same homogenate, we measured a greater content of epinephrine in pre-SHR ganglia (14.14±5.399 pg) compared with that measured in Wistar stellates (3.937±0.820 pg; P=0.0019), suggesting that a significant amount of norepinephrine is converted to epinephrine in prehypertensive states (Figure 5A).
Figure 5.
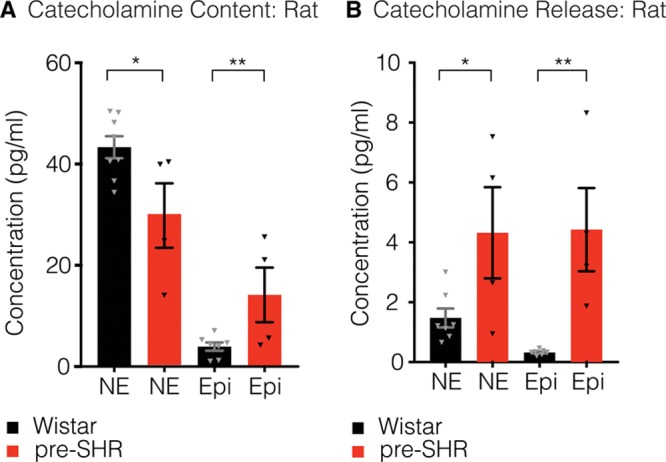
. Epinephrine (Epi) is released from pre-SHR but not Wistar whole-stellate ganglia. Using high-pressure liquid chromatography coupled to electrochemical detection, we measured significantly higher total norepinephrine (NE; A) in Wistar (43.3±2.173 pg; n=8) compared with pre-SHR neurons (29.82±6.366 pg; n=4; unpaired 2-tailed Student t test; P=0.0294). In the same stellate samples (A), we also measured a significantly greater total content of Epi in pre-SHR (14.14±5.399 pg) compared with that measured in Wistar ganglia (3.937±0.820 pg; unpaired 2-tailed Student t test; P=0.0019). Electric field stimulation of whole-rat stellate ganglia led to the release of NE (B) that was significantly higher in samples obtained from pre-SHR (4.32±1.523 pg) vs Wistar ganglia (1.477±0.316 pg; unpaired 2-tailed Student t test; P=0.0396). The concentrations of neurally-mediated Epi release (B) were also significantly higher in pre-SHR (4.424±1.391 pg; n=4) compared with Wistar stellates (0.3201±0.0325 pg; n=8; unpaired 2-tailed Student t test; P=0.0028).
We also investigated whether epinephrine is released from rat stellate ganglia with electric stimulation (Figure 5B). Electrically evoked concentrations of norepinephrine were significantly higher in samples obtained from pre-SHR (4.32±1.523 pg; n=4) versus Wistar ganglia (1.477±0.316 pg; n=8; P=0.0396). Moreover, in the same samples, the concentrations of electrically evoked epinephrine were significantly higher in pre-SHR ganglia (4.424±1.391 pg) compared with that measured in Wistar stellates (0.3201±0.0325 pg; P=0.0396).
Discussion
In this study, we have obtained evidence for β1AR and β2AR mRNA and protein expression on presynaptic postganglionic sympathetic neurons from human and rat ganglia. We have further demonstrated that in isolated sympathetic neurons, βAR agonists elevate cAMP and activate PKA. The effects were more pronounced in neurons from pre-SHR rats. We also observed that βAR agonists enhanced [Ca2+]iin response to depolarization by high K+ in pre-SHR neurons only. In addition, we demonstrate the presence of mRNA and protein expression of PNMT, the enzyme involved in the synthesis of epinephrine in human and rat sympathetic stellate neurons. Moreover, we observed that epinephrine is present in diseased states and is actively released from prehypertensive, but not healthy rat neurons, suggesting preferential switching of neurotransmitter synthesis in disease.
Single or combinatorial administration of β-blockers is a mainstay treatment strategy for diseases caused by sympathetic overactivity, although the precise mechanisms that underpin the long-term beneficial effects are not entirely clear.12 Current dogma suggests that the observed antihypertensive and cardioprotective effects of β-blockers are mediated through inhibition of cardiac and vascular βARs, reducing myocardial work and total peripheral resistance.52 Our findings suggest that the efficacy of clinical β-blockers may be attributed, at least in part, to a reduction in sympathetic hyperactivity and neurotransmission at the end-organ.
What is the cause for increased sympathetic neurotransmission before the onset of neurogenic hypertension? Emerging evidence suggests that impaired nitric oxide synthesis and reductions in cGMP–PKG (protein kinase G) signaling lead to pathological increases in [Ca2+]i and norepinephrine release at the end-organ.31,53 Recently, we demonstrated that decreased cGMP signaling leads to enhanced N-type Ca2+ channel (Cav2.2) currents and that this effect may be ameliorated by artificially increasing cytosolic cGMP.54,55 cN signaling is acutely regulated by phosphodiesterase enzymes, and in early prehypertensive states, phosphodiesterase signaling is impaired, resulting in an imbalance between cAMP and cGMP signaling.54 We, therefore, sought to ask the question, could high levels of neurotransmitter release act in an autocrine or paracrine fashion to increase neuronal cAMP and potentiate neurotransmission in a feed-forward manner?
Although it has been previously reported that presynaptic βARs are present56 and may be capable of facilitating norepinephrine release in several peripheral autonomic ganglia in rat, guinea pig, cat, rabbit, dog, and human13–17,19,57–62, the role of adrenergic signaling within the sympathetic stellate ganglia remains unclear, particularly in disease. In the present study, we confirmed the presence of both β1AR and β2AR isoforms in stellate ganglia from human and rat and found that activation of βARs on rat sympathetic neuron led to a significantly greater increase in intracellular cAMP generation, PKA activity in pre-SHR compared with control neurons (Figure 2).
To assess whether βAR signaling facilitates cardiac-sympathetic neurotransmission, [Ca2+]i was measured in response to KCl in the absence or presence of isoprenaline. Consistent with the observed increases in βAR-mediated cAMP–PKA signaling in pre-SHR neurons, isoprenaline also increased KCl-evoked [Ca2+]i in prehypertensive states; whereas there was no effect of isoprenaline in control neurons (Figure 2E and 2F). These data demonstrate that enhanced βAR-mediated signaling in sympathetic neurons contributes to the Ca2+ phenotype and increases sympathetic transmission. Previous work has demonstrated that the N-type calcium channel is the primary voltage-gated channel responsible for Ca2+ influx in sympathetic neurons and carries a significantly larger Ca2+ current in pre-SHR and SHR neurons compared with controls.55,63 N-type calcium channelactivity is differentially regulated by PKA and PKG.54,55 Therefore, we suggest that the isoprenaline-potentiated increases in [Ca2+]i in pre-SHR neurons primarily occurs as a result of βAR–cAMP activation that increases PKA-dependent phosphorylation of N-type calcium channel (Cav2.2).
To establish whether the observed increases in cAMP–PKA activity occur downstream of β1AR or β2AR signaling, cells were perfused with selective agonists for either β1AR or β2AR subtypes in the presence of either alternate βAR antagonist. We found that selective activation of β1AR or β2AR led to significantly greater increases in cAMP in pre-SHR neurons compared with Wistar. Indeed, there was no measureable effect of β1AR activation on [cAMP] in normotensive controls (Figure 3). Furthermore, stimulation of pre-SHR neurons with the β2AR agonist salbutamol led to cAMP generation that was almost twice as high as β1AR-evoked cAMP within pre-SHR neurons, suggesting a dominant role for β2AR compared with β1AR signaling. To establish whether increased βAR signaling in pre-SHR results from increases in βAR expression, we measured levels of β1AR and β2AR mRNA via qRT-PCR and RNAseq and quantified protein levels using sandwich ELISAs. Surprisingly, we observed that βAR transcripts and protein expression are reduced in pre-SHR stellates, as well as in aged SHR with established hypertension,6,29–31,33,35,36,42 compared with age-matched Wistar neurons, in a similar manner to that reported in the myocardium.64,65 We also report that in healthy ganglia, β1AR expression decreases with age, much like in the heart (Figure 1). Together, these data suggest that in diseased states, the potentiating effects of βAR agonists may be mediated through impaired second messengers coupled to cAMP and its effector PKA, probably via impairment of phosphodiesterases to hydrolyze cAMP54,55 rather than the G-protein coupled receptors themselves.
Which neurotransmitter preferentially activates presynaptic βARs? Several studies suggest limited involvement of norepinephrine in potentiating presynaptic neurotransmission but argue for a critical role for epinephrine in enhancing release, particularly in patients with essential hypertension66 or stress disorders.67,68 The role of epinephrine in the pathogenesis of essential hypertension has been termed the Adrenaline Hypothesis23; however, the origins of local concentration of epinephrine remain unclear. Most reports suggest that high circulating plasma epinephrine concentrations arise from the adrenal medulla with active reuptake into sympathetic nerve terminals.19,22,23,69–71 Others have identified heightened epinephrine synthesis within the central nervous system, specifically the nucleus tractus solitariuus72 and hypothalamus72,73 and suggest that this source of epinephrine may underpin the high plasma levels of epinephrine. Alternatively, some reports have identified in situ epinephrine synthesis within various sympathetic ganglia in rat and human, via a stress-inducible mechanism.19,24,66,72,74–77 Our identification of PNMT mRNA and protein expression in human and rat cardiac-sympathetic ganglia (Figure 4) supports the findings of these earlier studies that epinephrine is synthesized in sympathetic stellate ganglia in disease.
What is the relevance of epinephrine synthesis in prehypertensive sympathetic stellate ganglia? The Adrenaline Hypothesis of hypertension proposes that stress and subsequent small incremental increases in epinephrine plays a major role in the pathogenesis of hypertension, not via epinephrine directly, but as a result of increased sympathetic activity and enhanced norepinephrine release.23 This sustained increase in sympathetic activity caused by epinephrine leads to the development of hypertension. We have shown that epinephrine is synthesized in pre-SHR to a greater extent than in healthy sympathetic stellate ganglia and is only released from pre-SHR ganglia. Importantly, epinephrine has a 10-fold higher affinity for β2AR than norepinephrine (EC50 5.2, 53.7 nmol/L, respectively) and is capable of generating 3× more cAMP than norepinephrine via β2AR activation, a feature that may be mimicked by isoprenaline because of similarities in efficacy.78 Subsequently, the high efficacy of epinephrine (and the relatively low efficacy of norepinephrine) at β2AR has been shown to result in epinephrine-dependent norepinephrine transmission. Indeed, low concentrations of epinephrine (0.1–10 nmol/L) have been shown to be 100× to 500× more potent than norepinephrine in enhancing activity-dependent norepinephrine release.19,79–81 Moreover, epinephrine may have a more sustained effect on norepinephrine release because of the extended tissue half-life of epinephrine.79 Epinephrine-induced norepinephrine release has been identified in a wide variety of peripheral tissues in rat, rabbit, and human.18,19,22
We sought to investigate whether the presence of presynaptic PNMT plays a functional role in converting norepinephrine to epinephrine in rat stellate ganglia, by measuring total catecholamine content and electrically evoked catecholamines by high-pressure liquid chromatography coupled to electrochemical detection (Figure 5). We identified a significant decrease in total norepinephrine content in pre-SHR ganglia (74.8% of total catecholamine content) compared with norepinephrine calculated in Wistar ganglia (91.5% of total catecholamine content). We have also observed that the total content of epinephrine was significantly higher in pre-SHR ganglia (25.2% of total catecholamine content) compared with epinephrine levels quantified in Wistar ganglia (8.5% of total catecholamines measured). Furthermore, we found that on electric stimulation, the percentage ratio of norepinephrine:epinephrine released from Wistar ganglia was calculated as 91%:9%; whereas in pre-SHR ganglia, the ratio of catecholamines released (norepinephrine:epinephrine) was 44%:56% (Figure 5), although the total amounts of catecholamines released during electric stimulation remained fairly similar between the strains (≈11–12 pg).
One recurrent feature in human and animal models of hypertension is the reduction in norepinephrine reuptake transport (NET), leading to larger and more sustained extracellular catecholamine concentrations.24,82–84 Recently, it has been proposed that PNMT may also act as a DNA methylase, silencing NET transcription that may underpin the observed NET phenotype.24 We have previously identified reductions in NET activity in the pre-SHR cardiac-stellate ganglia.82
Limitations
In this study, we investigated the role and mechanisms involved in feed-forward presynaptic signaling in the cardiac-sympathetic ganglia. We performed a hypothesis neutral, nonbiased approach to sequencing the transcriptome of sympathetic stellate in adult rats that revealed the presence of RNA transcripts involved βAR receptor expression and epinephrine synthesis. We assessed the functional relevance of these findings by probing the adrenergic intracellular signaling pathways coupled to Ca2+-mediated exocytosis. There were several limitations to these approaches. First, the stellate ganglion comprises a heterogeneous population of cell types. Indeed, we identified markers of fibroblasts and astrocytes, including vimentin and glial fibrillary acidic protein, respectively; however, we identified that a high number of transcripts were neuronal in phenotype. We also found that the subunit profile of nicotinic acetylcholine receptors matches those described for sympathetic neurons.85 Moreover, immunocytochemistry highlighted the localization of β- and αARs on the soma and dendrites of TH-positive neurons. In support of these data, our collaborators have also identified the presence of transcripts encoding presynaptic βARs in sorted sympathetic mouse neurons (Ana Domingos, personal communication, 2018). Second, in the absence of cardiac tracing experiments, we rely on anatomic literature,25–27 and our own previous observations32,86–88 that the results presented here are relevant to cardiac-sympathetic communication because significant myocardial sympathetic innervation has been shown to arise from the cervicothoracic ganglia. Third, we used stellates obtained from male rats. Although sex differences in hypertension and cardiovascular disease incidence have been widely reported,89 in this study, we focused on investigating the transcriptome of the male rat stellate ganglia given that the prevalence for cardiovascular diseases is significantly higher in males than premenopausal women.89 Fourth, cNs and phosphodiesterases reside in distinct subcellular compartments, and their localization with βARs receptors is acutely regulated.90–92 Similarly, the regulation of Ca2+ channels by PKA/PKG occurs in distinct signalosomes, conferring site-specific regulation of Ca2+ entry coupled to neurotransmission. Furthermore, the rate of phosphodiesterase hydrolysis is critically dependent on the concentration of both cAMP and cGMP that is reported to be different between cell types.93 In this study, we measured global cytosolic cAMP, PKA, and Ca2+ concentrations, therefore we cannot ascertain precisely where the key pathways converge. Site-specific FRET and Ca2+ sensors will be required to resolve the question of microdomain impairments in cN and effector signaling.
Perspectives
Our data here demonstrate that in prehypertensive and hypertensive states, epinephrine is synthesized within presynaptic sympathetic nerve terminals and released on activation at the end-organ. In prehypertensive states, evoked release of epinephrine (that is exacerbated by decreased NET activity) may act preferentially on presynaptic β2ARs to increase cAMP generation and PKA activity, thereby enhancing Ca2+ levels and neurotransmission in disease, in a manner akin to positive feedback. We suggest that epinephrine release at the end-organ may play a role in the pathogenesis of hypertension. Figure 6 depicts the model signaling pathways in healthy and prehypertensive states and highlights potential sites for neural phenotypic targeting in disease. We suggest that in a model of early hypertension, activation of presynaptic βARs enhances cAMP generation, PKA activity, and [Ca2+]i to greater levels than that measured in healthy neurons, facilitating both norepinephrine and epinephrine release. Presynaptic activation of β2ARs (and β1ARs to a lesser extent) further enhances neurotransmission in a potentiating feed-forward manner, with activation of α2ARs playing a role in negative feedback. Additional studies aimed at investigating the relative roles of epinephrine and norepinephrine in positive and negative feedbacks signaling may be of therapeutic relevance. Indeed, these findings may have implications beyond neurogenic hypertension and may offer benefit in other diseases of sympathetic overactivity, such as modulation of renin–angiotensin–aldosterone release, chronic inflammatory diseases, and heart failure.
Figure 6.
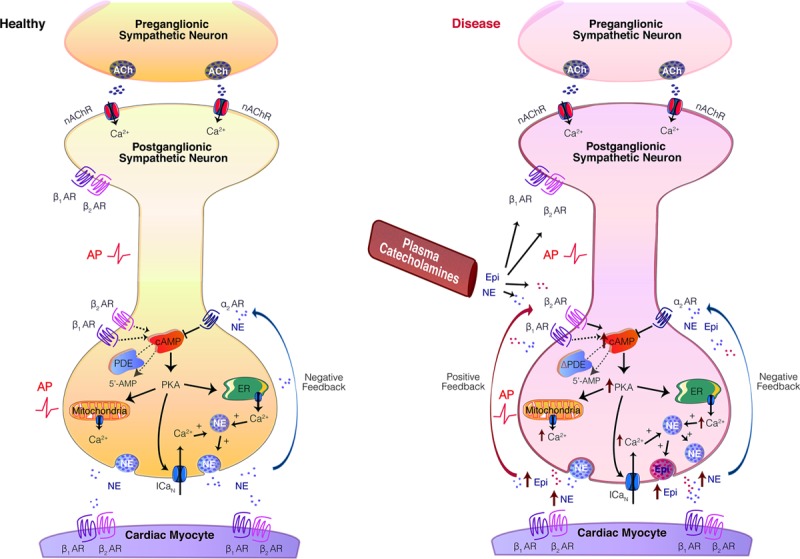
Model figure. The sympathetic stellate ganglia (cervicothoracic ganglia) are located alongside vertebrate T1 to T3. They are the primary sympathetic ganglia that innervate the heart and have been shown to exert the greatest control over increases in heart rate and contractility.25–28 In healthy postganglionic sympathetic neurons (A), Ca2+-dependent exocytosis facilitates the release of norepinephrine (NE) onto cardiac myocytes, where postsynaptic β1- and β2-adrenergic receptors (ARs) are activated. Increases in extracellular NE acts on presynaptic α2ARs, reducing adenylyl cyclase (AC) activity through activation of inhibitory Gαi G-proteins. Acute regulation of cAMP is maintained by phosphodiesterases (PDEs).90,91 cAMP-dependent PKA (protein kinase A) activity increases intracellular Ca2+ ([Ca2+]i) via phosphorylation of the N-type Ca2+ Channel (ICaN; CaV2.2)55; regulation of endoplasmic reticulum stores and mitochondrial Ca2+ release.31 In neurons obtained from the prehypertensive SHR, a young genetic model of hypertension (B), Ca2+-dependent exocytosis facilitates the release of NE and epinephrine (Epi). Activation of presynaptic βARs in prehypertensive states29–36 enhances cAMP generation, PKA activity, and [Ca2+]i to greater levels than in healthy neurons, facilitating neurotransmission in a potentiating feed-forward manner. This occurs preferentially via β2AR activation. Catecholamines may also be supplied from the circulation. We propose that β-blockers may have efficacy at βARs expressed on peripheral neurons, by reducing cardiac-sympathetic communication in hypertension and dysautonomias. ACh indicates acetylcholine; and nAChRs, nicotinic acetylcholine receptors.
Acknowledgments
We acknowledge the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z) for the generation of the sequencing data. We acknowledge our collaborators Drs Ajijola, Shivkumar, and Ardell at the University of California, Los Angeles, Cardiac Arrhythmia Center for kindly extracting and shipping human sympathetic stellate ganglia from donor patients no. 19, no. 23, and no. 24. We thank Dr Threlfell in our department for her help and expertise with high-pressure liquid chromatography coupled to electrochemical detection experimental design and protocols. E.N. Bardsley and D.J. Paterson planned the project. E.N. Bardsley performed all of the rat and human RNA extraction, validation, and quantitative real-time polymerase chain reaction experiments. E.N. Bardsley performed all cell culturing and performed Ca2+ imaging, Förster resonance energy transfer imaging, ELISAs, and immunocytochemistry. K.J. Buckler assisted with the Ca2+ experiments. H. Davis performed the catecholamine high-pressure liquid chromatography coupled to electrochemical detection experiments. E.N. Bardsley analyzed all experimental data. E.N. Bardsley and H. Davis performed the RNA sequencing differential expression analysis. E.N. Bardsley and D.J. Paterson cowrote the paper. All authors edited the manuscript.
Sources of Funding
This project was funded by the Wellcome Trust OXION initiative (105409/Z/14/Z), British Heart Foundation (BHF) Centre of Research Excellence and BHF (RG/17/14/33085), and National Institutes of Health SPARC (OT2OD023848) initiative.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.118.10844/-/DC1.
Novelty and Significance
What Is New?
We provide evidence for β1- and β2-adrenergic receptors in human stellate ganglia. These receptors are also conserved in rat stellates. We demonstrate that the cAMP–PKA signaling pathway coupled to activation of β-adrenergic receptors is impaired and exacerbates the Ca2+ phenotype in young prehypertensive rats.
We have also shown that prehypertensive rat stellate neurons synthesize and release epinephrine.
What Is Relevant?
Neurotransmitter switching to epinephrine may be an early cellular marker because this neurochemical phenotype may reflect sympathetic impairment in the early stages of disease progression.
Targeting the β-adrenergic intracellular signaling pathway and reducing PNMT (phenylethanolamine-N-methyltransferase) activity may be therapeutically relevant for the early treatment of sympathetic dysautonomia.
Summary
Using a combination of RNA sequencing, quantitative real-time polymerase chain reaction, immunocytochemistry, ELISA, Förster resonance energy transfer and Ca2+ imaging, we have shown the presence β-adrenergic receptors on presynaptic sympathetic neurons in human and rat. We have demonstrated that catecholaminergic stimulation of these receptors exacerbates the sympathetic Ca2+ phenotype in the pre-SHR before the onset of hypertension. Using high-pressure liquid chromatography coupled to electrochemical detection, we have also found that physiological concentrations of epinephrine are released from sympathetic ganglia before increases in blood pressure occur. This may reflect a site of neural impairment in disease progression.
References
- 1.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 2.Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, Nikolaev VO. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat Commun. 2015;6:6965. doi: 10.1038/ncomms7965. doi: 10.1038/ncomms7965. [DOI] [PubMed] [Google Scholar]
- 3.Najafi A, Sequeira V, Kuster DW, van der Velden J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest. 2016;46:362–374. doi: 10.1111/eci.12598. doi: 10.1111/eci.12598. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo G, Viola E, Nocco M, Durante M, Tarantini S, Marigliano V. Autonomic modulation of heart rate and blood pressure in normotensive offspring of hypertensive subjects. J Lab Clin Med. 2000;135:145–152. doi: 10.1067/mlc.2000.103428. doi: 10.1067/mlc.2000.103428. [DOI] [PubMed] [Google Scholar]
- 5.Lopes HF, Silva HB, Consolim-Colombo FM, Barreto Filho JA, Riccio GM, Giorgi DM, Krieger EM. Autonomic abnormalities demonstrable in young normotensive subjects who are children of hypertensive parents. Braz J Med Biol Res. 2000;33:51–54. doi: 10.1590/s0100-879x2000000100007. [DOI] [PubMed] [Google Scholar]
- 6.Thorén P, Ricksten SE. Recordings of renal and splanchnic sympathetic nervous activity in normotensive and spontaneously hypertensive rats. Clin Sci (Lond) 1979;57(suppl 5):197s–199s. doi: 10.1042/cs057197s. [DOI] [PubMed] [Google Scholar]
- 7.Shanks J, Herring N. Peripheral cardiac sympathetic hyperactivity in cardiovascular disease: role of neuropeptides. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1411–R1420. doi: 10.1152/ajpregu.00118.2013. doi: 10.1152/ajpregu.00118.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Paterson DJ. Cyclic nucleotide regulation of cardiac sympatho-vagal responsiveness. J Physiol. 2016;594:3993–4008. doi: 10.1113/JP271827. doi: 10.1113/JP271827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AA, Taylor T, Wortis SB. Abnormal catechol amine metabolism in familial dysautonomia. N Engl J Med. 1963;268:705–707. doi: 10.1056/NEJM196303282681304. doi: 10.1056/NEJM196303282681304. [DOI] [PubMed] [Google Scholar]
- 10.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension (review). Cochrane Database Syst Rev. 2017:1–93. doi: 10.1002/14651858.CD002003.pub5. doi: 10.1002/14651858.cd002003.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frishman WH. Beta-adrenergic receptor blockers in hypertension: alive and well. Prog Cardiovasc Dis. 2016;59:247–252. doi: 10.1016/j.pcad.2016.10.005. doi: 10.1016/j.pcad.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM. Cochrane corner: beta-blockers for hypertension. Heart. 2018;104:282–283. doi: 10.1136/heartjnl-2017-311585. doi: 10.1136/heartjnl-2017-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler-Graschinsky E, Langer SZ. Possible role of a beta-adrenoceptor in the regulation of noradrenaline release by nerve stimulation through a positive feed-back mechanism. Br J Pharmacol. 1975;53:43–50. doi: 10.1111/j.1476-5381.1975.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard OA, Abrahamsen J. Modulation of noradrenaline release by activation of presynaptic beta-adrenoceptors in the cardiovascular system. Ann NY Acad Sci. 1990;604:528–544. doi: 10.1111/j.1749-6632.1990.tb32018.x. [DOI] [PubMed] [Google Scholar]
- 15.Apparsundaram S, Eikenburg DC. Role of prejunctional beta adrenoceptors in rat cardiac sympathetic neurotransmission. J Pharmacol Exp Ther. 1995;272:519–526. [PubMed] [Google Scholar]
- 16.Costa M, Majewski H. Facilitation of noradrenaline release from sympathetic nerves through activation of ACTH receptors, beta-adrenoceptors and angiotensin II receptors. Br J Pharmacol. 1988;95:993–1001. doi: 10.1111/j.1476-5381.1988.tb11730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majewski H. Review modulation of noradrenaline release through activation of presynaptic beta-adrenoreceptors. J Auton Pharmacol. 1983;3:155–155. doi: 10.1111/j.1474-8673.1983.tb00496.x. doi: 10.1111/j.1474–8673.1983.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 18.Lokhandwala MF, Eikenburg DC. Presynaptic receptors and alterations in norepinephrine release in spontaneously hypertensive rats. Life Sci. 1983;33:1527–1542. doi: 10.1016/0024-3205(83)90693-8. [DOI] [PubMed] [Google Scholar]
- 19.Misu Y, Kubo T. Presynaptic beta-adrenoceptors. Med Res Rev. 1986;6:197–225. doi: 10.1002/med.2610060204. [DOI] [PubMed] [Google Scholar]
- 20.Marrazzi AS. Electrical studies on the pharmacology of autonomic synapses ii. The action of a sympathomimetic drug (epinephrine) on sympathetic ganglia. J Pharmacol Exp Ther. 2006;65:395–404. [Google Scholar]
- 21.Goodall M, Kirshner N. Biosynthesis of epinephrine and norepinephrine by sympathetic nerves and ganglia. Circulation. 1958;17:366–371. doi: 10.1161/01.cir.17.3.366. [DOI] [PubMed] [Google Scholar]
- 22.Floras JS. Epinephrine and the genesis of hypertension. Hypertension. 1992;19:1–18. doi: 10.1161/01.hyp.19.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Brown MJ, Macquin I. Is adrenaline the cause of essential hypertension? Lancet. 1981;2:1079–1082. doi: 10.1016/s0140-6736(81)91279-4. [DOI] [PubMed] [Google Scholar]
- 24.Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, El-Osta A, Guo L, Barton D, Pier C, Brenchley C, Dawood T, Jennings G, Lambert E. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann NY Acad Sci. 2008;1148:338–348. doi: 10.1196/annals.1410.064. doi: 10.1196/annals.1410.064. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol. 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. doi:10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 26.Korzina MB, Korobkin AA, Vasilieva OA, Maslyukov PM. Morphological characteristics of the stellate ganglion in white rats. Neurosci Behav Physiol. 2011;41:436–439. doi:10.1007/s11055-011-9433-6. [Google Scholar]
- 27.Ellison JP, Williams TH. Sympathetic nerve pathways to the human heart, and their variations. Am J Anat. 1969;124:149–162. doi: 10.1002/aja.1001240203. doi: 10.1002/aja.1001240203. [DOI] [PubMed] [Google Scholar]
- 28.Vaseghi M, Zhou W, Shi J, Ajijola OA, Hadaya J, Shivkumar K, Mahajan A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm. 2012;9:1303–1309. doi: 10.1016/j.hrthm.2012.03.052. doi:10.1016/j.hrthm.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Judy WV, Watanabe AM, Murphy WR, Aprison BS, Yu PL. Sympathetic nerve activity and blood pressure in normotensive backcross rats genetically related to the spontaneously hypertensive rat. Hypertension. 1979;1:598–604. doi: 10.1161/01.hyp.1.6.598. [DOI] [PubMed] [Google Scholar]
- 30.Dickhout JG, Lee RM. Blood pressure and heart rate development in young spontaneously hypertensive rats. Am J Physiol. 1998;274(3)(pt 2):H794–H800. doi: 10.1152/ajpheart.1998.274.3.H794. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Lee CW, Buckler K, Parekh A, Herring N, Paterson DJ. Abnormal intracellular calcium homeostasis in sympathetic neurons from young prehypertensive rats. Hypertension. 2012;59:642–649. doi: 10.1161/HYPERTENSIONAHA.111.186460. doi: 10.1161/HYPERTENSIONAHA.111.186460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanks J, Manou-Stathopoulou S, Lu CJ, Li D, Paterson DJ, Herring N. Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305:H980–H986. doi: 10.1152/ajpheart.00255.2013. doi: 10.1152/ajpheart.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijnen BF, Van Essen H, Schalkwijk CG, Janssen BJ, Struijker-Boudier HA. Renal inflammatory markers during the onset of hypertension in spontaneously hypertensive rats. Hypertens Res. 2014;37:100–109. doi: 10.1038/hr.2013.99. doi: 10.1038/hr.2013.99. [DOI] [PubMed] [Google Scholar]
- 34.Komolova M, Friberg P, Adams MA. Altered vascular resistance properties and acute pressure-natriuresis mechanism in neonatal and weaning spontaneously hypertensive rats. Hypertension. 2012;59:979–984. doi: 10.1161/HYPERTENSIONAHA.111.178194. doi: 10.1161/HYPERTENSIONAHA.111.178194. [DOI] [PubMed] [Google Scholar]
- 35.Ely DL, Friberg P, Nilsson H, Folkow B. Blood pressure and heart rate responses to mental stress in spontaneously hypertensive (SHB) and normotensive (WKY) rats on various sodium diets. Acta Physiol Scand. 1985;123:159–169. doi: 10.1111/j.1748-1716.1985.tb07573.x. doi: 10.1111/j.1748-1716.1985.tb07573.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AJ, Wang VY, Sands GB, Young AA, Nash MP, LeGrice IJ. Increased cardiac work provides a link between systemic hypertension and heart failure. Physiol Rep. 2017;5:e13104. doi: 10.14814/phy2.13104. doi: 10.14814/phy2.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pijacka W, McBryde FD, Marvar PJ, Lincevicius GS, Abdala AP, Woodward L, Li D, Paterson DJ, Paton JF. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J Physiol. 2016;594:6255–6266. doi: 10.1113/JP272708. doi: 10.1113/JP272708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira-Sales EB, Colombari E, Abdala AP, Campos RR, Paton JF. Sympathetic overactivity occurs before hypertension in the two-kidney, one-clip model. Exp Physiol. 2016;101:67–80. doi: 10.1113/EP085390. doi: 10.1113/EP085390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira-Sales EB, Toward MA, Campos RR, Paton JF. Revealing the role of the autonomic nervous system in the development and maintenance of Goldblatt hypertension in rats. Auton Neurosci. 2014;183:23–29. doi: 10.1016/j.autneu.2014.02.001. doi: 10.1016/j.autneu.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith TL, Hutchins PM. Central hemodynamics in the developmental stage of spontaneous hypertension in the unanesthetized rat. Hypertension. 1979;1:508–517. doi: 10.1161/01.hyp.1.5.508. [DOI] [PubMed] [Google Scholar]
- 42.Lee RM. Structural alterations of blood vessels in hypertensive rats. Can J Physiol Pharmacol. 1987;65:1528–1535. doi: 10.1139/y87-241. [DOI] [PubMed] [Google Scholar]
- 43.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szcześniak MW, Gaffney DJ, Elo LL, Zhang X, Mortazavi A. Erratum to: a survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:181. doi: 10.1186/s13059-016-0881-8. doi: 10.1186/s13059-016-1047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 45.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. doi:10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 46.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLoS One. 2015;10:e0122513. doi: 10.1371/journal.pone.0122513. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol Biosyst. 2011;7:52–58. doi: 10.1039/c0mb00079e. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- 48.Williams RS, Bishop T. Selectivity of dobutamine for adrenergic receptor subtypes: in vitro analysis by radioligand binding. J Clin Invest. 1981;67:1703–1711. doi: 10.1172/JCI110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cullum VA, Farmer JB, Jack D, Levy GP. Salbutamol: a new, selective beta-adrenoceptive receptor stimulant. Br J Pharmacol. 1969;35:141–151. doi: 10.1111/j.1476-5381.1969.tb07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ladage D, Schwinger RH, Brixius K. Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther. 2013;31:76–83. doi: 10.1111/j.1755-5922.2011.00306.x. doi: 10.1111/j.1755-5922.2011.00306.x. [DOI] [PubMed] [Google Scholar]
- 52.Ram CVS. Beta-blockers in hypertension. Am J Cardiol. 2010;106:1819–1825. doi: 10.1016/j.amjcard.2010.08.023. doi:10.1016/j.amjcard.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ, Paterson DJ. Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension. 2013;61:202–207. doi: 10.1161/HYPERTENSIONAHA.111.00105. doi: 10.1161/HYPERTENSIONAHA.111.00105. [DOI] [PubMed] [Google Scholar]
- 54.Bardsley EN, Larsen HE, Paterson DJ. Impaired cAMP-cGMP cross-talk during cardiac sympathetic dysautonomia. Channels (Austin) 2017;11:178–180. doi: 10.1080/19336950.2016.1259040. doi: 10.1080/19336950.2016.1259040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larsen HE, Bardsley EN, Lefkimmiatis K, Paterson DJ. Dysregulation of neuronal Ca2+ channel linked to heightened sympathetic phenotype in prohypertensive states. J Neurosci. 2016;36:8562–8573. doi: 10.1523/JNEUROSCI.1059-16.2016. doi: 10.1523/JNEUROSCI.1059-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazanietz MG, Enero MA. Role of cyclic AMP in the release of noradrenaline from isolated rat atria. Effect of pretreatment with clenbuterol. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:311–314. doi: 10.1007/BF00173544. [DOI] [PubMed] [Google Scholar]
- 57.Remie R, Knot HJ, Bos EA, Zaagsma J. Characterization of presynaptic beta-adrenoceptors facilitating endogenous noradrenaline release in the portal vein of permanently cannulated, freely moving rats. Eur J Pharmacol. 1988;157:37–43. doi: 10.1016/0014-2999(88)90468-2. [DOI] [PubMed] [Google Scholar]
- 58.Majewski H, Murphy TV. Beta-adrenoceptor blockade and sympathetic neurotransmission in the pithed rat. J Hypertens. 1989;7:991–996. doi: 10.1097/00004872-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Kazanietz MG, Enero MA. Modulation of noradrenaline release by presynaptic alpha-2 and beta adrenoceptors in rat atria. Effect of pretreatment with clenbuterol. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:274–278. doi: 10.1007/BF00168510. [DOI] [PubMed] [Google Scholar]
- 60.Tarizzo VI, Coppes RP, Dahlöf C, Zaagsma J. Pre- and postganglionic stimulation-induced noradrenaline overflow is markedly facilitated by a prejunctional beta 2-adrenoceptor-mediated control mechanism in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:570–577. doi: 10.1007/BF01258461. [DOI] [PubMed] [Google Scholar]
- 61.Berg T. β1-blockers lower norepinephrine release by inhibiting presynaptic, facilitating β1-adrenoceptors in normotensive and hypertensive rats. Front Neurol. 2014;5:51. doi: 10.3389/fneur.2014.00051. doi: 10.3389/fneur.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg T. Altered β1-3-adrenoceptor influence on α2-adrenoceptor-mediated control of catecholamine release and vascular tension in hypertensive rats. Front Physiol. 2015;6:120. doi: 10.3389/fphys.2015.00120. doi: 10.3389/fphys.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tu H, Liu J, Zhang D, Zheng H, Patel KP, Cornish KG, Wang WZ, Muelleman RL, Li YL. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am J Physiol Cell Physiol. 2014;306:C132–C142. doi: 10.1152/ajpcell.00223.2013. doi: 10.1152/ajpcell.00223.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blumenthal SJ, McConnaughey MM, Iams SG. Myocardial adrenergic receptors and adenylate cyclase in the developing spontaneously hypertensive rat. Clin Exp Hypertens A. 1982;4:883–901. doi: 10.3109/10641968209060760. [DOI] [PubMed] [Google Scholar]
- 65.Madamanchi A. Beta-adrenergic receptor signaling in cardiac function and heart failure. Mcgill J Med. 2007;10:99–104. [PMC free article] [PubMed] [Google Scholar]
- 66.Rumantir MS, Jennings GL, Lambert GW, Kaye DM, Seals DR, Esler MD. The ‘adrenaline hypothesis’ of hypertension revisited: evidence for adrenaline release from the heart of patients with essential hypertension. J Hypertens. 2000;18:717–723. doi: 10.1097/00004872-200018060-00009. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson DJ, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry. 1998;55:511–520. doi: 10.1001/archpsyc.55.6.511. [DOI] [PubMed] [Google Scholar]
- 68.Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- 69.Majewski H, Hedler L, Starke K. The noradrenaline release rate in the anaesthetized rabbit: facilitation by adrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:20–27. doi: 10.1007/BF00586343. doi:10.1007/BF00586343. [DOI] [PubMed] [Google Scholar]
- 70.Caramona MM, Soares-da-Silva P. The effects of chemical sympathectomy on dopamine, noradrenaline and adrenaline content in some peripheral tissues. Br J Pharmacol. 1985;86:351–356. doi: 10.1111/j.1476-5381.1985.tb08903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munch G, Nguyen NTB, Nekolla S, Ziegler S, Muzik O, Chakraborty P, Wieland DM, Schwaiger M. Evaluation of sympathetic nerve terminals with [11C]epinephrine and [11C]hydroxyephedrine and positron emission tomography. Circulation. 2000;101:516–523. doi: 10.1161/01.cir.101.5.516. doi:10.1161/01.CIR.101.5.516. [DOI] [PubMed] [Google Scholar]
- 72.Gianutsos G, Moore KE. Epinephrine contents of sympathetic ganglia and brain regions of spontaneously hypertensive rats of different ages. Proc Soc Exp Biol Med. 1978;158:45–49. doi: 10.3181/00379727-158-40136. [DOI] [PubMed] [Google Scholar]
- 73.Torda C. Hypothalamic adrenaline synthesis after stimulation of the medial forebrain bundle. Br J Pharmacol. 1977;61:5–8. doi: 10.1111/j.1476-5381.1977.tb09733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kennedy B, Elayan H, Ziegler MG. Lung epinephrine synthesis. Am J Physiol. 1990;258(4)(pt 1):L227–L231. doi: 10.1152/ajplung.1990.258.4.L227. doi: 10.1152/ajplung.1990.258.4.L227. [DOI] [PubMed] [Google Scholar]
- 75.Kubovcakova L, Micutkova L, Bartosova Z, Sabban EL, Krizanova O, Kvetnansky R. Identification of phenylethanolamine N-methyltransferase gene expression in stellate ganglia and its modulation by stress. J Neurochem. 2006;97:1419–1430. doi: 10.1111/j.1471-4159.2006.03832.x. doi: 10.1111/j.1471-4159.2006.03832.x. [DOI] [PubMed] [Google Scholar]
- 76.Zeman M, Petrák J, Stebelová K, Nagy G, Krizanova O, Herichová I, Kvetnanský R. Endocrine rhythms and expression of selected genes in the brain, stellate ganglia, and adrenals of hypertensive TGR rats. Ann N Y Acad Sci. 2008;1148:308–316. doi: 10.1196/annals.1410.069. doi: 10.1196/annals.1410.069. [DOI] [PubMed] [Google Scholar]
- 77.Gavrilovic L, Spasojevic N, Varagic V, Dronjak S. Gene expression of catecholamine synthesizing enzymes in stellate ganglia of stressed rats. Acta vet (Beogr) 2010;60:15–22. doi:10.2298/avb1001015g. [Google Scholar]
- 78.MacGregor DA, Prielipp RC, Butterworth JF, James RL, Royster RL. Relative efficacy and potency of β-adrenoceptor agonists for generating camp in human lymphocytes. Chest. 2016;109:194–200. doi: 10.1378/chest.109.1.194. doi:10.1378/chest.109.1.194. [DOI] [PubMed] [Google Scholar]
- 79.Majewski H, Rand MJ, Tung LH. Activation of prejunctional beta-adrenoceptors in rat atria by adrenaline applied exogenously or released as a co-transmitter. Br J Pharmacol. 1981;73:669–679. doi: 10.1111/j.1476-5381.1981.tb16802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahlöf C, Ljung B, Ablad B. Increased noradrenaline release in rat portal vein during sympathetic nerve stimulation due to activation of presynaptic beta-adrenoceptors by noradrenaline and adrenaline. Eur J Pharmacol. 1978;50:75–78. doi: 10.1016/0014-2999(78)90255-8. [DOI] [PubMed] [Google Scholar]
- 81.Westfall TC, Peach MJ, Tittermary V. Enhancement of the electrically induced release of norepinephrine from the rat portal vein: mediation by beta 2-adrenoceptors. Eur J Pharmacol. 1979;58:67–74. doi: 10.1016/0014-2999(79)90341-8. [DOI] [PubMed] [Google Scholar]
- 82.Shanks J, Mane S, Ryan R, Paterson DJ. Ganglion-specific impairment of the norepinephrine transporter in the hypertensive rat. Hypertension. 2013;61:187–193. doi: 10.1161/HYPERTENSIONAHA.112.202184. doi: 10.1161/HYPERTENSIONAHA.112.202184. [DOI] [PubMed] [Google Scholar]
- 83.Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens. 2001;14(6)(pt 2):139S–146S. doi: 10.1016/s0895-7061(01)02081-7. [DOI] [PubMed] [Google Scholar]
- 84.Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 85.Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–223. doi: 10.1080/14622200110050213. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- 86.Choate JK, Paterson DJ. Nitric oxide inhibits the positive chronotropic and inotropic responses to sympathetic nerve stimulation in the isolated guinea-pig atria. J Auton Nerv Syst. 1999;75:100–108. doi: 10.1016/s0165-1838(98)00173-8. [DOI] [PubMed] [Google Scholar]
- 87.Herring N, Lee CW, Sunderland N, Wright K, Paterson DJ. Pravastatin normalises peripheral cardiac sympathetic hyperactivity in the spontaneously hypertensive rat. J Mol Cell Cardiol. 2011;50:99–106. doi: 10.1016/j.yjmcc.2010.09.025. doi: 10.1016/j.yjmcc.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buttgereit J, Shanks J, Li D. C-type natriuretic peptide and natriuretic peptide receptor B signalling inhibits cardiac sympathetic neurotransmission and autonomic function. Cardiovasc Res. 2016;112:637–644. doi: 10.1093/cvr/cvw184. doi:10.1093/cvr/cvw184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stangherlin A, Zaccolo M. Phosphodiesterases and subcellular compartmentalized cAMP signaling in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2012;302:H379–H390. doi: 10.1152/ajpheart.00766.2011. doi: 10.1152/ajpheart.00766.2011. [DOI] [PubMed] [Google Scholar]
- 91.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- 92.Zoccarato A, Surdo NC, Aronsen JM, et al. Cardiac hypertrophy is inhibited by a local pool of cAMP regulated by phosphodiesterase 2. Circ Res. 2015;117:707–719. doi: 10.1161/CIRCRESAHA.114.305892. doi: 10.1161/CIRCRESAHA.114.305892. [DOI] [PubMed] [Google Scholar]
- 93.Zhao CY, Greenstein JL, Winslow RL. Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network. J Mol Cell Cardiol. 2016;91:215–227. doi: 10.1016/j.yjmcc.2016.01.004. doi:10.1016/j.yjmcc.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our RNA sequencing (RNAseq) raw FastQ files are deposited in the National Center for Biotechnology Information short reads archive under Short Reads Archive number SRP132271, and our quasi-mapped data will be available under Gene Expression Omnibus accession number (GSE110197).


