Abstract
Cardiovascular disease (CVD) is a major challenge in the management of type 2 diabetes mellitus. Glucose-lowering agents that reduce the risk of major cardiovascular events would be considered a major advance, as recently reported with liraglutide and semaglutide, 2 glucagon-like peptide-1 receptor agonists, and with empagliflozin and canagliflozin, 2 SGLT-2 (sodium-glucose cotransporter type 2) inhibitors, but not with DPP-4 (dipeptidyl peptidase-4) inhibitors. The present review is devoted to CV effects of new oral glucose-lowering agents. DPP-4 inhibitors (gliptins) showed some positive cardiac and vascular effects in preliminary studies, and initial data from phase 2 to 3 clinical trials suggested a reduction in major cardiovascular events. However, subsequent CV outcome trials with alogliptin, saxagliptin, and sitagliptin showed noninferiority but failed to demonstrate any superiority compared with placebo in patients with type 2 diabetes mellitus and high CV risk. An unexpected higher risk of hospitalization for heart failure was reported with saxagliptin. SGLT-2 inhibitors (gliflozins) promote glucosuria, thus reducing glucose toxicity and body weight, and enhance natriuresis, thus lowering blood pressure. Two CV outcome trials in type 2 diabetes mellitus patients mainly in secondary prevention showed remarkable positive results. Empagliflozin in EMPA-REG-OUTCOME (EMPAgliflozin Cardiovascular OUTCOME Events in Type 2 Diabetes Mellitus Patients) reduced major cardiovascular events, CV mortality, all-cause mortality, and hospitalization for heart failure. In CANVAS (Canagliflozin Cardiovascular Assessment Study), the reduction in CV mortality with canagliflozin failed to reach statistical significance despite a similar reduction in major cardiovascular events. The underlying protective mechanisms of SGLT-2 inhibitors remain unknown and both hemodynamic and metabolic explanations have been proposed. CVD-REAL studies (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors; with the limitation of an observational approach) suggested that these favorable results may be considered as a class effect shared by all SGLT-2 inhibitors (including dapagliflozin) and be extrapolated to a larger population of patients with type 2 diabetes mellitus in primary prevention. Ongoing CV outcome trials with other DPP-4 (linagliptin) and SGLT-2 (dapagliflozin, ertugliflozin) inhibitors should provide additional information about CV effects of both pharmacological classes.
Keywords: empagliflozin, heart failure, mortality, myocardial infarction, stroke
Cardiovascular disease (CVD) represents both an individual and a societal burden in patients with type 2 diabetes mellitus (T2DM). The life expectancy of a 50-year-old with diabetes mellitus is, on average, 6 years shorter than that of a counterpart without diabetes mellitus, with ≈60% of the difference in survival attributable to excess vascular deaths.1 Thanks to a better control of modifiable risk factors,2 a progressive decline in major cardiovascular events (MACE) has been reported during the last 2 decades, both in the United States3 and in Europe.4 Nevertheless, fatal CV outcomes declined less among patients with T2DM than among controls4 and the excess risk in patients with T2DM remains high compared with nondiabetic.3 CV effects of more intensive glucose control5,6 and of the different glucose-lowering agents7 remain a matter of controversy. A recent analysis of CV outcome trials showed that both the reduction in glycated hemoglobin (HbA1c) and the duration of the intensification of glycemic control are important factors that may influence CV outcome results.8
Since 2008 and the guidance document by the US Food and Drug Administration (FDA), all new glucose-lowering agents must prove CV safety.9 Therefore, numerous randomized controlled trials (RCTs) were primarily designed as noninferiority trials compared with placebo to exclude an unacceptable risk of CV events with these drugs in the shortest possible time period.10 Of note, all these placebo-controlled RCTs were performed in the setting of adjustment of alternative class glucose-lowering therapies to achieve local and individual glycemic targets. Almost all used as primary outcome a composite triple MACE combining CV mortality, nonfatal myocardial infarction, and nonfatal stroke.11,12 Secondary outcomes consider each individual component of the primary outcome, all-cause death and sometimes an expanded MACE (triple MACE plus hospitalization for unstable angina). Of note, the potential long-term benefits or risks were not assessed effectively as the median follow-up in these event-driven studies was limited to 1.5 to 3 years. These trials included patients with relatively long duration of T2DM, advanced atherosclerosis and higher CV risk, generally patients with established CVD (secondary prevention). These trials were not intended to assess CV benefit in the general population with T2DM (most patients being in primary prevention) and are best interpreted as evidence for CV safety of these new antihyperglycemic medications in patients with T2DM and very high risk.13
The aim of the present review is to discuss the most important recent findings concerning 2 classes of new oral glucose-lowering agents, DPP-4 (dipeptidyl peptidase-4) inhibitors14,15 and SGLT-2 (sodium-glucose cotransporter type 2) inhibitors,16,17 which are increasingly used for the management of T2DM.18,19 This review will not analyze the positive CV results with injectable therapies, that is, GLP-1 (glucagon-like peptide-1) receptor agonists, reported in LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) with liraglutide and in SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-Term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) with semaglutide.12,13 This article will focus only on human studies; a summary of animal data may be found in several reviews dealing with DPP-4 inhibitors14,20–23 and SGLT-2 inhibitors.24–26 We will summarize effects on surrogate vascular end points and CV risk factors before paying more attention on CV clinical outcomes (MACE), including hospitalization for heart failure (HF), and mortality. We will consider both preliminary data from phase 2 to 3 trials (although not designed to specifically assess CV outcomes) and, more importantly, dedicated prospective CV outcome trials. Indeed, the 2 types of data are complementary because the populations recruited were quite different, most T2DM patients without CVD in phase 2 to 3 trials contrasting with T2DM patients with established CVD in prospective CV outcome trials. The positive renal outcomes, which have also been reported with SGLT-2 inhibitors27–29 and to a lesser extent with DPP-4i,30,31 will not be discussed extensively here. However, because chronic kidney disease (CKD) is considered as an additional important CV risk and because SGLT-2 inhibitors exert more positive effects on renal outcomes than DPP-4 inhibitors, some renal findings are briefly presented whenever appropriate.
Cardiovascular Effects of DPP-4 Inhibitors
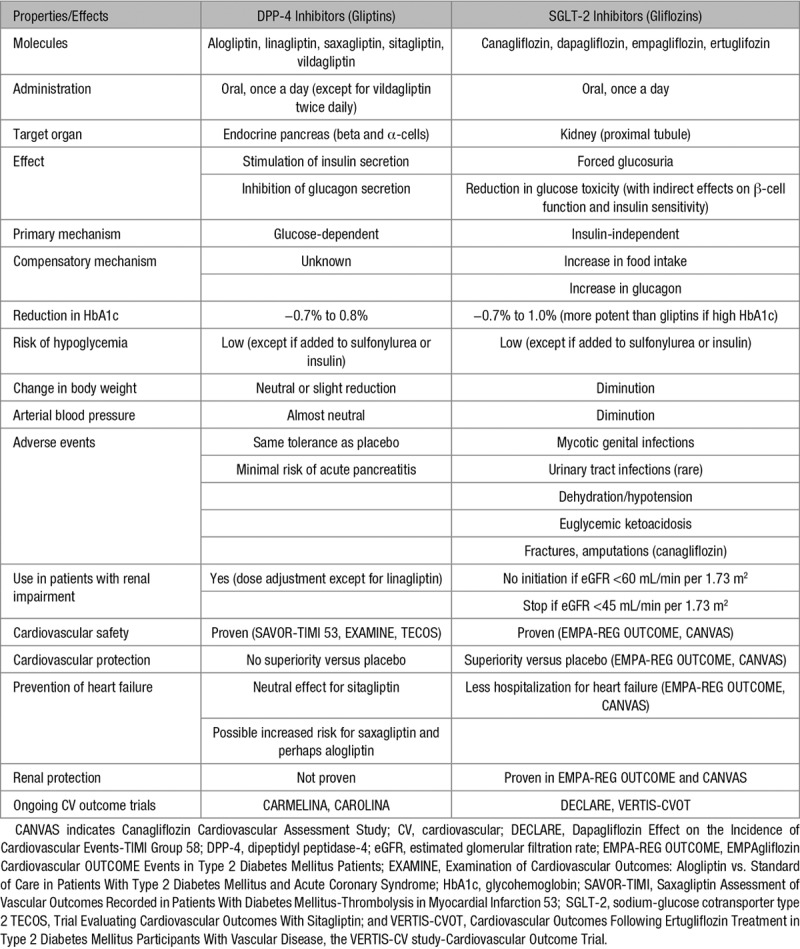
DPP-4 inhibitors inhibit the enzyme that degrades 2 gut-derived incretin hormones, GLP-1 and GIP (glucose-dependent insulinotropic polypeptide).32 Thereby, they stimulate insulin secretion and reduce glucagon secretion in a glucose-dependent manner, both effects contributing to the glucose-lowering activity (Figure 1). DPP-4 inhibitors occupy an increasing place in the management of T2DM, progressively replacing sulfonylureas in numerous countries.18,19 The reasons for this trend are that DPP-4 inhibitors are not associated with hypoglycemia or weight gain, have a good safety profile and are very easy to use (generally 1 tablet a day, without titration).15,33 They can be prescribed in patients with moderate to severe CKD, provided that the daily dose is adjusted to the estimated glomerular filtration rate (eGFR); of note, linagliptin does not require dose adjustment because of a biliary rather than a renal excretion.34
Figure 1.
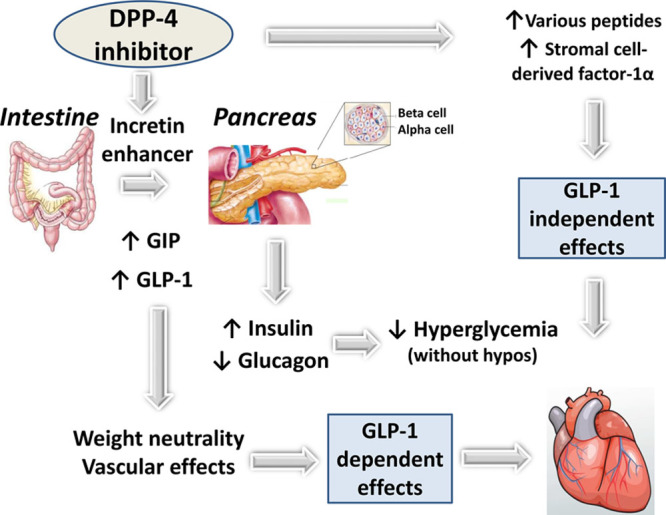
Illustration of the primary mechanisms of action of DPP-4 (dipeptidyl peptidase-4) inhibitors and their GLP (glucagon-like peptide)-1–dependent and GLP-1–independent effects. Positive effects may be counterbalanced by unknown negative effects so that the final resulting is the absence of improvement of myocardial function. GIP indicates glucose-dependent insulinotropic polypeptide.
Effects of DPP-4 Inhibitors on Surrogate Vascular End Points and CV Risk Factors
Beyond the glucose-lowering effect, DPP-4 inhibitors may positively influence surrogate vascular end points and other CV risk factors, as extensively discussed in previous reviews (Figure 1).14,21–23 DPP-4 inhibitors may improve several CV risk factors. Besides their positive effect on glucose control (mainly by reducing postprandial hyperglycemia), DPP-4 inhibitors showed neutral to modest beneficial effects on body weight, blood pressure (without an increase in heart rate), postprandial lipemia, inflammatory markers, oxidative stress, and endothelial function in patients with T2DM.21,22 Even if each of these effects may appear modest, one may hypothesize that taken all together they could result in positive CV outcomes.
GLP-1 is classically viewed as the primary DPP-4 substrate capable in modulating CV function.20 However, DPP-4 is widely expressed in most cells and tissues. It exhibits enzymatic activity against dozens of peptide hormones and chemokines with roles in vascular pathophysiology, inflammation, stem cell homing, and cell survival.14,20,22 Several studies focused on the role of DPP-4 in the inactivation of SDF-1α (stromal cell-derived factor-1α), a powerful chemoattractant of stem/progenitor cells. By inhibiting the degradation of SDF-1α, DPP-4 inhibitors may enhance homing of endothelial progenitor cells and thereby exert vascular protection.35 Thus, DPP-4 inhibitors may exert a possible beneficial action on vessels and heart, via both GLP-1–dependent and GLP-1–independent effects.20,22
Cardioprotective actions of DPP-4 inhibitors in preclinical models of ischemic injury and HF are contrasted with modest and often inconclusive results with these compounds in short-term human studies.20–22 Although some positive effects have been described on the function of the heart in patients with T2DM with or without ischemic heart disease or HF,36,37 yet their clinical relevance remains to be further investigated.
Finally, all these effects reported with DPP-4 inhibitors, even combined, seem to be insufficient to provide a positive impact on renal function.30 Indeed, as recently reviewed,30,31 prevention of new microalbuminuria or of progression of albuminuria has been reported in some clinical studies, but no significant effects on eGFR were noticed in most studies. The long-term effects of DPP-4 inhibitors on clinical renal outcomes and development of end-stage renal disease remain largely unknown and thus deserve further investigations in prospective trials or long-term observational studies.31
CV Outcomes in Meta-Analyses of Phase 2 to 3 Trials With DPP-4 Inhibitors
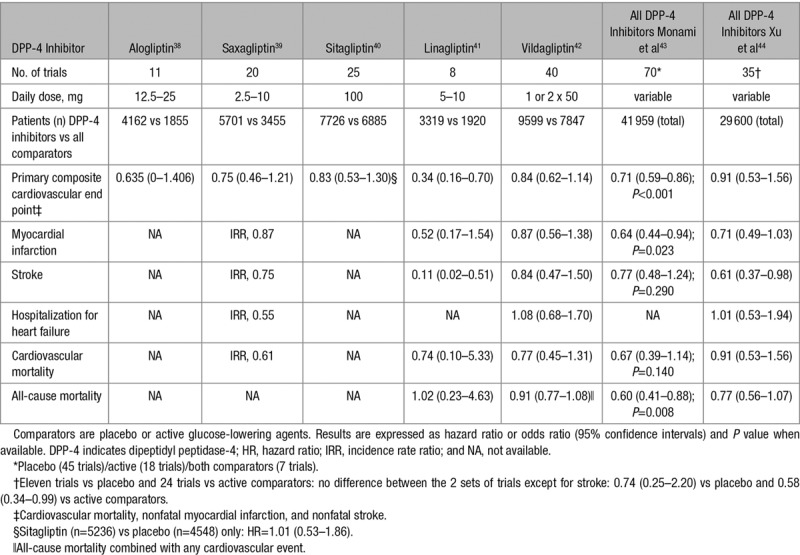
Several meta-analyses of RCTs with each of the DPP-4 inhibitor commercialized in the United States (vildagliptin is not available in the United States) and in Europe generally reported a nonsignificant trend toward a lower incidence of MACE compared with placebo or other active glucose-lowering compounds: alogliptin,38 saxagliptin,39 sitagliptin,40 linagliptin,41 and vildagliptin42 (Table 1). It is noteworthy, however, that none of these trials were designed to test CV safety/efficacy of the DPP-4 inhibitor; moreover, patients were at a rather low risk of CVD (primary prevention), the trial duration was quite short (generally ≤1 year) and CV events were not always properly adjudicated. Because of the rather low number of MACE in each individual DPP-4 inhibitor specific meta-analysis, the differences failed to reach statistical significance, thus paving the road to pooled analysis. Overall, data from meta-analyses did not show evidence of harm and showed neutral to beneficial effects for a variety of CV outcomes depending on the analysis (Table 1).43,44
Table 1.
Cardiovascular Events and Mortality Rates With DPP-4 Inhibitors in Meta-Analyses of Phase 2 to 3 Randomized Controlled Trials (Excluding the 3 Cardiovascular Outcome Trials)
Neutral CV effects were reported when pooling the results of all phase 2 to 3 trials and of the 3 CV outcome trials (EXAMINE [Examination of Cardiovascular Outcomes: Alogliptin vs. Standard of Care in Patients With Type 2 Diabetes Mellitus and Acute Coronary Syndrome], SAVOR-TIMI 53 [Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53], TECOS [Trial Evaluating Cardiovascular Outcomes with Sitagliptin]; Table 2).45,46 No significant differences were observed in meta-analyses that compared DPP-4 inhibitors with combined placebo or active glucose-lowering medications and in meta-analyses that compared DPP-4 inhibitors with placebo only (Table 2).45 Three meta-analyses of the 3 prospective CV outcome trials (EXAMINE, SAVOR-TIMI 53, TECOS: see below) failed to demonstrate any positive effect of DPP-4 inhibitors compared with placebo on CV outcomes and mortality (Table 3).44,47,48
Table 2.
Cardiovascular Events and Mortality Rates With DPP-4 Inhibitors in Meta-Analyses of Randomized Controlled Trials, Including the 3 Prospective Cardiovascular Outcome Trials (EXAMINE, SAVOR-TIMI 53, and TECOS)
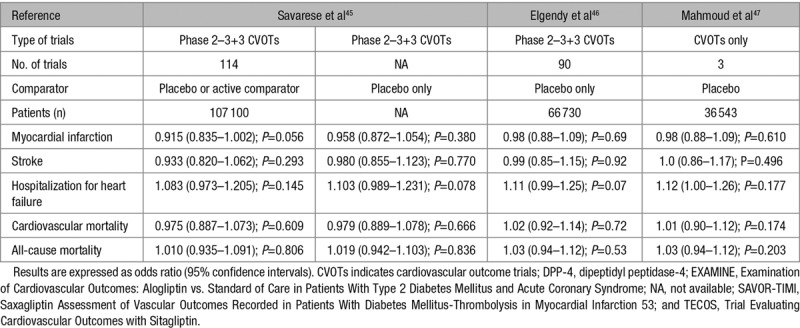
Table 3.
Cardiovascular Outcome Trials Comparing a DPP-4 Inhibitor With a Placebo
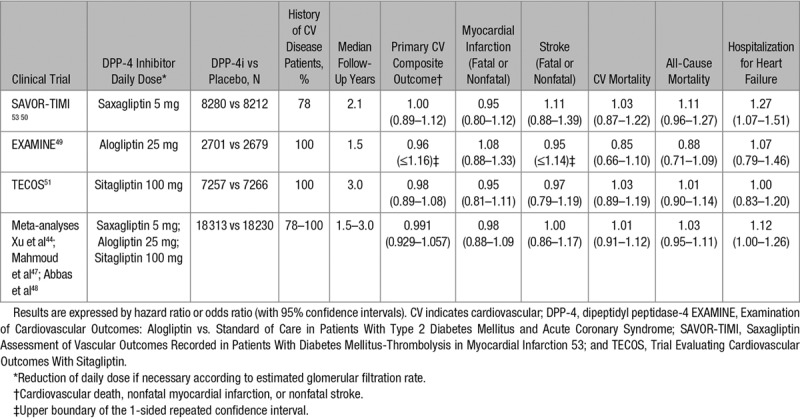
Results of Dedicated CV Outcome Trials With DPP-4 Inhibitors
All CV outcome trials with DPP-4 inhibitors compared a gliptin with placebo in T2DM patients with established CVD. Of note, T2DM patients included in these trials already at baseline received standard of care not only with diabetes mellitus management but also with cardiovascular protection as shown by the high proportion of patients treated with statins, antiplatelet agents, inhibitors of the renin–angiotensin system, and β-blockers. In agreement with the guidance by the FDA, EXAMINE49 and SAVOR-TIMI 5350 used a primary composite CV end point defined as the first confirmed event of the triple MACE. TECOS, which was planned before the 2008 guidance of the FDA, used an expanded 4 MACE as the primary end point and the triple MACE as the secondary end point. Even if all 3 trials were designed to primarily prove the safety of DPP-4 inhibitors, that is, noninferiority versus placebo, SAVOR-TIMI 53 and TECOS were powered to test superiority if the criterion of noninferiority was met.51
Alogliptin in EXAMINE
In EXAMINE, 5380 patients with T2DM and either an acute myocardial infarction or unstable angina requiring hospitalization within the previous 15 to 90 days were randomly assigned to receive alogliptin or matching placebo in addition to existing antihyperglycemic and CV drug therapy.49 After a median follow-up of 18 months and a mean difference of 0.36% in HbA1c, the primary end point (triple MACE) occurred in the similar proportion of T2DM patients assigned to alogliptin or placebo (P<0.001 for noninferiority). No significant differences were observed about the incidence rate of myocardial infarction, stroke, CV mortality, and all-cause mortality (Table 3)49 or of a 5-component composite end point, including coronary revascularization and CV hospitalization.52
A nonsignificant trend for a higher rate of hospital admission for HF was observed in the alogliptin group compared with the placebo group (Table 3).49 However, alogliptin had no effect on a composite end point combining CV death and hospital admission for HF in a post hoc analysis (hazard ratio [HR], 1.00; 95% confidence interval [CI], 0.82–1.21).53 Similar results were reported in T2DM patients treated with angiotensin-converting enzyme inhibitors (HR, 0.93; 95% CI, 0.72–1.20).54 These data are reassuring after the demonstration of an increased sympathetic activity during combined angiotensin-converting enzyme inhibition and DPP-4 inhibition.55 Furthermore, results did not differ by baseline brain natriuretic peptide concentration.53
Saxagliptin in SAVOR-TIMI 53
This trial randomly assigned 16 492 T2DM patients who had a history of, or were at risk for, CV events to blindly receive saxagliptin or matching placebo.50 After a median follow-up of 2.1 years, the primary end point (triple MACE) occurred in a similar proportion of patients receiving saxagliptin or placebo (P<0.001 for noninferiority; P=0.99 for superiority). No significant between-group differences were observed about myocardial infarction, ischemic stroke, CV mortality, and all-cause mortality (Table 3). Especially, the overall CV safety of saxagliptin was shown in a robust number of elderly and very elderly participants.56 There was no heterogeneity in the effect of saxagliptin on MACE or CV death by baseline HbA1c categories.57
Intriguingly, more patients in the saxagliptin group than in the placebo group were hospitalized for HF (3.5% versus 2.8%; P=0.007; Table 3).50 This increase in risk of HF was highest among T2DM patients with elevated levels of natriuretic peptides, prior HF, or CKD at entry of the study.58 The risk of HF hospitalization was increased irrespective of age category56 while high baseline HbA1c was not shown to be a risk factor.57 Of note, this increased risk of HF hospitalization was not associated with an increased risk of CV death or all-cause mortality in the group treated with saxagliptin, a finding that may be considered as reassuring. Nevertheless, caution is recommended and T2DM patients taking saxagliptin should be informed to contact their health professionals in case of symptoms (shortness of breath) and signs (swelling in the ankles) of HF.
Sitagliptin in TECOS
Sitagliptin has been extensively investigated in T2DM patients and is currently the most prescribed DPP-4 inhibitor worldwide.59 In the randomized, double-blind TECOS trial, 14 671 patients with T2DM and CVD were assigned to add either sitagliptin or matching placebo to their existing therapy.51 After a median follow-up of 3.0 years during which only a small difference in HbA1c (−0.29%; search of equipoise) in the sitagliptin group compared with the placebo group was observed, the primary outcome (expanded MACE) occurred in a similar proportion of patients treated with sitagliptin or placebo (P<0.001 for noninferiority; P=0.65 for superiority). No differences were observed between the 2 arms about each individual component of the primary end point or all-cause mortality (Table 3).51 The most common CV death was sudden death (27% of CV deaths) followed by acute myocardial infarction and stroke (21%) and HF (12%).60 Subgroup analyses showed no significant heterogeneity about prespecified primary outcomes (triple MACE).51 Also among older patients with well-controlled T2DM and CVD, sitagliptin had neutral effects on CV risk.61
In contrast with the 2 previous CV outcome trials with DPP-4 inhibitors, rates of hospitalization for HF did not differ between the 2 sitagliptin versus placebo groups (Table 3).51 The risk of specific CV death subcategories was lower among patients with no baseline history of HF.60 In a secondary analysis of TECOS, CV death and all-cause death occurring after hospitalization for HF were similar in the sitagliptin and placebo groups.62 Furthermore, no heterogeneity for the effect of sitagliptin on hospitalization for HF was observed in subgroup analyses across 21 factors. Although a signal for hospitalization for HF was seen within one trial (SAVOR-TIMI 53) but not within another (TECOS), heterogeneity of effect with the different agents across trials could not be established (I2=44.9, P=0.16).62
Linagliptin in CARMELINA and CAROLINA
CARMELINA (Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus; URL: http://www.clinicaltrials.gov. Unique identifier: NCT01897532) is comparing linagliptin 5 mg once daily with a placebo in patients with T2DM and high risk of CV events defined by albuminuria (micro or macro) and previous macrovascular disease and impaired renal function with predefined urinary albumin:creatinine ratio.63 Compared with the 3 previous placebo-controlled CV outcome trials with other DPP-4 inhibitors, CARMELINA has targeted a T2DM population with more advanced CKD, a condition known to be associated with a higher risk of CVD. The primary outcome is time to the first occurrence of any of the components of the classical 3-point MACE (all confirmed by adjudication).
CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes; URL: http://www.clinicaltrials.gov. Unique identifier: NCT01243424) is comparing linagliptin 5 mg once daily with an active comparator (glimepiride rather than placebo) in patients with T2DM and high CV risk defined as preexisting CVD or specified diabetes mellitus end-organ damage or age ≥70 years or 2 or more specified CV risk factors.64,65 CAROLINA is unique because it compares a DPP-4i with a sulfonylurea, a pharmacological class that raised controversy about its CV safety over the last 4 decades but is still widely used worldwide.66
Vildagliptin
No dedicated CV outcome trial has been performed with vildagliptin and this DPP-4 inhibitor is not marketed in the United States. A retrospective meta-analysis of prospectively adjudicated CV events pooled patient-level data from 40 phase 3 to 4 RCTs with vildagliptin (50 mg once or twice daily; n=9599) versus placebo or active comparator (n=7847).42 After a mean duration of exposure of about 50 weeks, a MACE occurred in 83 (0.86%) vildagliptin-treated patients and 85 (1.20%) comparator-treated patients, with a Mantel–Haenszel risk ratio of 0.82; 95% CI, 0.61 to 1.11. Nonsignificant between-group differences were observed for each of the individual events of the triple MACE as well as for confirmed HF events (Mantel–Haenszel risk ratio, 1.08; 95% CI, 0.68–1.70).42 These results are consistent with data reported in meta-analyses of phase 2 to 3 trials with other DPP-4 inhibitors as shown in Table 1.
In the recently published VIVIDD trial (Vildagliptin in Ventricular Dysfunction Diabetes), patients with T2DM and HF (New York Heart Association functional class I to III and left ventricular ejection fraction <0.40) were randomized to 52-week treatment with vildagliptin (50 mg twice daily or 50 mg once daily if treated with a sulfonylurea) or matching placebo. Compared with placebo, vildagliptin had no major effect on left ventricular ejection fraction but did lead to an increase in left ventricular volumes, the cause and clinical significance of which are unknown.67 A noninterventional analytic cohort study, using real-world data from 5 European electronic healthcare databases, suggested CV safety of vildagliptin versus other noninsulin antidiabetic drugs, including the risk of HF (range of adjusted incidence rate ratios for composite CV outcomes: 0.22–1.02).68
Interpretation of the CV Outcomes With DPP-4 Inhibitors
How to Explain Noninferiority?
DPP-4 inhibitors have several advantages over some other glucose-lowering agents, such as sulfonylureas.69 Especially they do not induce weight gain or hypoglycemia, 2 factors that may be associated with an increased CV risk.33 Furthermore, they are not associated with fluid retention or lipid disturbances, responsible for HF and possibly increased ischemic heart disease as reported with rosiglitazone. Finally, the trial designs to adjust nonclass glucose-lowering medications minimized differences in glucose control (search for glucose equipoise) between treatment groups and thus would contribute to attenuate potential benefit of DPP-4 inhibitors versus placebo.
How to Explain the Absence of Superiority?
The positive cardiac and vascular effects described in different animal models with DPP-4 inhibitors20–22 were not confirmed in clinical studies, especially in CV outcome trials,70 which showed the only noninferiority compared with placebo. The reasons for disappointing results in humans are unknown, but several tempting hypotheses may be proposed at least.
First of all, these CV outcome trials were primarily designed to prove safety by showing noninferiority versus placebo and not to demonstrate superiority. Second, equipoise in glucose control between the DPP-4 inhibitor arm and the placebo arm was the objective; therefore, open-label use of antihyperglycemic therapy was encouraged in both groups to reach individually appropriate glycemic targets in all patients. Although equipoise was not fully met, the overall HbA1c difference between the 2 groups was minimal. It averaged almost 0.3% to 0.4% throughout the 3 studies, thus far below the HbA1c difference reported in previous double-blind RCTs that compared DPP-4 inhibitors versus placebo in the absence of any other glucose-lowering therapy adjustment15 or in studies that compared intensive glucose-lowering regimen versus standard regimen.71 Third, the duration of these trials was rather short (2–3 years), so that the difference in hyperglycemia exposure between the 2 arms was probably too low to show any difference in CV outcomes, especially in T2DM patients with already advanced CVD.8 Fourth, the rather moderate increase in GLP-1 levels observed with DPP-4 inhibitors might explain why the patients did not benefit from the beneficial CV effects of GLP-1.20,22,72 The CV actions of DPP-4 inhibitors and GLP-1 receptor agonists were compared, with a focus on the translation of mechanisms derived from preclinical studies to complementary findings in clinical studies.23 Although continued research is needed to better define the mechanisms leading to the benefits seen with GLP-1 receptor agonists but not with DPP-4 inhibitors, it is speculated that the reductions in CV events seen with liraglutide and semaglutide are a direct result of GLP-1 receptor signaling, given the numerous actions of GLP-1 within the heart and blood vessels, and possibly other tissues that impact risk factors.23
It may be also hypothesized that some unknown deleterious effects of DPP-4 inhibitors, resulting from out-of-target effects, could neutralize any positive effects from the reduction in HbA1c (Figure 1). However, even if DPP-4 inhibitors can interfere with many other substrates than DPP-4 itself,14,20 obvious negative effects have not been shown to date, except perhaps the question of HF with some DPP-4 inhibitors (see below unresolved issues). Another possible explanation is that positive effects in patient subgroups may be compensated for by negative effects in other subgroups. This has been suggested by a recent meta-analysis of the 3 CV outcome trials with DPP-4 inhibitors that compared the effects in patients treated or not treated by metformin at baseline. The results showed a trend for reduction in the incidence of primary CV outcomes in metformin-treated patients contrasting with a trend for an increase in MACE in patients not receiving metformin.73 As the between-group was statistically significant, it was considered that metformin may act as a CV moderator of DPP-4 inhibitors, but obviously these hypothesis-generating findings require further confirmation.74 Indeed, possible bias could not be excluded, especially because a major reason for not prescribing metformin may have been CKD, a condition known to be associated with higher CV risk as already mentioned.
CV Outcomes Versus Overall Safety Profile
The overall safety profile of DPP-4 inhibitors is excellent, even in special populations such as elderly subjects or patients with renal impairment33 or T2DM patients with established CVD or high CV risk.48 Thus, even if DPP-4 inhibitors do not succeed to demonstrate CV protection, they are not associated with harm whereas controversy still persists with sulfonylureas.66 Of note, however, none of the CV outcome trials performed with DPP-4 inhibitors were specific to the elderly population with T2DM. A concern about a possible higher risk of acute pancreatitis associated with the use of DPP-4is was raised soon after the commercialization of this pharmacological class. A pooled analysis of data from the 3 prospective CV outcome trials with alogliptin,49 saxagliptin,50 and sitagliptin51 showed an increased risk of acute pancreatitis with DPP-4 inhibitors (odds ratio versus placebo 1.79 [95% CI, 1.13–2.82]; P=0.013), but the difference in the absolute risk was small (0.13%).75 This has been confirmed in a broader meta-analysis of all clinical trials with DPP-4 inhibitors which showed that the overall risk of acute pancreatitis remains minimal (5.5 extra cases/10 000 patients per year and a number needed to harm of 1940 per year).48 No increased risk of pancreatic cancer was noticed in any CV outcome trial.49–51
Unresolved Issues
The relative effect of DPP-4 inhibitors on the risk of HF in patients with T2DM remains uncertain.76 In a nationwide T2DM cohort, DPP-4 inhibitor use was not associated with a higher risk of hospitalization for HF even in patients with preexisting HF.77 However, a meta-analysis of both RCTs and observational studies suggested that DPP-4 inhibitors may increase the risk of hospital admission for HF in those patients with existing CVDs or multiple risk factors for vascular diseases, compared with no use.76 Nevertheless, another meta-analysis pointed out a differential effect of each DPP-4 inhibitor on the risk of HF: the use of saxagliptin significantly increased the risk of HF by 21%, especially among patients with high CV risk, while no signals were detected with other DPP-4 inhibitors.78 Finally, according to another analysis, despite pooled data from 79 867 patients, including the data from the 3 CV outcome trials, whether DPP-4 inhibitors increase HF overall or exhibit within-class differences remains unresolved.79
The reason for the increase in hospitalization for HF in patients treated with saxagliptin is unclear and a chance finding could not be excluded.80 From a methodological point of view, the statistical analysis has been criticized.81,82 By using an alternative measure to the HR, no substantial clinically relevant difference in the risk of hospitalization for HF was shown between saxagliptin and placebo, as it was for alogliptin and sitagliptin.83 The saxagliptin nonclinical and clinical pharmacology programs did not identify evidence of myocardial injury and CV harm that may have predicted or may explain this imbalance in the rate of hospitalization for HF seen in SAVOR-TIMI 53.84 Nevertheless, recent in vitro experimental data provided possible new mechanisms for off-target deleterious effects of saxagliptin on cardiac function. Saxagliptin internalized into cardiomyocytes and induced several biochemical changes that resulted in reduced sarcoplasmic reticulum Ca2+ content, diastolic Ca2+ overload, systolic dysfunction, and impaired contractile force. These findings may support a possible link between saxagliptin and an increased risk of HF.85 Whether such changes may also be observed with other DPP-4 inhibitors is unknown. Currently, the safety of saxagliptin about the risk of HF remains a matter of controversy, which justifies a warning in the FDA and European Medicines Agency (EMA) labels of the compound.
All published CV outcome trials compared a DPP-4 inhibitor with a placebo and demonstrated noninferiority. Therefore, it remains unknown whether DPP-4 inhibitors might offer CV superiority (for instance compared with sulfonylureas)66 or possibly show CV inferiority compared with other glucose-lowering agents, for instance GLP-1 receptor agonists (especially liraglutide)72 or SGLT-2 inhibitors (empagliflozin, canagliflozin), agents that are recommended in T2DM with established CVD by the most recent guidelines of the American Diabetes Association.19 A recent study based on a large nationwide diabetic cohort of 113 051 patients with T2DM showed that DPP inhibitors as a second- or third-line add-on treatment provided CV benefits compared with other glucose-lowering agents, including sulfonylureas.86 A systematic research of published data showed that the combination therapy of metformin plus DPP-4 inhibitor significantly decreased the relative risk of nonfatal CV events, CV mortality, and all-cause mortality, compared with the combination therapy of metformin plus sulfonylurea.87 In this respect, the results of the CV outcome trial CAROLINA that is comparing linagliptin with the sulfonylurea glimepiride are awaited with interest.64,65
Finally, previous CV outcome studies have recruited a large majority of T2DM patients with preserved renal function so that the CV effects of DPP-4 inhibitors in patients with more advanced CKD remain largely unknown. This condition is currently investigated in the CARMELINA trial that compares linagliptin versus placebo in T2DM patients with impaired renal function with predefined urinary albumin:creatinine ratio.63
Cardiovascular Effects of SGLT-2 Inhibitors
SGLT-2 inhibitors exert a glucose-lowering effect via a specific renal action by enhancing glucosuria, independently of insulin.16 As a consequence, they promote weight loss and do not induce hypoglycemia. Furthermore, by reducing glucotoxicity, they indirectly improve both β-cell function and insulin sensitivity (Figure 2).16,88 SGLT-2 inhibitors can be used in all stages of the natural history of T2DM, except in presence of moderate to severe CKD.17,89 Overall, this new pharmacological class is characterized by a good efficacy/risk balance.90
Figure 2.
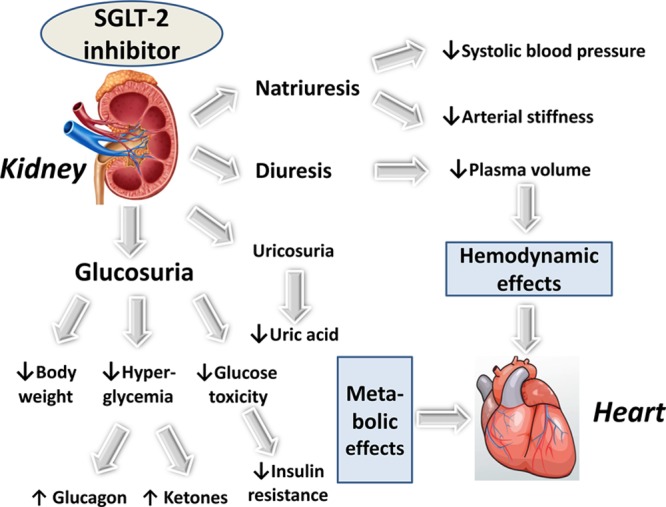
Illustration of the primary mechanisms of action of SGLT-2 (sodium-glucose cotransporter type 2) inhibitors and their hemodynamic and metabolic effects resulting in improved myocardial function and a reduced risk of heart failure.
Effects of SGLT-2 Inhibitors on CV Risk Factors and Surrogate End Points
Along with the primary antihyperglycemic effect, SGLT-2 inhibitors possess multidimensional properties that may favorably influence CV prognosis (Figure 2).16,17,91–93 They affect positively several recognized CV risk factors: a weight reduction results from calorie loss because of glucosuria,94 despite some compensatory increase in appetite and food intake95; a drop in arterial blood pressure is commonly explained by natriuresis and a diuretic effect;96,97 a reduction in serum uric acid levels is attributed to enhanced urinary excretion.98 Furthermore, an increase of hematocrit presumed to involve enhancement of erythropoiesis in addition to hemoconcentration (because of osmotic diuresis) may also exert positive effects.99 The clinical significance of minimal changes in lipids (minor increases in LDL [low-density lipoprotein], HDL [high-density lipoprotein], and non-HDL cholesterol levels and inconsistent changes in triglyceride levels) is unclear.100 SGLT-2 inhibitors induce small increases in serum concentrations of magnesium,101 potassium, and phosphate, yet the potential role of these increases in serum electrolyte levels in the CV protection remains unknown.102 Finally, preliminary data pointed toward additional benefits, including reduction of inflammation and oxidative markers, lowering of albuminuria in diabetic nephropathy and attenuation of fatty liver disease in experimental models.92 Positive effects on CV risk factors have been reported with canagliflozin,103,104 dapagliflozin,105 and empagliflozin.106
Finally, SGLT-2 inhibitors have proven their capacity to dampen the deterioration in renal function and reduce renal outcomes, a condition associated with a higher CV risk. They have the potential to exert nephroprotection not only through improving glycemic control but also through glucose-independent effects, such as blood pressure-lowering and direct renal effects.27,28
CV Outcomes in Meta-Analyses of Phase 2 to 3 Trials With SGLT-2 Inhibitors
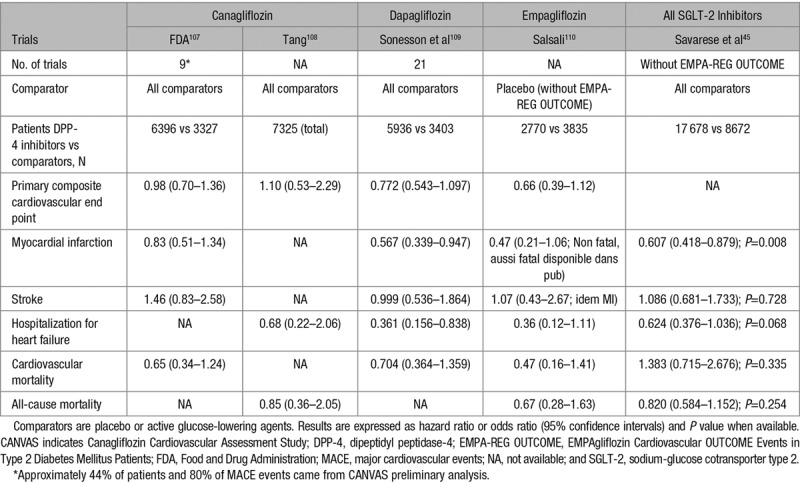
Several meta-analyses of RCTs with each of the 3 SGLT-2 inhibitors commercialized in the United States and in Europe generally reported a nonsignificant trend toward a lower incidence of MACE compared with placebo or other active glucose-lowering compounds: canagliflozin,107,108 dapagliflozin,109 and empagliflozin.110 Again, as already pointed out for DPP-4 inhibitors, none of these phase 2 to 3 trials were designed to test CV safety/efficacy of the SGLT-2 inhibitor and patients were at rather low risk of CVD (mainly primary prevention, except for canagliflozin for which preliminary data from CANVAS (Canagliflozin Cardiovascular Assessment Study) in patients with established CVD were included to more quickly fulfill the request by the FDA) and the trial duration was quite short (generally ≤1 year). As a consequence, the number of MACE was rather low for each individual SGLT-2 inhibitor so that the differences between the incidence of CV events in the SGLT-2 inhibitor and the comparator (placebo or active) groups failed to reach statistical significance in most instances (Table 4). However, in a pooled analysis of all RCTs (excluding the data from EMPA-REG OUTCOME [EMPAgliflozin Cardiovascular OUTCOME Events in Type 2 Diabetes Mellitus Patients] and from CANVAS), a significant reduction in the incidence of myocardial infarction and a trend for a reduction in the rate of hospitalization for HF were reported (Table 4).45
Table 4.
Cardiovascular Events and Mortality Rates With SGLT-2 Inhibitors in Meta-Analyses of Phase 2 to 3 Randomized Controlled Trials (Excluding Final Results of CANVAS Program and Results of EMPA-REG OUTCOME)
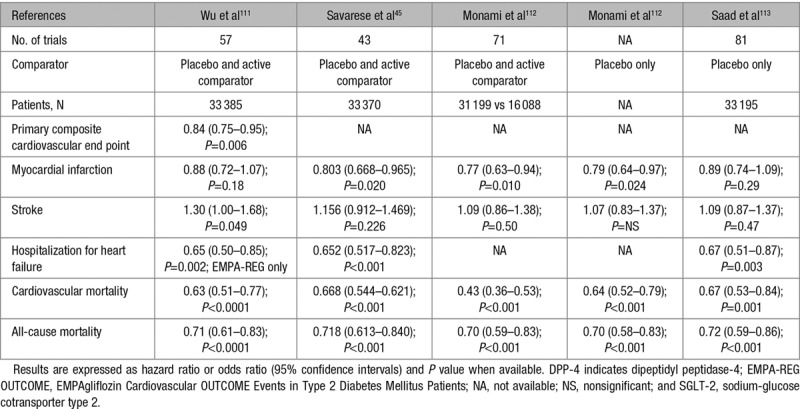
Several meta-analyses that have pooled all phase 2 to 3 RCTs plus EMPA-REG OUTCOME were published.45,111–113 Because of the size of EMPA-REG OUTCOME and the amplitude of the effect of empagliflozin in this trial114 (see below), significant reductions in the incidence of hospitalization for HF, CV mortality, and all-cause mortality were noticed (Table 5). The findings were more heterogeneous about the reductions in myocardial infarction and stroke (Table 5).
Table 5.
Cardiovascular Events and Mortality Rates With SGLT-2 Inhibitors in Meta-Analyses of Randomized Controlled Trials (Phase 2 to 3 Trials Plus EMPA-REG OUTCOME)
Dedicated CV Outcome Trials With SGLT-2 Inhibitors
Empagliflozin in EMPA-REG OUTCOME
The EMPA-REG OUTCOME trial randomly assigned 7020 patients with T2DM and established CVD (secondary prevention) to receive 10 mg or 25 mg of empagliflozin or matching placebo once daily added to standard care.114 After a median observation time of 3.1 years, empagliflozin (pooled data of the 2 doses) was associated with a moderate, but significant, reduction in the primary CV composite outcome (triple MACE) and with a more marked reduction in a CV and all-cause mortality (Table 6).114 There was no significant between-group difference about myocardial infarction or stroke as well as expanded MACE (P=0.08 for superiority).114
Table 6.
Cardiovascular Outcome Trials and Observational Studies With SGLT-2 Inhibitors
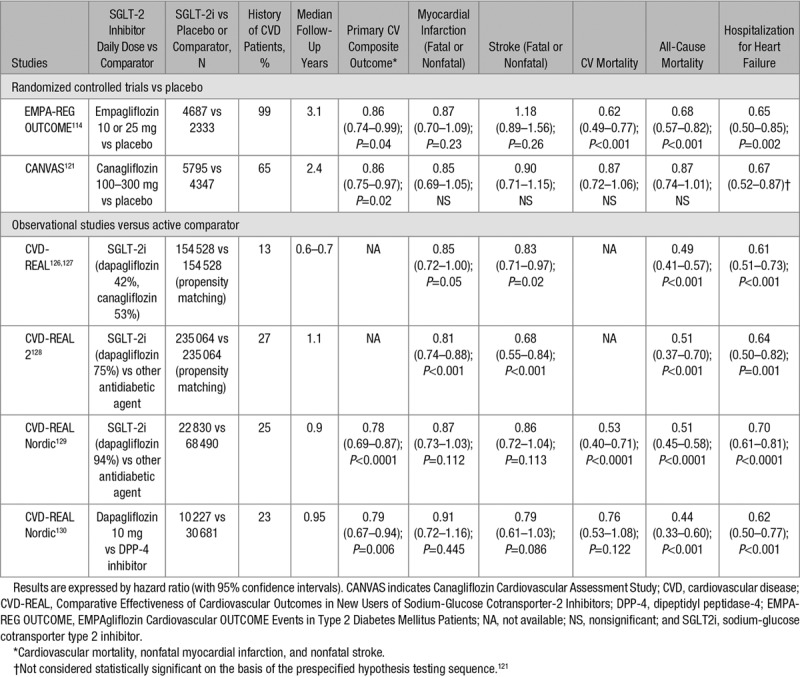
A significant reduction (−35%, P=0.002) in hospitalization for HF was noticed in patients treated with empagliflozin compared with those receiving placebo (Table 6).115 When the patients without HF at baseline (89.9%) were classified as low-to-average (<10%), high (10% to 20%), and very high (≥20%) 5-year risk for incident HF, the effect on combined CV death and HF hospitalization with empagliflozin was consistent across these 3 groups (HR, 0.71; 95% CI, 0.52–0.96; HR, 0.52; 95% CI, 0.36–0.75, and HR, 0.55; 95% CI, 0.30–1.00), respectively].116 These favorable results on HF were observed rather quickly, within the first 6 months after the treatment initiation, a finding that is suggestive of a predominant diuretic effect (although different from classical diuretics, see discussion below).117
Because CKD is considered as an independent CV risk, the positive effects of empagliflozin on renal outcomes should also be taken into account. Significant reductions in incident or worsening nephropathy (HR, 0.61; 95% CI, 0.53–0.70), progression to macroalbuminuria (HR, 0.62; 95% CI, 0.54–0.72), doubling of serum creatinine accompanied by eGFR ≤45 mL/min per 1.73 m2 (HR, 0.56; 95% CI, 0.39–0.59) and initiation of renal replacement (HR, 0.45; 95% CI, 0.21–0.97) were reported in the group treated with empagliflozin compared with the group treated with placebo.118
Intriguingly, a trend for an increased risk of stroke was observed in the empagliflozin group compared with the placebo group (HR, 1.18; 95% CI, 0.89–1.56; P=0.26; Table 6),114 a surprising observation for a drug that reduces arterial blood pressure.119 However, these data were reanalyzed in a further article specifically devoted to this topic, with quite reassuring results.120 Indeed, the numeric difference in stroke between the SGLT-2 inhibitor and placebo was primarily because of 18 patients in the empagliflozin group with a first event >90 days after the last intake of study drug corresponding to study end (versus only 3 on placebo). The reason why such an imbalance occurred >90 days after the end of the trial is unknown. No significant relationships with changes in arterial blood pressure (including rebound effects) or in hematocrit could be detected. In a sensitivity analysis restricted to events during treatment or ≤90 days after the last dose of study drug, the HR for stroke with empagliflozin versus placebo became close to one (HR 1.08; 95% CI 0.81–1.45; P=0.60). Furthermore, there were no differences in risk of recurrent, fatal, or disabling strokes, or transient ischemic attack, with empagliflozin versus placebo.120
Canagliflozin in CANVAS Program
The CANVAS program integrated data from 2 trials (CANVAS and CANVAS-R [Canagliflozin Cardiovascular Assessment Study-Renal]) involving a total of 10 142 participants with T2DM and high CV risk (65.6% had a history of CVD, 34.4% had only CV risk factors and were thus in primary prevention).121 Participants were randomly assigned to receive canagliflozin (100–300 mg) or placebo and were followed for a mean of 3.6 years (median 2.4 years). The rate of the primary outcome (triple MACE) was significantly lower with canagliflozin than with placebo (Table 6). All 3 components of the primary outcome showed a trend for the benefit (including nonfatal strokes), although the individual effects did not reach significance. Especially the reduction in CV mortality was only moderate and not statistically significant. Superiority was not shown for the first secondary outcome in the testing sequence (death from any cause; P=0.24). Therefore, subsequent differences between canagliflozin and placebo in death from CV causes and hospitalization for HF were not considered to be significant.121
The positive effects on renal outcomes reported with empagliflozin in EMPA-REG OUTCOME were confirmed with canagliflozin in CANVAS although on the basis of the prespecified hypothesis testing sequence the renal outcomes are not viewed as statistically significant. Nevertheless, the results showed a possible benefit of canagliflozin with respect to the progression of albuminuria (HR, 0.73; 95% CI, 0.67–0.79) and the composite outcome of a sustained 40% reduction in the eGFR, the need for renal-replacement therapy, or death from renal causes (HR, 0.60; 95% CI, 0.47–0.77).121
The adaptative design of the CANVAS program with different populations in CANVAS and CANVAS-R has been criticized, especially because a series of modifications have been made to the initially planned analyses. However, as emphasized by the Authors,122 the specification of the analysis strategy before knowledge of the trial results, their careful planning by the independent scientific trial Steering Committee, the detailed a priori definition of the analysis plans, and the external review provided by the US FDA all provide maximally efficient and robust utilization of the data. Thus, despite some weakness in the design of the CANVAS program, no major concern could be raised in the interpretation of the data.
Dapagliflozin in DECLARE TIMI-58
A prespecified meta-analysis of CV events from 21 phase 2b/3 RCTs with dapagliflozin suggested the potential for a beneficial CV effect in any of the populations investigated.109 The ongoing DECLARE trial (Dapagliflozin Effect on the Incidence of Cardiovascular Events-TIMI Group 58; URL: http://www.clinicaltrials.gov. Unique identifier: NCT01730534) that is comparing dapagliflozin 10 mg once daily with placebo recruited a large proportion of T2DM patients in primary CV prevention (10 228 out of 17 276 individuals).123 The results will allow to decide whether the CV protection by SGLT-2 inhibitors could be extended to all patients at risk of CVD (both in primary and secondary prevention) and whether the awaited findings with dapagliflozin support a class effect without significant heterogeneity.124
Ertugliflozin in VERTIS-CVOT
Ertugliflozin is a new SGLT-2 inhibitor that has been extensively investigated in the large VERTIS (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease, the VERTIS-CV study) clinical program and is currently in a final phase of clinical development.125 VERTIS-CVOT (URL: http://www.clinicaltrials.gov. Unique identifier: NCT0198688) is a placebo-controlled RCT to assess CV outcomes following treatment with ertugliflozin (5 or 15 mg once daily) in patients with T2DM and established vascular disease, a population almost similar to that enrolled in EMPA-REG OUTCOME.
CV Outcomes in Observational Studies: CVD-REAL Registries
In a large multinational study (CVD-REAL [Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors]: 6 European countries and the United States), a treatment with SGLT-2 inhibitors versus other glucose-lowering agents was associated with a lower risk of hospitalization for HF and all-cause death (Table 6).126 A further subanalysis of CVD-REAL showed that initiation of SGLT-2i versus other antidiabetic agents was associated with a modestly lower risk of myocardial infarction and stroke (Table 6).127 Confirmatory findings were reported in another similar study performed in other countries (CVD-REAL 2: South Korea, Japan, Singapore, Israel, Australia, and Canada; Table 6).128 As a majority of these T2DM patients did not exhibit established CVD (only 25% to 27% were in secondary prevention) and were treated with dapagliflozin, these data suggest that the benefits seen with empagliflozin and canagliflozin in RCTs may be a class effect applicable to a broad population of patients with T2DM in real-world practice.126–128 These data were confirmed in 2 substudies using the CVD-REAL Nordic database, the first one comparing matched SGLT-2 inhibitor (94% users of dapagliflozin) and other glucose-lowering drug groups,129 the second one comparing new users of dapagliflozin and new users of DPP-4 inhibitors (Table 6).130
These findings of CVD-REAL are consistent with the results of 2 placebo-controlled RCTs in T2DM patients at high CV risk, EMPA-REG OUTCOME, and CANVAS. Because of the observational design of the CVD-REAL studies, possible biases (differences likely exaggerated by immortal time and time-lag biases) could not be excluded so that caution is required before drawing any definite conclusion.131
Interpretation of the CV Outcomes With SGLT-2 Inhibitors
How to Explain Noninferiority?
SGLT-2 inhibitors do not exert negative effects such as weight gain or hypoglycemia, in contrast to what may occur with sulfonylureas. The reduction in blood pressure is not accompanied by a stimulation of sympathetic activity. The side effects resulting from volume depletion are rather rare and generally not severe.90 A suspicion of increased risk of stroke in EMPA-REG OUTCOME114 could not be related to the increase in hematocrit or orthostatic hypotension.120 Furthermore, it was not confirmed neither in a dedicated post hoc analysis,120 as already mentioned, nor in CANVAS.121
How to Explain the Superiority?
It is generally considered that the favorable effects of empagliflozin in EMPA-REG OUTCOME could not be explained neither by the modest reduction in HbA1c (search for equipoise in glucose control), nor by the slight reduction in arterial blood pressure in already well-controlled patients (mean systolic blood pressure <140 mm Hg),119 nor by the moderate body weight loss94 nor by the lowering effect of serum uric acid levels98 nor by the increase in hematocrit alone. Even a combination of all these mechanisms seems insufficient to explain the marked reduction in a CV and all-cause mortality observed with empagliflozin in EMPA-REG OUTCOME.114 If results of this landmark trial raised much interest among cardiologists,132,133 the mechanisms explaining the reduction in MACE and mortality remain highly debated,117,134 and an extensive discussion of the different proposals is beyond the scope of this review. Schematically, hemodynamic28,133 or metabolic135,136 mechanistic explanations have been proposed. Importantly, they are not mutually exclusive and the relative impact of each mechanism may vary over time (for instance, the hemodynamic component being predominant early after the administration of the drug and the metabolic component becoming more important later on during the observation period). What so ever, all the proposed mechanisms are still hypothetical and remain to be specifically investigated in further studies.26,28,93,117
The observation that the reduction in hospitalization for HF and CV mortality occurred rather quickly (<6 months), without significant changes in the incidence of myocardial infarction and stroke put forward a hemodynamic hypothesis, emphasizing the diuretic activity of SGLT-2 inhibitors.117,137 However, the diuretic properties of SGLT-2 inhibitors are quite different from those of classical diuretics.138 On the one hand, it has been hypothesized that, by reducing interstitial fluid space to a greater extent than blood volume, SGLT-2 inhibitors might provide better control of congestion without reducing arterial filling and perfusion, thus explaining the reduction in hospitalization for HF.139 On the other hand, it has been shown that SGLT-2 inhibitors, by promoting natriuresis and osmotic diuresis, also lead to some plasma volume contraction and reduced preload; in addition, they induce decreases in blood pressure, arterial stiffness, and afterload as well, thereby improving subendocardial blood flow especially in patients with HF.140 In a recent exploratory analysis from the EMPA-REG OUTCOME trial with multivariable models, changes in markers of plasma volume were the most important mediators of the reduction in risk of CV death; indeed, changes from baseline in hematocrit and hemoglobin mediated 51.8% and 48.9%, respectively, of the overall effect of empagliflozin versus placebo on CV death.141
Besides this hemodynamic hypothesis, SGLT-2 inhibitors are also associated with hormonal and metabolic changes that may also contribute to improve the CV prognosis of T2DM patients at high CV risk. A possible beneficial effect of the observed rise in glucagon levels with SGLT-2 inhibitors has been hypothesized.142,143 The most popular metabolic hypothesis is based on the slight increase in plasma β-hydroxybutyrate levels that results in a shift substrate utilization.136,144,145 It is postulated that the CV (and renal) benefits of empagliflozin may be because of a shift in myocardial (and renal) fuel metabolism away from free fatty acid and glucose oxidation, which are energy inefficient in the setting of the heart (and kidney) in patients with advanced T2DM, toward a more energy-efficient fuel like ketone bodies, which improves myocardial (renal) work efficiency and function.136,144,145
Finally, it has been recently hypothesized that the benefits of SGLT-2 inhibitors in HF may be mediated by the inhibition of sodium-hydrogen exchange in both the kidneys and the myocardium.146 In the kidneys, SGLT-2 functionally interacts with the sodium-hydrogen exchanger, which is responsible for the majority of sodium tubular reuptake after filtration. The activity of sodium-hydrogen exchanger is markedly increased in patients with HF. This abnormality may be responsible for resistance to both endogenous natriuretic peptides and diuretics and could be at least in part reverted with SGLT-2 inhibitors. In the heart, empagliflozin also seems to inhibit sodium-hydrogen exchange, which may lead to a reduction in cardiac injury, hypertrophy, fibrosis, and remodeling, and thus to attenuation of myocardial dysfunction.146 Further studies should validate this hypothesis.
CV Outcomes Versus Overall Safety Profile
The clinical value of SGLT-2 inhibitors should be assessed considering the risk-benefit ratio of the pharmacological class, which may depend on the individual patient characteristics.90 Besides their positive effects on CV outcomes, SGLT-2 inhibitors have the potential to exert nephroprotection, as already discussed. Nephroprotective effects were confirmed in both EMPA-REG OUTCOME118 and CANVAS121 and are now considered as a potential major add-on value of SGLT-2 inhibitors.27,28
The most common adverse events reported with SGLT-2 inhibitors are explained by the specific renal mechanism of action of this pharmacological class.90,147 Mycotic genital infections occurred 4 to 6× more frequently with SGLT-2 inhibitors than with placebo or other antidiabetic agents, more frequently in women than in men and generally not severe. Urinary tract infections are rare and not significantly increased when compared with comparators in most studies, including EMPA-REG OUTCOME114 and CANVAS.121 Side effects related to dehydration are more frequent with SGLT-2 inhibitors (mostly hypotension) than with other glucose-lowering agents but rather rare and not severe in published RCTs.90 Nevertheless, caution is recommended in the elderly more frailty population.90,144 The overall good safety profile of canagliflozin,104,148 dapagliflozin,149 and empagliflozin150 have been recently reported in dedicated extensive reviews of pooled data from clinical trials. However, 2 more severe adverse events have been attributed to SGLT-2 inhibitors, diabetic ketoacidosis episodes, and peripheral amputations.
A higher risk of euglycemic ketoacidosis has been reported in patients treated with SGLT-2 inhibitors. Reduction in insulin dose, stimulation of the release of glucagon and enhanced ketone reabsorption in the renal tubuli could contribute to the increase in the concentration of ketone bodies, whereas enhanced glucosuria limits the amplitude of hyperglycemia.151 Euglycemic ketoacidosis was considered as a predictable, detectable, and preventable safety concern with SGLT-2 inhibitors.152 Hospitalization for surgery was shown to be a predisposing condition, but it may be speculated that hospitalization for an acute coronary syndrome should also be considered as a risky condition that requires the interruption of the SGLT-2 inhibitor during the acute phase. In a meta-analysis of RCTs, no signal of increased risk for ketoacidosis was observed for SGLT-2 inhibitors as a class (MH-OR, 1.14; 95% CI, 0.45–2.88; P=0.78) or as individual molecules,153 and this was also the case in EMPA-REG OUTCOME114 and in CANVAS.121 However, the risk may be higher in real-life conditions with less well-selected patients under less strict supervision.154,155 Thus, the increased risk of ketoacidosis with SGLT-2 inhibitors should be considered at the time of initiating prescription of any SGLT-2 inhibitor and throughout therapy, if patients present with symptoms (mainly nausea and vomiting) suggestive of ketoacidosis.
The second severe complication is peripheral amputation.156 An unexpected increased risk of amputation was observed in the CANVAS program when comparing canagliflozin and placebo groups (respectively, 6.3 versus 3.4 participants per 1000 patient-years; HR, 1.97; 95% CI, 1.41–2.75). These amputations were primarily at the level of the toe or metatarsal.121 In a pharmacovigilance analysis using the US FDA Adverse Event Reporting System, canagliflozin was associated with a higher risk of amputation relative to empagliflozin and dapagliflozin, although the records may not be sufficient to explain a precise causal relationship between canagliflozin exposure and amputation.157 Furthermore, the US FDA Adverse Event Reporting System is exposed to possible reporting biases, that may limit the interpretation of these data. A US real-world study observed no evidence of increased risk of below-knee lower extremity amputation for new users of canagliflozin compared with non-SGLT-2 inhibitor antihyperglycemic agents in a broad population of patients with T2DM.158 More detailed approach considering individual clinical course potentially involved in the amputation, notably in the CANVAS program, would help to further unravel the cause for suspected risk of amputation with canagliflozin. If confirmed, this complication may attenuate the overall CV benefit attributed to canagliflozin and perhaps, in the absence of clear explanation, may also affect the whole pharmacological class of SGLT-2 inhibitors. Of note, however, no increased risk of lower limb amputations was reported in the EMPA-REG OUTCOME trial with empagliflozin versus placebo (HR, 1.00; 95% CI, 0.70–1.44). Despite the limitation of post hoc analyses of cases of lower limb amputations that were manually identified, these findings were consistent across subgroups by established risk factors for amputation,159 including patients with established peripheral artery disease.160 What so ever, possible adverse events should not hide the CV protection associated with SGLT-2 inhibitors: for instance in CANVAS, the number needed to harm for peripheral amputation was ≈ 330 per year, whereas the number-needed-to-treat to avoid a MACE was ≈200 per year.121
Finally, in the CANVAS program, canagliflozin was also associated with a higher fracture incidence of all bone fractures (HR, 1.26; 95% CI, 1.04–1.52) and low-trauma fracture events (HR, 1.23; 95% CI, 0.99–1.52); the risk of fractures was significantly higher in the canagliflozin group than in the placebo group in CANVAS but not in CANVAS-R, a heterogeneity that remains unexplained, yet the follow-up was almost twice longer in CANVAS than in CANVAS-R.121 An increased risk of fractures as not reported with empagliflozin in EMPA-REG OUTCOME114 and in 2 pooled analyses of phase 2 to 3 trials with empagliflozin150 and dapagliflozin.149 The reason for increased fracture risk with canagliflozin treatment is unknown but is likely not related to a direct effect of canagliflozin on bone-related biomarkers.161
Unresolved Issues
There are some differences between the results observed in CANVAS121 and those reported in EMPA-REG OUTCOME.114 Indeed, in contrast to the marked reduction in a CV and all-cause mortality reported in EMPA-REG OUTCOME,114 no such significant reduction was noticed in CANVAS.121 This difference may be at least partially explained by the characteristics of the T2DM patients: in CANVAS, almost 35% of the population were in primary prevention, whereas in EMPA-REG OUTCOME, nearly all patients were in secondary prevention. In CANVAS, the interaction test between the 2 subgroups of T2DM patients with and without previous CV complications did not reach statistical significance (P=0.18).121 However, the reduction in CV mortality among the subgroup of patients in secondary prevention in CANVAS was much lower than the corresponding reduction noticed in EMPA-REG OUTCOME. The reasons for such difference are unclear and it is unknown whether it is molecule-dependent.
As already discussed, a higher risk of bone fractures and amputations was reported with canagliflozin and not with empagliflozin or dapagliflozin. The underlying mechanisms of peripheral amputations attributed to canagliflozin remain quite obscure and whether these adverse effects are specific to canagliflozin or might be a class effect remains an open question.162
Whether the results obtained in EMPA-REG OUTCOME and to a lesser extend CANVAS may be extrapolated to real-life conditions in patients with T2DM and without established CVD is still to be proven. Indeed, only a minority of T2DM patients in the United States163 or in the United Kingdom164 population have the criteria that had allowed them to be included in EMPA-REG OUTCOME. The findings of the CVD-REAL studies provide arguments supporting the concept that benefits could be expected from SGLT-2 inhibitors also in T2DM patients in primary prevention.126 Because of possible bias inherent to observational findings,130 the final answer will come from the results of DECLARE that enrolled almost two-thirds of T2M patients without established CVD.123
Another question is whether the positive findings of EMPA-REG OUTCOME with empagliflozin may be extrapolated to dapagliflozin. The results of the CVD-REAL observational studies suggested that it may be the case.126–130 However, here again, only the ongoing RCT DECLARE123 comparing dapagliflozin with a placebo in a large cohort of T2DM with and without CVD will allow to conclude if the CV protection observed with empagliflozin and to a lesser extent to canagliflozin can be attributed to a class effect or not.
Besides SGLT-2 inhibitors, the GLP-1 receptor agonist liraglutide has also proven CV benefit in the LEADER trial.165 Thus, the question emerges which drug to use in T2DM patients with established CVD, either a SGLT-2 inhibitor (empagliflozin) or a GLP-1 receptor agonist (liraglutide and possibly when available semaglutide according to the results of SUSTAIN-6)?12 In patients with or at risk of HF, the answer is easy and SGLT-2 inhibitors should be preferred considering the mitigated results with GLP-1 receptor agonists on this complication.166 Although SGLT-2 inhibitors seem to exert their CV protective actions mainly by hemodynamic effects,28 GLP-1 receptor agonists most probably work via antiatherogenic/anti-inflammatory mechanisms. This raises the possibility that combined therapy with these 2 classes may produce additive CV benefits and be considered to further improve the CV prognosis in T2DM patients at very high risk of CVD.167
Finally, the reduction in the incidence of hospitalization for HF in EMPA-REG OUTCOME and CANVAS is impressive. These findings raise the possibility of using SGLT-2 inhibitors as therapies not only in the prevention of HF but also for the treatment of patients with established HF regardless of the presence or absence of diabetes mellitus. Several large trials are currently exploring this working hypothesis.168
Personalized Approach: DPP-4 or SGLT-2 Inhibitor?
Although DPP-4 inhibitors showed the only noninferiority versus placebo, SGLT-2 inhibitors exhibited superiority. Thus, from the point of view of the cardiologist, the preference should be given to SGLT-2 inhibitors, at least in T2DM patients at high CV risk. This is in agreement with the 2017–2018 updated standards of medical care in diabetes mellitus from the American Diabetes Association (Figure 3).19,169 However, from the point of view of the endocrinologist, both DPP-4 and SGLT-2 inhibitors have advantages and disadvantages so that a personalized approach based on the properties of the medication and the individual characteristics of the patient is the recommended best approach (Table 7).170 The use of glucose-lowering medications should be optimized according to a patient-centered approach, and the emergence of precision medicine may help to have a better strategy for the management of T2DM in the future.171
Figure 3.
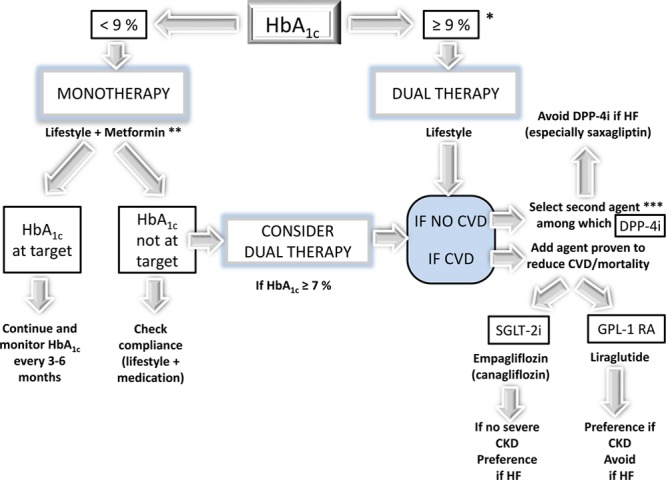
Pharmacological approaches to glycemic treatment in type 2 diabetes mellitus according to standards of medical care in diabetes mellitus 2018. After the failure of metformin monotherapy, a patient-centered approach is recommended, and the choice of the add-on medication is triggered by the presence or not of cardiovascular disease, heart failure (HF), and chronic kidney disease (CKD). *If glycated hemoglobin (HbA1c) ≥10%, blood glucose ≥300 mg/dL (16.7 mmol/L), or symptoms, consider combination injectable therapy. **If no contraindications. ***A patient-centered approach should be used to guide the choice of the second pharmacological agent. For instance, if a patient has no cardiovascular disease (CVD), one may consider an SGLT-2i (sodium-glucose cotransporter type 2 inhibitor; as opposed to a DPP-4i [dipeptidyl peptidase-4 inhibitor]) if weight loss or improved blood pressure control were being considered. GLP-1 RA indicates glucagon-like peptide-1 receptor agonist.
Table 7.
Characteristics of DPP-4 and SGLT-2 Inhibitors for the Management of Type 2 Diabetes Mellitus
DPP-4 Inhibitors for Which Patients?
The safety profile of DPP-4 inhibitors is excellent33 and the clinical experience with this pharmacological class is rather broad worldwide over the last 10 years. DPP-4 inhibitors may be used safely in the frailty patient at higher risk of hypoglycemia or volume depletion/dehydration.33 Interestingly for the clinician, they may be used whatever the renal function provided that the daily dose is adjusted to eGFR.34 This contrasts with SGLT-2 inhibitors whose use is currently contraindicated in patients with moderate to severe renal impairment.89 In real life, numerous patients with T2DM and advanced CVD or HF have also some deterioration of kidney function that may contraindicate the use of SGLT-2 inhibitors. In these patients, DPP-4 inhibitors seem to be used safely from a cardiology standpoint.
However, in patients with or at risk of HF, the prescription of a DPP-4 inhibitor may be a matter of concern even if sitagliptin seems to be safe according to the results of TECOS.51,62 Despite the absence of significant heterogeneity when comparing the results of the 3 trials about the risk of hospitalization for HF,62 it is prudent to avoid the use of saxagliptin after the results of the SAVOR-TIMI 53 trial50,58 and also alogliptin that was associated with a nonsignificant trend to an increase in hospitalization for HF in EXAMINE.49 This caution is mentioned within the US FDA and EMA labels of the 2 products and the recommendations of the American Diabetes Association.19,169
SGLT-2 Inhibitors for Which Patients?
On the basis of the landmark trials with SGLT-2 inhibitors, especially EMPA-REG OUTCOME, a paradigm shift in the management of patients with T2DM, specifically in those with the prior macrovascular disease has been proposed. It implies a transition from current algorithms based primarily on glucose control and HbA1c changes to a more comprehensive strategy additionally focused on CV prevention.172 In December 2016, the FDA added a new indication for empagliflozin, to reduce the risk of CV death in adults with T2DM and CVD, and this approach is now recognized in the standards of medical care in diabetes mellitus published early 2017 by the American Diabetes Association 2017169 and confirmed in 2018 (Figure 3).19 The following specific recommendation (class B) is stated: in patients with long-standing suboptimally controlled T2DM and established CVD, empagliflozin (or liraglutide) should be considered as they have been shown to reduce CV and all-cause mortality when added to standard care.169
According to the 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic HF, empagliflozin should be considered in patients with T2DM to prevent or delay the onset of HF and prolong life (Class IIa, level B).173 Considering the recent data of CANVAS,121 with a similar reduction in hospitalization for HF as in EMPA-REG-OUTCOME114 (Table 6), it seems reasonable to extend this recommendation to canagliflozin.19 The favorable results about the risk of hospitalization for HF with dapagliflozin in the observational CVD-REAL studies126–130 require further confirmation in the upcoming DECLARE CV outcome RCT.123
DPP-4 Plus SGLT-2 Inhibitor Combined Therapy
Despite the emergence of a large variety of new antidiabetic agents, the management of T2DM remains highly challenging18,19 and so-called treatment-resistant T2DM is leading to poor glucose control is a common phenomenon in clinical practice for many reasons.174 There is a strong rationale for combining a DPP-4i and an SGLT-2i in patients with T2DM because the 2 drugs exert different and complementary glucose-lowering effects.175,176 Furthermore, the increase of glucagon levels induced by SGLT-2 inhibitors may be blunted by the coadministration of DPP-4 inhibitors. Dual therapy with a DPP-4 inhibitor and an SGLT-2 inhibitor (initial combination or stepwise approach) is more potent than either monotherapy in patients treated with diet and exercise or already receiving with metformin. Combining the 2 pharmacological options is safe and does not induce hypoglycemia. It is currently unknown whether the addition of a DPP-4 inhibitor could reduce the risk of euglycemic ketoacidosis reported with SGLT-2 inhibitor therapy. A reduction in the incidence of genital infections associated with SGLT-2 inhibitors has been reported when a DPP-4 inhibitor is added, perhaps because of a better glucose control although other possible mechanisms remain to be investigated.177 Of note, the CV effects of the combined therapy have not been investigated. Two fixed-dose combinations are already available (saxagliptin-dapagliflozin and linagliptin-empagliflozin) and a third one (sitagliptin-ertugliflozin) has been recently approved by the US FDA and the EMA.175,176
GLP-1 Receptor Agonist Plus SGLT-2 Inhibitor
Liraglutide and empagliflozin are recognized to be able to reduce the incidence of MACE in T2DM patients with established CVD (Figure 3).19,169 Because GLP-1 receptor agonists result in CV protection presumably by different mechanisms than those hypothesized for SGLT-2 inhibitors, it might be of interest to combine a GLP-1 inhibitor (such as liraglutide) and an SGLT-2 inhibitor (such as empagliflozin) to offer the best CV (and renal) protection in very high-risk patients with T2DM. This combined therapy of a GLP-1 receptor agonist and an SGLT-2 inhibitor has only been tested using surrogate end points such as glucose-lowering efficacy, weight loss, and blood pressure reduction.167,178,179 Whether this dual therapy may result in a better CV and renal protection remains unknown. Of note, the combination of a GLP-1 receptor agonist and a DPP-4 inhibitor, 2 compounds that act at least partially via similar mechanisms, does not provide any obvious added value and thus is not recommended.180
Conclusions
New oral glucose-lowering agents have been evaluated in specific CV outcome RCTs. DPP-4 inhibitors showed noninferiority versus placebo in patients with T2DM and high CV risk, thus demonstrating the CV safety of this pharmacological class, but failed to show superiority. In contrast, 2 SGLT-2 inhibitors empagliflozin and canagliflozin showed a significant reduction in triple MACE, all-cause mortality and hospitalization for HF (and also CV mortality for empagliflozin). These findings open new perspectives in the management of patients with T2DM and established CVD, especially those with or at high risk of HF. Whether these results may be extended to all the pharmacological class of SGLT-2 inhibitors, to patients with T2DM and lower CV risk and perhaps also to nondiabetic patients with CVD and HF require further studies. Obviously, the prevention of CVD in patients with T2DM has reached an exciting new era.
Disclosures
A.J. Scheen has received lecturer/advisor/investigator fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis, NovoNordisk, Sanofi, and Servier. He worked as a clinical investigator in the TECOS, EMPA-REG OUTCOME, CANVAS-R, and DECLARE trials.
Footnotes
Nonstandard Abbreviations and Acronyms
- CARMELINA
- Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus
- CAROLINA
- Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes
- CI
- confidence interval
- CKD
- chronic kidney disease
- CV
- cardiovascular
- CVD
- cardiovascular disease
- DPP-4
- dipeptidyl peptidase-4
- eGFR
- estimated glomerular filtration rate
- FDA
- Food and Drug Administration
- GIP
- glucose-dependent insulinotropic polypeptide
- GLP-1
- glucagon-like peptide-1
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- HF
- heart failure
- HR
- hazard ratio
- LDL
- low-density lipoprotein
- MACE
- major cardiovascular events
- RCT
- randomized controlled trial
- SDF-1α
- stromal cell-derived factor-1α
- SGLT-2
- sodium-glucose cotransporter type 2
- T2DM
- type 2 diabetes mellitus
- TECOS
- Trial Evaluating Cardiovascular Outcomes with Sitagliptin
References
- 1.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. 2017;70:883–893. doi: 10.1016/j.jacc.2017.07.001. doi: 10.1016/j.jacc.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 4.Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 6.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;11:CD008143. doi: 10.1002/14651858.CD008143.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Scheen AJ, Charbonnel B. Effects of glucose-lowering agents on vascular outcomes in type 2 diabetes: a critical reappraisal. Diabetes Metab. 2014;40:176–185. doi: 10.1016/j.diabet.2014.03.004. doi: 10.1016/j.diabet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Roussel R, Steg PG, Mohammedi K, Marre M, Potier L. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: a perspective on glucose-lowering interventions. Diabetes Obes Metab. 2018;20:238–244. doi: 10.1111/dom.13033. doi: 10.1111/dom.13033. [DOI] [PubMed] [Google Scholar]
- 9.Goldfine AB. Assessing the cardiovascular safety of diabetes therapies. N Engl J Med. 2008;359:1092–1095. doi: 10.1056/NEJMp0805758. doi: 10.1056/NEJMp0805758. [DOI] [PubMed] [Google Scholar]
- 10.Zannad F, Stough WG, Lipicky RJ, et al. Assessment of cardiovascular risk of new drugs for the treatment of diabetes mellitus: risk assessment vs. risk aversion. Eur Heart J Cardiovasc Pharmacother. 2016;2:200–205. doi: 10.1093/ehjcvp/pvw007. doi: 10.1093/ehjcvp/pvw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288–2296. doi: 10.1093/eurheartj/ehv239. doi: 10.1093/eurheartj/ehv239. [DOI] [PubMed] [Google Scholar]
- 12.Standl E, Schnell O, McGuire DK, Ceriello A, Rydén L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5:391–402. doi: 10.1016/S2213-8587(17)30033-5. doi: 10.1016/S2213-8587(17)30033-5. [DOI] [PubMed] [Google Scholar]
- 13.Chawla H, Tandon N. Interpreting cardiovascular endpoints in trials of antihyperglycemic drugs. Am J Cardiovasc Drugs. 2017;17:203–215. doi: 10.1007/s40256-017-0215-6. doi: 10.1007/s40256-017-0215-6. [DOI] [PubMed] [Google Scholar]
- 14.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheen AJ. A review of gliptins for 2014. Expert Opin Pharmacother. 2015;16:43–62. doi: 10.1517/14656566.2015.978289. doi: 10.1517/14656566.2015.978289. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–531. doi: 10.1210/er.2010-0029. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 17.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 18.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2018 executive summary. Endocr Pract. 2018;24:91–120. doi: 10.4158/CS-2017-0153. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 20.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84. doi: 10.1038/nrcardio.2012.183. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- 22.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 23.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 24.Michel MC, Mayoux E, Vallon V. A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:801–816. doi: 10.1007/s00210-015-1134-1. doi: 10.1007/s00210-015-1134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Cardiol. 2017;120:S28–S36. doi: 10.1016/j.amjcard.2017.05.013. doi: 10.1016/j.amjcard.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38. doi: 10.1016/j.cmet.2017.04.011. doi: 10.1016/j.cmet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. doi: 10.2337/dcS15-3006. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 28.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 29.Scheen AJ, Delanaye P. Effects of reducing blood pressure on renal outcomes in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Metab. 2017;43:99–109. doi: 10.1016/j.diabet.2016.12.010. doi: 10.1016/j.diabet.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Scheen AJ, Delanaye P. Renal outcomes with dipeptidyl peptidase-4 inhibitors. Diabetes Metab. 2018;44:101–111. doi: 10.1016/j.diabet.2017.07.011. doi: 10.1016/j.diabet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Coppolino G, Leporini C, Rivoli L, Ursini F, di Paola ED, Cernaro V, Arturi F, Bolignano D, Russo E, De Sarro G, Andreucci M. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol Res. 2018;129:274–294. doi: 10.1016/j.phrs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 33.Scheen AJ. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2 diabetes. Expert Opin Drug Saf. 2015;14:505–524. doi: 10.1517/14740338.2015.1006625. doi: 10.1517/14740338.2015.1006625. [DOI] [PubMed] [Google Scholar]
- 34.Scheen AJ. Pharmacokinetics and clinical use of incretin-based therapies in patients with chronic kidney disease and type 2 diabetes. Clin Pharmacokinet. 2015;54:1–21. doi: 10.1007/s40262-014-0198-2. doi: 10.1007/s40262-014-0198-2. [DOI] [PubMed] [Google Scholar]
- 35.Anderluh M, Kocic G, Tomovic K, Kocic R, Deljanin-Ilic M, Smelcerovic A. Cross-talk between the dipeptidyl peptidase-4 and stromal cell-derived factor-1 in stem cell homing and myocardial repair: potential impact of dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2016;167:100–107. doi: 10.1016/j.pharmthera.2016.07.009. doi: 10.1016/j.pharmthera.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 36.McCormick LM, Kydd AC, Read PA, Ring LS, Bond SJ, Hoole SP, Dutka DP. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ Cardiovasc Imaging. 2014;7:274–281. doi: 10.1161/CIRCIMAGING.113.000785. doi: 10.1161/CIRCIMAGING.113.000785. [DOI] [PubMed] [Google Scholar]
- 37.Yamada H, Tanaka A, Kusunose K, et al. PROLOGUE Study Investigators. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16:63. doi: 10.1186/s12933-017-0546-2. doi: 10.1186/s12933-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White WB, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, Menon V. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:668–673. doi: 10.1111/dom.12093. doi: 10.1111/dom.12093. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal N, Parker A, Frederich R, Donovan M, Hirshberg B. Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: pooled analysis of 20 clinical trials. Cardiovasc Diabetol. 2014;13:33. doi: 10.1186/1475-2840-13-33. doi: 10.1186/1475-2840-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engel SS, Golm GT, Shapiro D, Davies MJ, Kaufman KD, Goldstein BJ. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovasc Diabetol. 2013;12:3. doi: 10.1186/1475-2840-12-3. doi: 10.1186/1475-2840-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. doi: 10.1186/1475-2840-11-3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McInnes G, Evans M, Del Prato S, Stumvoll M, Schweizer A, Lukashevich V, Shao Q, Kothny W. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17 000 patients. Diabetes Obes Metab. 2015;17:1085–1092. doi: 10.1111/dom.12548. doi: 10.1111/dom.12548. [DOI] [PubMed] [Google Scholar]
- 43.Monami M, Ahrén B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:112–120. doi: 10.1111/dom.12000. doi: 10.1111/dom.12000. [DOI] [PubMed] [Google Scholar]
- 44.Xu S, Zhang X, Tang L, Zhang F, Tong N. Cardiovascular effects of dipeptidyl peptidase-4 inhibitor in diabetic patients with and without established cardiovascular disease: a meta-analysis and systematic review. Postgrad Med. 2017;129:205–215. doi: 10.1080/00325481.2017.1255537. doi: 10.1080/00325481.2017.1255537. [DOI] [PubMed] [Google Scholar]
- 45.Savarese G, D’Amore C, Federici M, De Martino F, Dellegrottaglie S, Marciano C, Ferrazzano F, Losco T, Lund LH, Trimarco B, Rosano GM, Perrone-Filardi P. Effects of dipeptidyl peptidase 4 inhibitors and sodium-glucose linked cotransporter-2 inhibitors on cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis. Int J Cardiol. 2016;220:595–601. doi: 10.1016/j.ijcard.2016.06.208. doi: 10.1016/j.ijcard.2016.06.208. [DOI] [PubMed] [Google Scholar]
- 46.Elgendy IY, Mahmoud AN, Barakat AF, Elgendy AY, Saad M, Abuzaid A, Wayangankar SA, Bavry AA. Cardiovascular safety of dipeptidyl-peptidase IV inhibitors: a meta-analysis of placebo-controlled randomized trials. Am J Cardiovasc Drugs. 2017;17:143–155. doi: 10.1007/s40256-016-0208-x. doi: 10.1007/s40256-016-0208-x. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud AN, Saad M, Mansoor H, Elgendy AY, Barakat AF, Abuzaid A, Mentias A, Elgendy IY. Cardiovascular safety of incretin-based therapy for type 2 diabetes: a meta-analysis of randomized trials. Int J Cardiol. 2017;230:324–326. doi: 10.1016/j.ijcard.2016.12.113. doi: 10.1016/j.ijcard.2016.12.113. [DOI] [PubMed] [Google Scholar]
- 48.Abbas AS, Dehbi HM, Ray KK. Cardiovascular and non-cardiovascular safety of dipeptidyl peptidase-4 inhibition: a meta-analysis of randomized controlled cardiovascular outcome trials. Diabetes Obes Metab. 2016;18:295–299. doi: 10.1111/dom.12595. doi: 10.1111/dom.12595. [DOI] [PubMed] [Google Scholar]
- 49.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 50.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 51.Green JB, Bethel MA, Armstrong PW, et al. for the TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 52.Shimada YJ, Cannon CP, Liu Y, Wilson C, Kupfer S, Menon V, Cushman WC, Mehta CR, Bakris GL, Zannad F, White WB EXAMINE Investigators. Ischemic cardiac outcomes and hospitalizations according to prior macrovascular disease status in patients with type 2 diabetes and recent acute coronary syndrome from the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care trial. Am Heart J. 2016;175:18–27. doi: 10.1016/j.ahj.2016.01.011. doi: 10.1016/j.ahj.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 54.White WB, Wilson CA, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Heller SK, Mehta CR, Nissen SE, Zannad F, Kupfer S EXAMINE Investigators. Angiotensin-converting enzyme inhibitor use and major cardiovascular outcomes in type 2 diabetes mellitus treated with the dipeptidyl peptidase 4 inhibitor alogliptin. Hypertension. 2016;68:606–613. doi: 10.1161/HYPERTENSIONAHA.116.07797. doi: 10.1161/HYPERTENSIONAHA.116.07797. [DOI] [PubMed] [Google Scholar]
- 55.Devin JK, Pretorius M, Nian H, Yu C, Billings FT, IV, Brown NJ. Substance P increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. Hypertension. 2014;63:951–957. doi: 10.1161/HYPERTENSIONAHA.113.02767. doi: 10.1161/HYPERTENSIONAHA.113.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leiter LA, Teoh H, Braunwald E, Mosenzon O, Cahn A, Kumar KM, Smahelova A, Hirshberg B, Stahre C, Frederich R, Bonnici F, Scirica BM, Bhatt DL, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145–1153. doi: 10.2337/dc14-2868. doi: 10.2337/dc14-2868. [DOI] [PubMed] [Google Scholar]
- 57.Cavender MA, Scirica BM, Raz I, Steg PG, McGuire DK, Leiter LA, Hirshberg B, Davidson J, Cahn A, Mosenzon O, Im K, Braunwald E, Bhatt DL. Cardiovascular outcomes of patients in SAVOR-TIMI 53 by baseline hemoglobin A1c. Am J Med. 2016;129:340.e1–340.e8. doi: 10.1016/j.amjmed.2015.09.022. doi: 10.1016/j.amjmed.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 58.Scirica BM, Braunwald E, Raz I, et al. SAVOR-TIMI 53 Steering Committee and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 59.Scott LJ. Sitagliptin: a review in type 2 diabetes. Drugs. 2017;77:209–224. doi: 10.1007/s40265-016-0686-9. doi: 10.1007/s40265-016-0686-9. [DOI] [PubMed] [Google Scholar]
- 60.Sharma A, Green JB, Dunning A, et al. TECOS Study Group. Causes of death in a contemporary cohort of patients with type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care. 2017;40:1763–1770. doi: 10.2337/dc17-1091. doi: 10.2337/dc17-1091. [DOI] [PubMed] [Google Scholar]
- 61.Bethel MA, Engel SS, Green JB, Huang Z, Josse RG, Kaufman KD, Standl E, Suryawanshi S, Van de Werf F, McGuire DK, Peterson ED, Holman RR TECOS Study Group. Assessing the safety of sitagliptin in older participants in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Care. 2017;40:494–501. doi: 10.2337/dc16-1135. doi: 10.2337/dc16-1135. [DOI] [PubMed] [Google Scholar]
- 62.McGuire DK, Van de Werf F, Armstrong PW, et al. Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–135. doi: 10.1001/jamacardio.2016.0103. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 63.Rosenstock J, Perkovic V, Alexander JH, et al. CARMELINA® investigators. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17:39. doi: 10.1186/s12933-018-0682-3. doi: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenstock J, Marx N, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Bluhmki E, Patel S, Johansen OE, Woerle HJ. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10:289–301. doi: 10.1177/1479164112475102. doi: 10.1177/1479164112475102. [DOI] [PubMed] [Google Scholar]
- 65.Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Espeland MA, Bluhmki E, Mattheus M, Ryckaert B, Patel S, Johansen OE, Woerle HJ. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®). Diab Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelmoneim AS, Eurich DT, Light PE, Senior PA, Seubert JM, Makowsky MJ, Simpson SH. Cardiovascular safety of sulphonylureas: over 40 years of continuous controversy without an answer. Diabetes Obes Metab. 2015;17:523–532. doi: 10.1111/dom.12456. doi: 10.1111/dom.12456. [DOI] [PubMed] [Google Scholar]
- 67.McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, Lewsey JD, Krum H VIVIDD Trial Committees and Investigators. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018;6:8–17. doi: 10.1016/j.jchf.2017.08.004. doi: 10.1016/j.jchf.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Williams R, de Vries F, Kothny W, Serban C, Lopez-Leon S, Chu C, Schlienger R. Cardiovascular safety of vildagliptin in patients with type 2 diabetes: a European multi-database, non-interventional post-authorization safety study. Diabetes Obes Metab. 2017;19:1473–1478. doi: 10.1111/dom.12951. doi: 10.1111/dom.12951. [DOI] [PubMed] [Google Scholar]
- 69.Scheen AJ, Paquot N. Gliptin versus a sulphonylurea as add-on to metformin. Lancet. 2012;380:450–452. doi: 10.1016/S0140-6736(12)60859-9. doi: 10.1016/S0140-6736(12)60859-9. [DOI] [PubMed] [Google Scholar]
- 70.Scheen AJ. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med. 2013;125:7–20. doi: 10.3810/pgm.2013.05.2659. doi: 10.3810/pgm.2013.05.2659. [DOI] [PubMed] [Google Scholar]
- 71.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 72.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–216. doi: 10.1111/dom.12591. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crowley MJ, Williams JW, Jr, Kosinski AS, D’Alessio DA, Buse JB. Metformin use may moderate the effect of DPP-4 inhibitors on cardiovascular outcomes. Diabetes Care. 2017;40:1787–1789. doi: 10.2337/dc17-1528. doi: 10.2337/dc17-1528. [DOI] [PubMed] [Google Scholar]
- 74.Scheen AJ. Diabetes: Metformin - a cardiovascular moderator of DPP4 inhibitors? Nat Rev Endocrinol. 2018;14:8–9. doi: 10.1038/nrendo.2017.154. doi: 10.1038/nrendo.2017.154. [DOI] [PubMed] [Google Scholar]
- 75.Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284–286. doi: 10.2337/dc15-1707. doi: 10.2337/dc15-1707. [DOI] [PubMed] [Google Scholar]
- 76.Li L, Li S, Deng K, et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ. 2016;352:i610. doi: 10.1136/bmj.i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ou SM, Chen HT, Kuo SC, Chen TJ, Shih CJ, Chen YT. Dipeptidyl peptidase-4 inhibitors and cardiovascular risks in patients with pre-existing heart failure. Heart. 2017;103:414–420. doi: 10.1136/heartjnl-2016-309687. doi: 10.1136/heartjnl-2016-309687. [DOI] [PubMed] [Google Scholar]
- 78.Kongwatcharapong J, Dilokthornsakul P, Nathisuwan S, Phrommintikul A, Chaiyakunapruk N. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: a meta-analysis of randomized clinical trials. Int J Cardiol. 2016;211:88–95. doi: 10.1016/j.ijcard.2016.02.146. doi: 10.1016/j.ijcard.2016.02.146. [DOI] [PubMed] [Google Scholar]
- 79.Verma S, Goldenberg RM, Bhatt DL, Farkouh ME, Quan A, Teoh H, Connelly KA, Leiter LA, Friedrich JO. Dipeptidyl peptidase-4 inhibitors and the risk of heart failure: a systematic review and meta-analysis. CMAJ Open. 2017;5:E152–E177. doi: 10.9778/cmajo.20160058. doi: 10.9778/cmajo.20160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Standl E, Erbach M, Schnell O. Dipeptidyl-peptidase-4 inhibitors and heart failure: class effect, substance-specific effect, or chance effect? Curr Treat Options Cardiovasc Med. 2014;16:353. doi: 10.1007/s11936-014-0353-y. doi: 10.1007/s11936-014-0353-y. [DOI] [PubMed] [Google Scholar]
- 81.Cebrián-Cuenca AM, Núñez E, Núñez-Villota J, Consuegra-Sánchez L. What would be the fate of the association between saxagliptin and heart failure admission in the SAVOR-TIMI 53 trial if appropriate statistical methods should have been applied? Diabetes Res Clin Pract. 2017;126:320–321. doi: 10.1016/j.diabres.2017.02.029. doi: 10.1016/j.diabres.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 82.Cebrian Cuenca AM, Orozco Beltran D, Navarro Perez J, Alvarez-Guisasola F, Nunez Villota J, Consuegra-Sanchez L. Saxagliptin and heart failure in the SAVOR-TIMI 53 trial: reflections on the Bradford Hill criteria. Rev Esp Cardiol (Engl Ed) 2017;70:1143–1144. doi: 10.1016/j.rec.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Kaneko M, Narukawa M. Assessment of the risk of hospitalization for heart failure with dipeptidyl peptidase-4 inhibitors, saxagliptin, alogliptin, and sitagliptin in patients with type 2 diabetes, using an alternative measure to the hazard ratio. Ann Pharmacother. 2017;51:570–576. doi: 10.1177/1060028017698496. doi: 10.1177/1060028017698496. [DOI] [PubMed] [Google Scholar]
- 84.Pollack PS, Chadwick KD, Smith DM, Billger M, Hirshberg B, Iqbal N, Boulton DW. Nonclinical and clinical pharmacology evidence for cardiovascular safety of saxagliptin. Cardiovasc Diabetol. 2017;16:113. doi: 10.1186/s12933-017-0595-6. doi: 10.1186/s12933-017-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koyani CN, Kolesnik E, Wölkart G, et al. Dipeptidyl peptidase-4 independent cardiac dysfunction links saxagliptin to heart failure. Biochem Pharmacol. 2017;145:64–80. doi: 10.1016/j.bcp.2017.08.021. doi: 10.1016/j.bcp.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 86.Ou HT, Chang KC, Li CY, Wu JS. Comparative cardiovascular risks of dipeptidyl peptidase 4 inhibitors with other second- and third-line antidiabetic drugs in patients with type 2 diabetes. Br J Clin Pharmacol. 2017;83:1556–1570. doi: 10.1111/bcp.13241. doi: 10.1111/bcp.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang F, He Y, Zhang R, Zeng Q, Zhao X. Combination therapy of metformin plus dipeptidyl peptidase-4 inhibitor versus metformin plus sulfonylurea and their association with a decreased risk of cardiovascular disease in type 2 diabetes mellitus patients. Medicine (Baltimore) 2017;96:e7638. doi: 10.1097/MD.0000000000007638. doi: 10.1097/MD.0000000000007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab. 2014;40:S4–S11. doi: 10.1016/S1262-3636(14)72689-8. doi: 10.1016/S1262-3636(14)72689-8. [DOI] [PubMed] [Google Scholar]
- 89.Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691–708. doi: 10.1007/s40262-015-0264-4. doi: 10.1007/s40262-015-0264-4. [DOI] [PubMed] [Google Scholar]
- 90.Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep. 2016;16:92. doi: 10.1007/s11892-016-0789-4. doi: 10.1007/s11892-016-0789-4. [DOI] [PubMed] [Google Scholar]
- 91.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imprialos K, Faselis C, Boutari C, Stavropoulos K, Athyros V, Karagiannis A, Doumas M. SGLT-2 inhibitors and cardiovascular risk in diabetes mellitus: a comprehensive and critical review of the literature. Curr Pharm Des. 2017;23:1510–1521. doi: 10.2174/1381612823666170124123927. doi: 10.2174/1381612823666170124123927. [DOI] [PubMed] [Google Scholar]
- 93.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. doi: 10.1007/s00125-016-4157-3. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai X, Ji L, Chen Y, Yang W, Zhou L, Han X, Zhang S, Ji L. Comparisons of weight changes between sodium-glucose cotransporter 2 inhibitors treatment and glucagon-like peptide-1 analogs treatment in type 2 diabetes patients: a meta-analysis. J Diabetes Investig. 2017;8:510–517. doi: 10.1111/jdi.12625. doi: 10.1111/jdi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6:e004007. doi: 10.1161/JAHA.116.004007. doi: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S, Abbate A, Dixon DL. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005686. doi: 10.1161/JAHA.117.005686. doi: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20:458–462. doi: 10.1111/dom.13101. doi: 10.1111/dom.13101. [DOI] [PubMed] [Google Scholar]
- 99.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bays HE, Sartipy P, Xu J, Sjostrom CD, Underberg JA. Dapagliflozin in patients with type II diabetes mellitus, with and without elevated triglyceride and reduced high-density lipoprotein cholesterol levels. J Clin Lipidol. 2017;11:450 e1–458 e1. doi: 10.1016/j.jacl.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 101.Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, Zhai S, Song Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59:2546–2551. doi: 10.1007/s00125-016-4101-6. doi: 10.1007/s00125-016-4101-6. [DOI] [PubMed] [Google Scholar]
- 102.Filippatos TD, Tsimihodimos V, Liamis G, Elisaf MS. SGLT2 inhibitors-induced electrolyte abnormalities: an analysis of the associated mechanisms. Diabetes Metab Syndr. 2018;12:59–63. doi: 10.1016/j.dsx.2017.08.003. doi: 10.1016/j.dsx.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Budoff MJ, Wilding JPH. Effects of canagliflozin on cardiovascular risk factors in patients with type 2 diabetes mellitus. Int J Clin Pract. 2017;71:e12948. doi: 10.1111/ijcp.12948. doi: 10.1111/ijcp.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deeks ED, Scheen AJ. Canagliflozin: a review in type 2 diabetes. Drugs. 2017;77:1577–1592. doi: 10.1007/s40265-017-0801-6. [DOI] [PubMed] [Google Scholar]
- 105.Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751–759. doi: 10.2215/CJN.10180916. doi: 10.2215/CJN.10180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vettor R, Inzucchi SE, Fioretto P. The cardiovascular benefits of empagliflozin: SGLT2-dependent and -independent effects. Diabetologia. 2017;60:395–398. doi: 10.1007/s00125-016-4194-y. doi: 10.1007/s00125-016-4194-y. [DOI] [PubMed] [Google Scholar]
- 107.Food and Drug Administration: Center for Drug Evaluation and Research. Canagliflozin (Invokana): Summary Review (Application Number: 204042Orig1s000). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000ClinPharmR.pdf. 2013. Accessed April 19, 2018.
- 108.Tang H, Fang Z, Wang T, Cui W, Zhai S, Song Y. Meta-analysis of effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes mellitus. Am J Cardiol. 2016;118:1774–1780. doi: 10.1016/j.amjcard.2016.08.061. doi: 10.1016/j.amjcard.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 109.Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol. 2016;15:37. doi: 10.1186/s12933-016-0356-y. doi: 10.1186/s12933-016-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salsali A, Kim G, Woerle HJ, Broedl UC, Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes Metab. 2016;18:1034–1040. doi: 10.1111/dom.12734. doi: 10.1111/dom.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. doi: 10.1016/S2213-8587(16)00052-8. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 112.Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2017;54:19–36. doi: 10.1007/s00592-016-0892-7. doi: 10.1007/s00592-016-0892-7. [DOI] [PubMed] [Google Scholar]
- 113.Saad M, Mahmoud AN, Elgendy IY, Abuzaid A, Barakat AF, Elgendy AY, Al-Ani M, Mentias A, Nairooz R, Bavry AA, Mukherjee D. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors in patients with type II diabetes mellitus: a meta-analysis of placebo-controlled randomized trials. Int J Cardiol. 2017;228:352–358. doi: 10.1016/j.ijcard.2016.11.181. doi: 10.1016/j.ijcard.2016.11.181. [DOI] [PubMed] [Google Scholar]
- 114.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 115.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME® Trial Investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE EMPA-REG OUTCOME® Trial Investigators. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39:363–370. doi: 10.1093/eurheartj/ehx511. doi: 10.1093/eurheartj/ehx511. [DOI] [PubMed] [Google Scholar]
- 117.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 119.Scheen AJ. Effects of reducing blood pressure on cardiovascular outcomes and mortality in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Res Clin Pract. 2016;121:204–214. doi: 10.1016/j.diabres.2016.09.016. doi: 10.1016/j.diabres.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 120.Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, Mattheus M, Woerle HJ, Broedl UC, Johansen OE, Albers GW, Diener HC EMPA-REG OUTCOME Investigators (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017;48:1218–1225. doi: 10.1161/STROKEAHA.116.015756. doi: 10.1161/STROKEAHA.116.015756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 122.Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR CANVAS Program Collaborative Group. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19:926–935. doi: 10.1111/dom.12924. doi: 10.1111/dom.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raz I, Mosenzon O, Bonaca MP, Cahn A, Kato ET, Silverman MG, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Gause-Nilsson IAM, Langkilde AM, Johansson PA, Sabatine MS, Wiviott SD. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018;20:1102–1110. doi: 10.1111/dom.13217. doi: 10.1111/dom.13217. [DOI] [PubMed] [Google Scholar]
- 124.Avogaro A, Giaccari A, Fioretto P, Genovese S, Purrello F, Giorgino F, Del Prato S. A consensus statement for the clinical use of the renal sodium-glucose co-transporter-2 inhibitor dapagliflozin in patients with type 2 diabetes mellitus. Expert Rev Clin Pharmacol. 2017;10:763–772. doi: 10.1080/17512433.2017.1322507. doi: 10.1080/17512433.2017.1322507. [DOI] [PubMed] [Google Scholar]
- 125.Cinti F, Moffa S, Impronta F, Cefalo CM, Sun VA, Sorice GP, Mezza T, Giaccari A. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2017;11:2905–2919. doi: 10.2147/DDDT.S114932. doi: 10.2147/DDDT.S114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P CVD-REAL Investigators and Study Group*. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kosiborod M, Birkeland KI, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Jørgensen ME, Wittbrodt ET, Thuresson M, Bodegård J, Hammar N, Fenici P CVD-REAL Investigators and Study Group. Rates of myocardial infarction and stroke in patients initiated on SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: results from the CVD-REAL study [published online ahead of print March 22, 2018]. Diabetes Obes Metab. doi: 10.1111/dom.13299. doi: 10.1111/dom.13299. https://onlinelibrary.wiley.com/doi/epdf/10.1111/dom.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, Surmont F, Hammar N, Fenici P CVD-REAL Investigators and Study Group. Lower cardiovascular risk associated with SGLT-2i in >400,000 patients: the CVD-REAL 2 Study [published online ahead of print March 7, 2018]. J Am Coll Cardiol. doi: 10.1016/j.jacc.2018.03.009. https://www.sciencedirect.com/science/article/pii/S0735109718335289?via%3Dihub. [Google Scholar]
- 129.Birkeland KI, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Nyström T, Eriksson JW, Bodegård J, Norhammar A. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709–717. doi: 10.1016/S2213-8587(17)30258-9. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 130.Persson F, Nyström T, Jørgensen ME, Carstensen B, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Eriksson JW, Norhammar A, Bodegard J, Birkeland KI. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20:344–351. doi: 10.1111/dom.13077. doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41:6–10. doi: 10.2337/dc17-1223. doi: 10.2337/dc17-1223. [DOI] [PubMed] [Google Scholar]
- 132.Scheen AJ. Diabetes: time for reconciliation between cardiologists and diabetologists. Nat Rev Cardiol. 2016;13:509–510. doi: 10.1038/nrcardio.2016.113. doi: 10.1038/nrcardio.2016.113. [DOI] [PubMed] [Google Scholar]
- 133.Pham SV, Chilton R. EMPA-REG OUTCOME: the cardiologist’s point of view. Am J Med. 2017;130:S57–S62. doi: 10.1016/j.amjmed.2017.04.006. doi: 10.1016/j.amjmed.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 134.Scheen AJ. Reduction in cardiovascular and all-cause mortality in the EMPA-REG OUTCOME trial: a critical analysis. Diabetes Metab. 2016;42:71–76. doi: 10.1016/j.diabet.2015.12.005. doi: 10.1016/j.diabet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 135.Perreault L. EMPA-REG OUTCOME: the endocrinologist’s point of view. Am J Cardiol. 2017;120:S48–S52. doi: 10.1016/j.amjcard.2017.05.010. doi: 10.1016/j.amjcard.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 136.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? a unifying hypothesis. Diabetes Care. 2016;39:1115–1122. doi: 10.2337/dc16-0542. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 137.McMurray J. EMPA-REG - the “diuretic hypothesis”. J Diabetes Complications. 2016;30:3–4. doi: 10.1016/j.jdiacomp.2015.10.012. doi: 10.1016/j.jdiacomp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 138.Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 2016;42:224–233. doi: 10.1016/j.diabet.2016.05.006. doi: 10.1016/j.diabet.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 139.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obesity Metab. 2018;20:479–487. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 140.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41:356–363. doi: 10.2337/dc17-1096. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 142.Ceriello A, Genovese S, Mannucci E, Gronda E. Understanding EMPA-REG OUTCOME. Lancet Diabetes Endocrinol. 2015;3:929–930. doi: 10.1016/S2213-8587(15)00426-X. doi: 10.1016/S2213-8587(15)00426-X. [DOI] [PubMed] [Google Scholar]
- 143.Ceriello A, Genovese S, Mannucci E, Gronda E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. 2016;15:123. doi: 10.1186/s12933-016-0440-3. doi: 10.1186/s12933-016-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME Trial: a “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 145.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 146.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–1029. doi: 10.1001/jamacardio.2017.2275. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 147.Scheen AJ. SGLT2 inhibition: efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf. 2015;14:1879–1904. doi: 10.1517/14740338.2015.1100167. doi: 10.1517/14740338.2015.1100167. [DOI] [PubMed] [Google Scholar]
- 148.Qiu R, Balis D, Xie J, Davies MJ, Desai M, Meininger G. Longer-term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33:553–562. doi: 10.1080/03007995.2016.1271780. doi: 10.1080/03007995.2016.1271780. [DOI] [PubMed] [Google Scholar]
- 149.Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–628. doi: 10.1111/dom.13124. doi: 10.1111/dom.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017;34:1707–1726. doi: 10.1007/s12325-017-0573-0. doi: 10.1007/s12325-017-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–2852. doi: 10.1210/jc.2015-1884. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–1642. doi: 10.2337/dc15-1380. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 153.Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53–60. doi: 10.1016/j.diabres.2017.04.017. doi: 10.1016/j.diabres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 154.Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376:2300–2302. doi: 10.1056/NEJMc1701990. doi: 10.1056/NEJMc1701990. [DOI] [PubMed] [Google Scholar]
- 155.Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385–1389. doi: 10.1007/s00125-017-4301-8. doi: 10.1007/s00125-017-4301-8. [DOI] [PubMed] [Google Scholar]
- 156.Tanaka A, Node K. Increased amputation risk with canagliflozin treatment: behind the large cardiovascular benefit? Cardiovasc Diabetol. 2017;16:129. doi: 10.1186/s12933-017-0611-x. doi: 10.1186/s12933-017-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fadini GP, Avogaro A. SGTL2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 2017;5:680–681. doi: 10.1016/S2213-8587(17)30257-7. doi: 10.1016/S2213-8587(17)30257-7. [DOI] [PubMed] [Google Scholar]
- 158.Yuan Z, DeFalco FJ, Ryan PB, Schuemie MJ, Stang PE, Berlin JA, Desai M, Rosenthal N. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: a retrospective cohort study. Diabetes Obes Metab. 2018;20:582–589. doi: 10.1111/dom.13115. doi: 10.1111/dom.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:e4–e5. doi: 10.2337/dc17-1551. [DOI] [PubMed] [Google Scholar]
- 160.Verma S, Mazer CD, Al-Omran M, Inzucchi SE, Fitchett D, Hehnke U, George JT, Zinman B. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 161.Blevins TC, Farooki A. Bone effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus. Postgrad Med. 2017;129:159–168. doi: 10.1080/00325481.2017.1256747. doi: 10.1080/00325481.2017.1256747. [DOI] [PubMed] [Google Scholar]
- 162.Scheen AJ. Does lower-limb amputation concern all SGLT-2 inhibitors [published online ahead of print April 6, 2018]? Nature Rev Endocrinol. doi: 10.1038/s41574-018-0001-9. doi: 10.1038/s41574-018-0001-9. [DOI] [PubMed] [Google Scholar]
- 163.Arnold SV, Inzucchi SE, Tang F, McGuire DK, Mehta SN, Maddox TM, Goyal A, Sperling LS, Einhorn D, Wong ND, Khunti K, Lam CS, Kosiborod M. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR(R) Research to Practice project. Eur J Prev Cardiol. 2017;24:1637–1645. doi: 10.1177/2047487317729252. [DOI] [PubMed] [Google Scholar]
- 164.McGovern A, Feher M, Munro N, de Lusignan S. Sodium-glucose co-transporter 2 (SGLT2) inhibitor: comparing trial data and real-world use. Diabetes Ther. 2017;8:365–376. doi: 10.1007/s13300-017-0254-7. doi: 10.1007/s13300-017-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scheen AJ. GLP-1 receptor agonists and heart failure in diabetes. Diabetes Metab. 2017;43(suppl 1):2S13–2S19. doi: 10.1016/S1262-3636(17)30068-X. [DOI] [PubMed] [Google Scholar]
- 167.DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353–1362. doi: 10.1111/dom.12982. doi: 10.1111/dom.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Butler J, Hamo CE, Filippatos G, et al. EMPEROR Trials Program. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19:1390–1400. doi: 10.1002/ejhf.933. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 169.American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40:S1–S135. [Google Scholar]
- 170.Scheen AJ. SGLT2 versus DPP4 inhibitors for type 2 diabetes. Lancet Diabetes Endocrinol. 2013;1:168–170. doi: 10.1016/S2213-8587(13)70095-0. doi: 10.1016/S2213-8587(13)70095-0. [DOI] [PubMed] [Google Scholar]
- 171.Scheen AJ. Precision medicine: the future in diabetes care? Diabetes Res Clin Pract. 2016;117:12–21. doi: 10.1016/j.diabres.2016.04.033. doi: 10.1016/j.diabres.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 172.Ismail-Beigi F, Moghissi E, Kosiborod M, Inzucchi SE. Shifting paradigms in the medical management of type 2 diabetes: reflections on recent cardiovascular outcome trials. J Gen Intern Med. 2017;32:1044–1051. doi: 10.1007/s11606-017-4061-7. doi: 10.1007/s11606-017-4061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ponikowski P, Voors AA, Anker SD, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 174.Scheen AJ. Pharmacotherapy of ‘treatment resistant’ type 2 diabetes. Expert Opin Pharmacother. 2017;18:503–515. doi: 10.1080/14656566.2017.1297424. doi: 10.1080/14656566.2017.1297424. [DOI] [PubMed] [Google Scholar]
- 175.Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016;12:1407–1417. doi: 10.1080/17425255.2016.1215427. doi: 10.1080/17425255.2016.1215427. [DOI] [PubMed] [Google Scholar]
- 176.Dey J. SGLT2 inhibitor/DPP-4 inhibitor combination therapy - complementary mechanisms of action for management of type 2 diabetes mellitus. Postgrad Med. 2017;129:409–420. doi: 10.1080/00325481.2017.1307081. doi: 10.1080/00325481.2017.1307081. [DOI] [PubMed] [Google Scholar]
- 177.Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018;20:740–744. doi: 10.1111/dom.13130. doi: 10.1111/dom.13130. [DOI] [PubMed] [Google Scholar]
- 178.Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 179.Busch RS, Kane MP. Combination SGLT2 inhibitor and GLP-1 receptor agonist therapy: a complementary approach to the treatment of type 2 diabetes. Postgrad Med. 2017;129:686–697. doi: 10.1080/00325481.2017.1342509. doi: 10.1080/00325481.2017.1342509. [DOI] [PubMed] [Google Scholar]
- 180.Nauck MA, Kahle M, Baranov O, Deacon CF, Holst JJ. Addition of a dipeptidyl peptidase-4 inhibitor, sitagliptin, to ongoing therapy with the glucagon-like peptide-1 receptor agonist liraglutide: a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:200–207. doi: 10.1111/dom.12802. doi: 10.1111/dom.12802. [DOI] [PubMed] [Google Scholar]


