Abstract
Background:
Plasma concentrations of the S-enantiomer of citalopram were different between extensive and poor CYP2C19 metabolizers in healthy subjects and depressed patients. However, most studies applied dose-corrected concentrations. Thus, we studied the effects of polymorphisms of the CYP2C19 gene on raw plasma drug concentrations in Japanese patients with depression.
Methods:
Subjects in this study consisted of 412 depressed patients receiving 5, 10, 15, or 20 mg of escitalopram once a day. Plasma concentrations of escitalopram and desmethylescitalopram were quantified using HPLC. CYP2C19 genotypes were identified using polymerase chain reaction methods.
Results:
There were no differences in the steady-state plasma concentrations of escitalopram or desmethylescitalopram in each dose group (5, 10, 15, or 20 mg of escitalopram) among CYP2C19 genotype groups. However, 1-way analysis of variance showed significant effects of CYP2C19 genotypes on the dose-adjusted plasma concentration of escitalopram but not in the dose-adjusted plasma concentration of desmethylescitalopram. Analysis of covariance including age, sex, and body weight showed significant effects of CYP2C19 genotypes on the dose-adjusted plasma concentration of escitalopram and the ratio of desmethylescitalopram to escitalopram.
Conclusions:
These findings suggest that the CYP2C19 variants are associated with steady-state plasma concentrations of escitalopram to some extent but are not associated with desmethylescitalopram.
Key Words: escitalopram, desmethylescitalopram, CYP2C19, depression
INTRODUCTION
Escitalopram is the therapeutically active S-enantiomer of racemic citalopram, a commonly prescribed selective serotonin reuptake inhibitor. Escitalopram and citalopram have been used to treat patients with anxiety and major depressive disorders.1–3 Escitalopram exerts a highly selective, potent, and dose-dependent inhibitory effect on human serotonin transport.1 By inhibiting the reuptake of serotonin into presynaptic nerve endings, it enhances the activity of serotonin in the central nervous system.4 Escitalopram is approved at clinical doses of 10 and 20 mg (5 mg in certain subpopulations or as an initial dose). When taken orally, escitalopram reaches tmax within 5 hours and is 56% bound to protein. Escitalopram reaches steady-state concentrations in the blood within 1–2 weeks.5,6
Escitalopram is primarily metabolized by cytochrome P450 (CYP) 2C19, which is a highly polymorphic enzyme known to cause interindividual differences in pharmacokinetics.5,6 In a previous study, we demonstrated that compared with the extensive metabolizers of mephenytoin after racemic citalopram was given,7 the AUC of the S-enantiomer of citalopram was significantly higher in the poor metabolizers of mephenytoin.
Approximately 15%–25% of the East Asian population consists of poor metabolizers (PM) of S-mephenytoin, whereas the PM frequency in whites is less than 5% (Man et al, 2010).8 Most poor metabolism by CYP2C19 is attributable to the CYP2C19*2 and *3 alleles.9,10 The allele frequencies of the CYP2C19*2 and CYP2C19*3 alleles were 0.30–0.35 and 0.11–0.13 in Asians, respectively, whereas they were 0.15 and 0.001 in whites, respectively.8–10
Although several studies indicate the relationship between the CYP2C19 genotypes and the steady-state plasma concentration of escitalopram in the Asian and white population,11–14 there is still little information about these associations in the Japanese population. In addition, these studies' design was only dose corrected. Therefore, we examined the impact of the CYP2C19 genotypes on the raw (noncorrected) steady-state plasma concentration of escitalopram in depressed Japanese patients at each dose. Furthermore, various factors other than CYP2C19 genotypes such as age, body weight, and sex difference were examined.
MATERIALS AND METHODS
Subjects
The subjects of this study consisted of 412 depressed Japanese outpatients (121 men and 291 women) who fulfilled the criteria for major depressive disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The mean ± SD (range) age and body weight were 43.1 ± 17.3 (19–80) years and 60.3 ± 13.4 (34–191) kg, respectively. Subjects' characteristics at each dose are shown in Table 1. The study was approved by the Ethics Committee of Hirosaki University Hospital, Ehime University Hospital, and Niigata University Hospital. Written informed consent to participate in this study was obtained from all the patients.
TABLE 1.
Characteristics of Patients
Protocol
The subjects received 5, 10, 15, or 20 mg of escitalopram once a day (8–10 pm) for 2–52 weeks. The elimination half-lives of escitalopram and desmethylescitalopram were previously reported to be 26 hours and 20–29 hours, respectively.5 Therefore, plasma concentrations of these compounds already reached steady state in all the subjects before initiating the study. We confirmed adherence from patients. In the case in which the plasma drug concentration of escitalopram could not be detected, the data were excluded from the study (n = 10). We excluded the patients who had potential hepatic/renal dysfunction. The drugs coadministered were zolpidem 5–10 mg/d in 38 cases, brotizolam 0.25 mg/d in 26 cases, flunitrazepam 1–2 mg/d in 12 cases, lorazepam 1–3 mg/d in 19 cases, alprazolam 0.8–2.4 mg/d in 18 cases, and sennoside 24 mg/d in 56 cases.
Assays for Escitalopram and Desmethylescitalopram
Blood samples (5 mL) were obtained 14–16 hours after the last dose of escitalopram. The plasma samples were frozen and maintained at −20°C until analysis. The plasma concentrations of escitalopram and desmethylescitalopram were analyzed in duplicates using high-performance liquid chromatographic (HPLC) methods that were developed in our laboratory.15 In brief, the extraction procedure was as follows: 500 μL of 0.5 M NaOH, 100 μL of internal standard solution (trifluperidol 200 mcg/mL), and 100 μL of methanol were added to 2000 μL of plasma. Tubes were vortexed for 10 seconds, and 5 mL of n-heptane-chloroform (70:30, vol/vol) was added as an extraction solvent. After 10 minutes of shaking, the mixture was centrifuged at 2500g for 10 minutes at 4°C, and the organic phase was evaporated in vacuum conditions at 40°C until dry (TAITEC VC-960; Shimadzu, Kyoto, Japan). The residue was dissolved in 500 μL in the mobile phase, and 400 μL was injected onto the HPLC system. The HPLC system consisted of Shimadzu LC-10AT high-pressure pumps, a Shimadzu CTO-10AVP column oven, a Shimadzu Workstation CLASS-VP chromatography integrator (Kyoto, Japan), a Shimadzu SPD-10AVP (Kyoto, Japan), a Shimadzu SIL-10ADVP (500-μL injection volume) (Tokyo, Japan), and a column (STR-ODS II C18 150 × 4.6, 3 μm) (Shimadzu, Tokyo, Japan). The mobile phase was composed of phosphate buffer (0.02 M, pH = 4.6), acetonitrile, and perchloric acid (60%) (57.25: 42.5: 0.25, vol/vol/vol). The lower limits of detection were 1.0 ng/mL, and the values of the interassay coefficient of variation were less than 8.7% at all concentrations (2.5–150 ng/mL) of the calibration curve for escitalopram and desmethylescitalopram.
Analyses of CYP2C19 Genotypes
Genomic DNA was extracted from 5 mL of peripheral blood using a DNA purification kit [Qiagen (Ed3)]. Genotyping of CYP2C19 was performed using an AmpliChip CYP450 Test DNA tip (Roche Diagnostics). The AmpliChip CYP450 Test provides materials for genotyping 2 cytochrome genes, encompassing 2 mutations in the CYP2C19 gene (CYP2C19*2 and *3). The subjects were allocated into 3 groups based on the number of mutated alleles for CYP2C19: homogygous extensive metabolizers (Homo EM) (*1/*1), heterozygous extensive metabolizers (Hetero EM) (*1/*2 or *1/*3), and PM (*2/*2, *2/*3 or *3/*3).
Data Analyses
The comparison of plasma drug concentrations among CYP2C19 genotypes was performed using 1-way analysis of variance. Analysis of covariance was used for analyses of the effects of age, sex, body weight, and CYP2C19 genotype on plasma drug concentrations. Dummy variables for sex were as follows; male = 1, female = 2. A P value of 0.05 or less was regarded statistically significant. SPSS 22.0 for Windows SPSS, Japan Inc, Tokyo was used for these statistical analyses.
RESULTS
The patients had the following CYP2C19 genotypes: *1/*1 (134 patients), *1/*2 (165), *1/*3 (70), *2/*2 (39), and *2/*3 (14). Patients with *2/*2 and *2/*3 genotypes were regarded as poor metabolizers. Data from 7 patients in which no plasma concentration of escitalopram was found were excluded from the analyses.
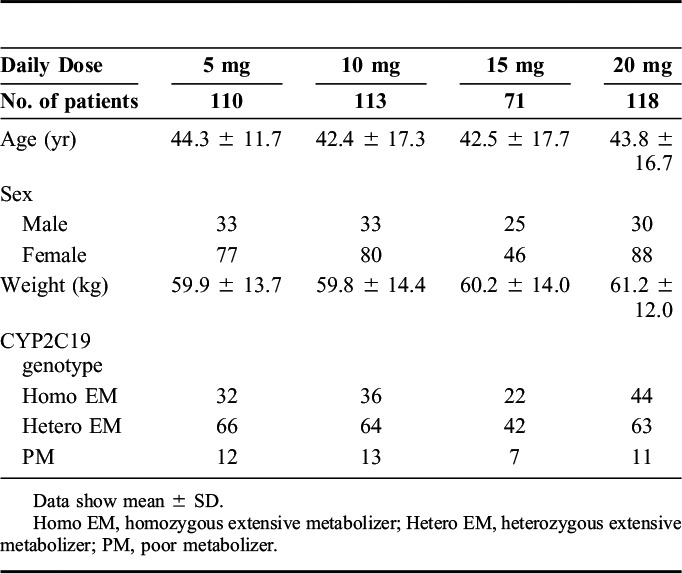
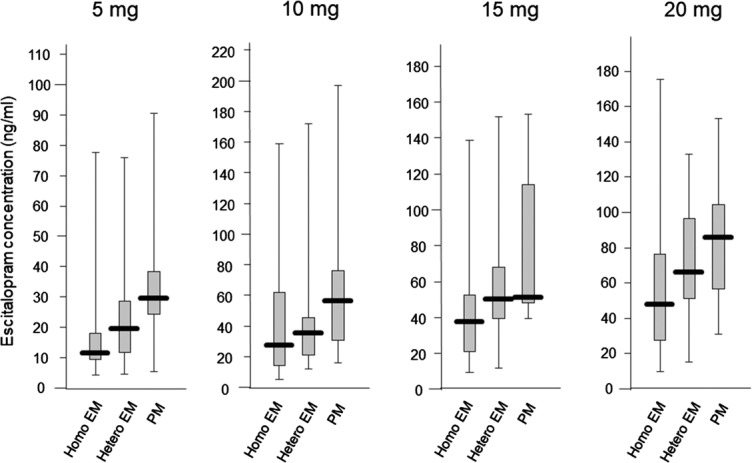
The average (±SD) plasma concentrations of escitalopram in homo EM, hetero EM, and PM were 18.2 ± 18.3, 26.2 ± 28.7, and 35.8 ± 26.4 ng/mL in 5 mg/d, 41.8 ± 40.8, 40.1 ± 31.7, and 66.1 ± 49.4 ng/mL in 10 mg/d, 43.5 ± 33.4, 55.6 ± 30.8, and 79.3 ± 44.2 ng/mL in 15 mg/d, and 61.3 ± 48.5, 68.8 ± 30.8, and 82.9 ± 40.3 ng/mL in 20 mg/d, respectively. Although there was a trend in the increased concentration with the number of mutated alleles at each dose, there were no significant differences in the steady-state plasma concentrations of escitalopram and desmethylescitalopram among the genotype groups at each dosage (Figs. 1, 2).
FIGURE 1.
Influence of CYP2C19 genotypes on the steady-state plasma concentration of escitalopram in each dose with box-and-whisker plots.
FIGURE 2.
Influence of CYP2C19 genotypes on the steady-state plasma concentration of desmethylescitalopram in each dose, with box-and-whisker plots.
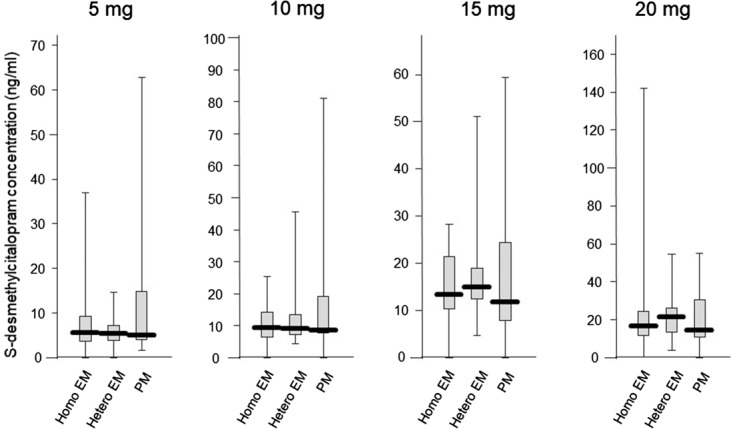
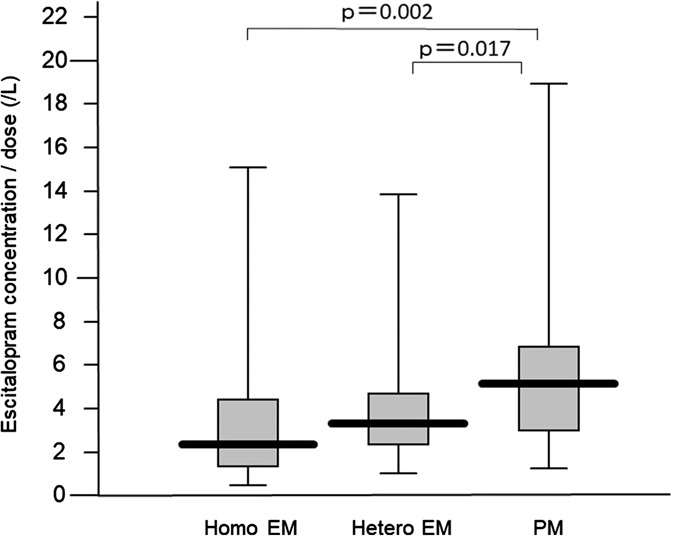
Steady-state plasma concentration was correlated with escitalopram dose (r = 0.415, P < 0.001). The average (±SD) concentrations corrected by a dose of escitalopram, desmethylescitalopram, and desmethylescitalopram/escitalopram in homo EM, hetero EM, and PM were 3.5 ± 3.3, 4.2 ± 3.8, and 6.1 ± 4.3/L for escitalopram, 1.2 ± 1.2, 1.2 ± 0.8, and 1.9 ± 2.5/L for desmethylescitalopram, and 0.55 ± 0.55, 0.35 ± 0.23, and 0.30 ± 0.42/L for desmethylescitalopram/escitalopram, respectively. There was a significant difference among genotype groups in the dose-corrected steady-state plasma concentration and metabolite ratio (Figs. 3, 4).
FIGURE 3.
Influence of CYP2C19 genotypes on the steady-state plasma concentration of escitalopram adjusted by dose, with box-and-whisker plots.
FIGURE 4.
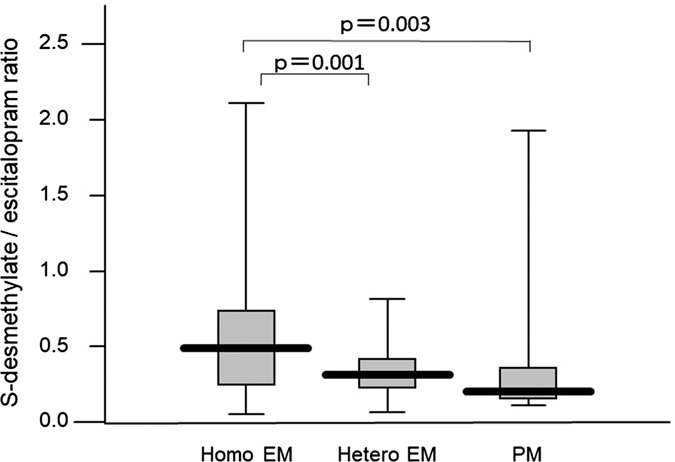
Influence of CYP2C19 genotypes on the steady-state plasma concentration of escitalopram adjusted by dose, with box-and-whisker plots.
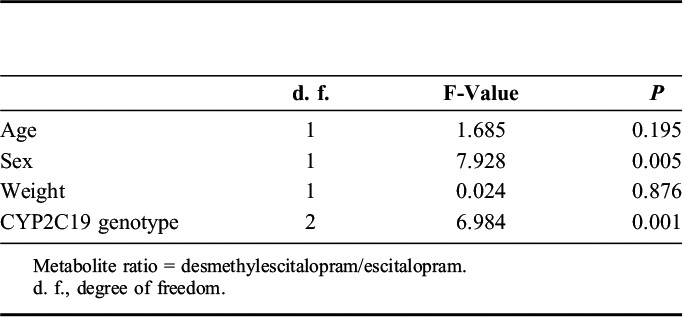
Analysis of covariance showed that CYP2C19 genotypes and age were correlated with the steady-state plasma concentration of escitalopram, whereas CYP2C19 genotypes and sex were correlated with the metabolite ratio (Tables 2 and 3).
TABLE 2.
Influence of Each Factor Including CYP2C19 Genotype on Steady-State Plasma Concentration of Escitalopram Adjusted by Dose
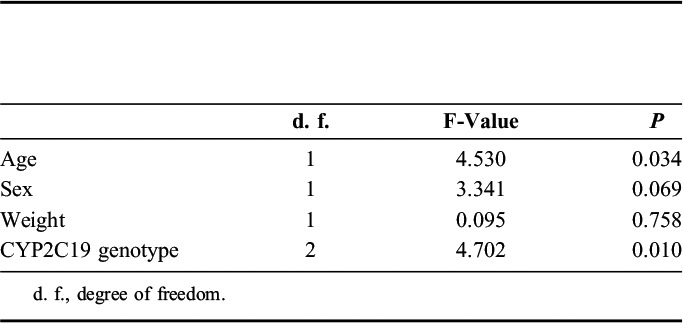
TABLE 3.
Influence of Each Factor Including CYP2C19 Genotype on Metabolite Ratio
DISCUSSION
The results of this study suggested that there was a significant correlation between the number of mutated alleles for CYP2C19 and the steady-state plasma concentration of escitalopram corrected by dose. However, no significant difference was found in the steady-state plasma concentration of escitalopram in the patients receiving 5, 10, 15, or 20 mg of escitalopram, probably because of the large variability in steady-state plasma concentrations of escitalopram. This finding is in accordance with previous studies.11–14 Therefore, CYP2C19 genotypes have an impact, to some extent, on the steady-state plasma concentration of escitalopram in depressed patients.
A recent meta-analysis showed that compared with subjects with the homo EM, the exposure to (es) citalopram increased by 95% (95% CI 40–149, P < 0.0001) in the PM and 30% (95% CI 4–55, P < 0.05) in the hetero EM.16 Our study indicated a 73% increase in the PM and a 20% increase in the hetero EM, which was shown as a weaker effect of CYP2C19. However, another study reported 276%–230% in PM and 97%–78% in hetero EM in 541 patients, showing a much stronger effect of CYP2C19.14 In the meta-analysis, 800 patients were included, whereas the number of subjects in this study and that in the study by Waade et al14 were over 400 and 500, respectively. Therefore, repeated analyses together with additional studies should be conducted to calculate the true effect.
Contrary to our prediction, compared between CYP2C19 genotypes, no differences were found at each dose. These findings may be explained by the large interindividual variability in the plasma concentrations of escitalopram. An in vitro study showed that not only CYP2C19 but also CYP3A4 is involved in the N-demethylation of escitalopram.17 It has been shown that there is a great interindividual variation in the activity of CYP3A4.18 Therefore, the variability in CYP3A4 possibly influenced the variability in plasma concentrations of escitalopram. In addition, deamination and dehydrogenation to a propionic acid derivative, N-oxidation, and glucuronide conjugations other than N-demethylation were confirmed.19,20 These minor metabolic pathways could confound the results and might have been activated after repeated doses. Finally, a steady-state plasma concentration in repeated doses was influenced by various factors, such as compliance, drugdrug and/or drugfood interaction, and renal and hepatic impairment.
A significant correlation was found between the steady-state plasma concentration of escitalopram and age. This is a novel finding, but it is not surprising because renal function tends to decrease with advanced age. A previous study showed that in patients with mild to moderate renal impairment [creatinine clearance (CLCR) 10–53 mL/min], the t1/2 of a single dose of citalopram increased by approximately 35%, with oral and renal clearance being approximately 15% and 40% lower than that in healthy volunteers, respectively.6 Therefore, careful dose setting and possibly therapeutic drug monitoring are needed not only in CYP2C19 PM but also in elderly patients. However, Waade et al14 showed that for CYP2C19 PMs, compared with those <40 years, a 1.4-fold higher mean concentration/dose ratio of escitalopram was observed in patients >65 years, but the difference was not statistically significant (P = 0.1). In the other CYP2C19 phenotype subgroups, similar degrees of differences in mean concentration/dose ratios of escitalopram were found to be significant among patients aged >65 versus <40 years (P < 0.02).14
Furthermore, in this study, the metabolite ratio was significantly associated with CYP2C19 but also with sex. Waade et al14 showed that metabolic ratios in women were 15% lower than those in men. Although we do not have a clear explanation for the sex difference, the different CYP3A4 activity between the sexes may be attributable to our findings because CYP3A4 is a major enzyme catalyzing N-demethylation of escitalopram.21
There are several limitations to our study. First, our sample had a small number of patients with PM because genotypes were retrospectively identified, which has some methodological weaknesses. These issues include uncertain or unconfirmed information regarding partial adherence and the concurrent use of potentially interacting drugs or herbal agents. Second, we did not detect CYP2C19*17, which may be contributing to steady-state plasma concentrations of escitalopram22,23 because it has been shown to be very rare (0.3%–1.3%) in Japanese populations.24–26
CONCLUSIONS
The present study suggests that CYP2C19 genotypes and age are determinants affecting dose-adjusted escitalopram concentrations. Particularly careful administration is necessary in elderly CYP2C19 PM patients.
ACKNOWLEDGMENTS
The authors thank all their coworkers who participated in this study for their skillful contributions in collecting and managing the data.
Footnotes
Supported by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Research (JSPS, 15K19239, 15K19710, and 15H04754) and a grant from the Hirosaki Research Institute for Neurosciences.
N. Yasui-Furukori has received grant/research support or honoraria from and has been on the panels of Dainippon, Mochida, MSD. The remaining authors declare no conflict of interest.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs. 2010;24:769–796. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin D, Woods R, Lawson R, et al. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199. [DOI] [PubMed] [Google Scholar]
- 3.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. [DOI] [PubMed] [Google Scholar]
- 4.Kirino E. Escitalopram for the management of major depressive disorder: a review of its efficacy, safety, and patient acceptability. Patient Prefer Adherence. 2012;6:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastoor D, Gobburu J. Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin Drug Metab Toxicol. 2014;10:121–128. [DOI] [PubMed] [Google Scholar]
- 6.Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46:281–290. [DOI] [PubMed] [Google Scholar]
- 7.Herrlin K, Yasui-Furukori N, Tybring G, et al. Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy Swedes. Br J Clin Pharmacol. 2003;56:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man M, Farmen M, Dumaual C, et al. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major east Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929–940. [DOI] [PubMed] [Google Scholar]
- 9.Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27:9–54. [DOI] [PubMed] [Google Scholar]
- 10.Ota T, Kamada Y, Hayashida M, et al. Combination analysis in genetic polymorphisms of drug-metabolizing enzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A5 in the Japanese population. Int J Med Sci. 2015;12:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudberg I, Hendset M, Uthus LH, et al. Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram). Ther Drug Monit. 2006;28:102–105. [DOI] [PubMed] [Google Scholar]
- 12.Noehr-Jensen L, Zwisler ST, Larsen F, et al. Impact of CYP2C19 phenotypes on escitalopram metabolism and an evaluation of pupillometry as a serotonergic biomarker. Eur J Clin Pharmacol. 2009;65:887–894. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MH, Lin KM, Hsiao MC, et al. Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics. 2010;11:537–546. [DOI] [PubMed] [Google Scholar]
- 14.Waade RB, Hermann M, Moe HL, et al. Impact of age on serum concentrations of venlafaxine and escitalopram in different CYP2D6 and CYP2C19 genotype subgroups. Eur J Clin Pharmacol. 2014;70:933–940. [DOI] [PubMed] [Google Scholar]
- 15.Yasui-Furukori N, Tsuchimine S, Kubo K, et al. The effects of fluvoxamine on the steady-state plasma concentrations of escitalopram and desmethylescitalopram in depressed Japanese patients. Ther Drug Monit. 2016;38:483–486. [DOI] [PubMed] [Google Scholar]
- 16.Chang M, Tybring G, Dahl ML, et al. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: a systematic review and meta-analysis. Clin Pharmacokinet. 2014;53:801–811. [DOI] [PubMed] [Google Scholar]
- 17.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- 18.Lamba JK, Lin YS, Schuetz EG, et al. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. [DOI] [PubMed] [Google Scholar]
- 19.Oyehaug E, Ostensen ET, Salvesen B. High-performance liquid chromatographic determination of citalopram and four of its metabolites in plasma and urine samples from psychiatric patients. J Chromatogr. 1984;308:199–208. [PubMed] [Google Scholar]
- 20.Rochat B, Amey M, Van Gelderen H, et al. Determination of the enantiomers of citalopram, its demethylated and propionic acid metabolites in human plasma by chiral HPLC. Chirality. 1995;7:389–395. [DOI] [PubMed] [Google Scholar]
- 21.Diczfalusy U, Miura J, Roh HK, et al. 4β-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics. 2008;18:201–208. [DOI] [PubMed] [Google Scholar]
- 22.Rosenborg SO, Mwinyi J, Andersson M, et al. Kinetics of omeprazole and escitalopram in relation to the CYP2C19*17 allele in healthy subjects. Eur J Clin Pharmacol. 2008;64:1175–1179. [DOI] [PubMed] [Google Scholar]
- 23.Rudberg I, Mohebi B, Hermann M, et al. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83:322–327. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto K, Uno T, Yamazaki H, et al. Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br J Clin Pharmacol. 2008;65:437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Lei HP, Li Z, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65:281–285. [DOI] [PubMed] [Google Scholar]
- 26.Ramsjö M, Aklillu E, Bohman L, et al. CYP2C19 activity comparison between Swedes and Koreans: effect of genotype, sex, oral contraceptive use, and smoking. Eur J Clin Pharmacol. 2010;66:871–877. [DOI] [PubMed] [Google Scholar]


