Abstract
Maternal separation (MS) plays a central role in developing physiology and psychology during the individual ontogeny process. MS is used to research the neurobiological mechanisms of mental disorders and early life stress. In this study, we investigated the effects of repeated MS and early handling (EH) on locomotor activity in an open-field test, a light–dark box test and an elevated plus-maze test of adolescent rats. The results showed that MS reduced locomotor activities in the open-field test, and increased anxiety-like behaviours in the light–dark box test and the elevated plus-maze test in adolescent rats. These tests indicated that early life stress caused by MS might induce anxiety-like behaviours during adolescence. However, compared with the control group, both the MS and EH groups showed conflicting anxiety levels. The results also suggested that females were more prone to showing anxiety-like behaviour compared with males when suffering from high-intensity stimulation. However, because of the low anxiety level associated with EH, the sex difference in behaviour was not significant. The present study provides novel insights into the effects of MS and EH on behaviour, which shows unique anxiety levels different in adolescent male and female rats.
Keywords: anxiety, early handling, locomotor activity, maternal separation
Introduction
Early life stress is one of the risk factors for psychiatric disorders 1. Growing evidence shows that stress during early life not only leads to lasting structure brain changes and functional deficits but also increases the risk of mental disorders in youth and adulthood 2.
Early social and environmental deprivation includes early handling (EH) and maternal separation (MS) 3. EH is defined as the separation of rat pups from their mother for 3–15 min each day. This short-time separation between mothers and pups simulated mothers leaving to find food, not wanting to deprive her pups 4. MS indicates that mothers and their pups need to be separated for long time periods (≥1 h), which leads to separation anxiety and anxiety-like behaviours 5.
MS is often used to model early life stress in rats 6 and has led to abnormal behaviours, such as increase in locomotor activity in an open-field test (OFT) 7, anxiety-related behaviours in an elevated plus-maze (EPM), prepulse inhibition deficits and impaired spatial learning in the Morris water-maze test 8–12. Similarly, stress responses related to the hypothalamic–pituitary–adrenal (HPA) axis were reported in previous studies 13. Nevertheless, MS anxiety-like behaviours were inconsistent in previous studies examining separation frequency and duration tests, where further research is needed.
The key stage of the HPA axis was found to be 3−14 days postpartum, which was also shown to be the stress hyporesponsive period 14. Numerous studies have focused on abnormal structural brain abilities, associated with MS, which include the amygdala, hippocampus, medial prefrontal cortex and nucleus accumbens. The hippocampus is believed to play a central role in learning and memorizing 15, similar to its regulation of the HPA axis 16. The decreased number of neuronal dendritic branches in the hippocampus was related to an increase in glucocorticoid levels and morphological changes in hippocampal neurons that could affect synaptic inputs and anxiety pathways 17,18. Studies have confirmed that MS caused the release of glucocorticoids, an end product of the HPA axis, which affects the behaviour of rats 19. Thus, these studies provide a certain basis for separation frequency.
The repeated effects of MS on the anxiety level of rodents were explored in a dynamic and developmental view. An EH procedure was implemented to contrast to an MS procedure. We investigated behavioural changes in adolescent rats after performing repeated MS [3 h/day from postnatal day (PND) 1 to 21] and EH (15 min/day from PND 1 to 21) studies to clarify the influence of different duration and separation frequencies. In a previous study, frequent separation led to aberrant individual behaviour and neural development 9. This study also proved that long separation could lead to significantly abnormal behaviour 20. These studies indicated that the duration and frequency of separation are a matter of concern. The current study was designed to evaluate the varying degrees of anxiety caused by MS or EH.
Materials and methods
Experimental animals and maternal separation
Male and female adult Wistar rats were bred by the Animal breeding centre of the Weifang Medical University and were treated under controlled environmental conditions (ambient temperature 22°C, 12 h light/dark cycles, lights on at 8:00 a.m.). The rats received food and water ad libitum, and experiments followed the guidelines of the Beijing laboratory animal centre and the national institutes of health guide for the care and use of laboratory animals (NIH Publications No. 80-23).
The rats were mated and gave birth to an average of 8–12 pups in one nest. Both dams and pups were used in the experiment. The date of the pups’ births was designated as PND 1. On PND 1, all pups in one nest were assigned randomly to the MS group (n=22, eight males and 14 females), the EH group (n=14, six males and eight females) and the control (Con) group (n=22, eight males, and 14 females). According to the previous methods, in the MS group, the dams were separated from the pups for three consecutive hours (from 8:00 to 11:00) every day. The EH group was similar to the MS group, but the separation was only for 15 min (from 8:00 to 08:15) every day. These experiments were conducted from PND 1 to 21. The experimental manipulation of the EH group is associated with increased maternal care 21, and reduced behavioural and endocrine stress reactivity in the pups 16. On PND 21, pups were weaned, and after PND 42, behavioural tests in pubescent rats were performed in the following order: light–dark box (LDB), OFT and EPM.
The light–dark box
The LDB test started on PND 42. Pups were placed in a dark compartment (25 cm×25 cm×25 cm) of a dual chamber apparatus and were allowed to freely explore both compartments for 5 min as described previously 22. The time that rats spent in the light compartment (25 cm×25 cm×25 cm) and the rate at which they entered the light compartment were analysed using SPSS (version 18.0; SPSS Inc., Chicago, Illinois, USA). The apparatus was cleaned with 90% ethanol between each test.
The open-field test
On PND 45, the OFT evaluated movements and anxiety-related behaviours of the rats to assess response to separation anxiety 23. The open-field apparatus (80 cm×80 cm×50 cm) was illuminated by an intensity of 80 lx. Every pup was placed in the centre of the open field, and the number of lines crossed (all four limbs) and the number of times the central zone of the apparatus was entered were recorded for 5 min. Between each test, the field was cleaned with 90% ethanol to remove olfactory cues. The field was divided into 25 equal-sized squares including one central zone and 24 squares corresponding to the peripheral zones using maze software (SMART; Panlab, SL, Barcelona, Spain).
The elevated plus-maze
On PND 49, all pups were evaluated using the EPM test, which was illuminated using 80 lx illumination. The following parameters were obtained from each pup: the number of times that they entered the open arm of the maze, the number of times that they entered the closed arm of the maze and the time that they spent in the open arm of the maze. Two anxiety indices were determined in the experiments: (i) the number of times the rats entered the open arm of the maze expressed as a percentage of the total number of entries and (ii) the amount of time spent in the open arm of the maze expressed as a percentage of the total time 24. The maze was cleaned with 90% ethanol after each test to ensure that the environment was free of olfactory cues. The basic design (time course) of this experiment is shown in Fig. 1.
Fig. 1.
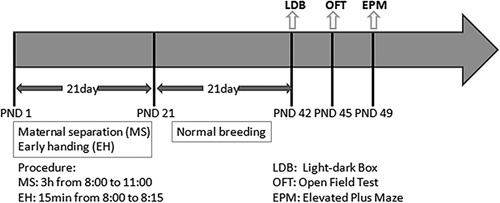
The basic time course of the study is presented. EH, early handling; EPM, elevated plus-maze; LDB, light–dark box; MS, maternal separation; OFT, open-field test; PND, postnatal day.
Statistical analysis
All data were shown as ±SD of the mean. The analyses were carried out using SPSS 18 software. All behaviour test data were analysed using univariate analysis of variance. We compared two and three data groups and analysed the data using one-way analysis of variance, followed by least significant difference post-hoc tests. Significance was defined as P less than 0.05.
Results
Anxiety-like behaviours in the light–dark box
In the LDB, the time that rats spent in the light box is shown in Fig. 2a. A significant effect because of early life conditions [F (2, 58)=120.204; P<0.01] was found, but the sex of the rats did not affect the test results significantly [F (1, 58)=1.972; P=0.166]. No significant interactions were also found between early life conditions and the sex of the rats [F (2, 58)=0.497; P=0.611]. Post-hoc analysis showed that the MS and EH group rats spent much less time in the light box than the Con group rats. In addition, a significant difference was found between male and female rats in the MS group [F (1, 21)=4.643; P<0.05].
Fig. 2.
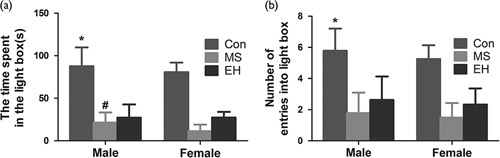
The effects of each group on behaviour using the light–dark box test include the time spent in the light compartment (a) and the number of entries into the light compartment (b). Results are expressed as mean±SDM (*Comparison among groups, P<0.05; #Intragroup comparison, P<0.05). Con, control; EH, early handling; MS, maternal separation.
The number of rats that entered into the light box is summarized in Fig. 2b. These results indicate that a significant effect was found in the early life condition [F (2, 58)=52.793; P<0.01], but no significant difference was found between male and female rats [F (1, 58)=1.156; P=0.287]. Similarly, no significant interaction was found between the early life condition and the sex of the rats [F (2, 58)=0.062; P=0.940]. In the same group, the sex of the rats did not affect the times that the rats entered the light compartment in LDB and the post-hoc analysis showed that the number of entries into the light box of the Con rats was more significant than both the MS and EH group rats.
Locomotor activity in the open-field test
The number of lines crossed in the open field is summarized in Fig. 3a. The OFT data found significant results in the early life condition [F (2, 58)=34.607; P<0.01] and the sex of rats also significantly affected the results [F (1, 58)=5.792; P<0.05]. A significant interaction between the early life condition and the sex of the rats was found in the number of lines crossed [F (2, 58)=3.195; P<0.05]. In terms of data of each group, post-hoc (least significant difference) comparisons showed that the number of lines that the MS rats crossed was significantly larger than that of the Con and the EH rats. At the same time, significant differences were observed between male and female MS rats [F (1, 21)=9.302; P<0.01].
Fig. 3.
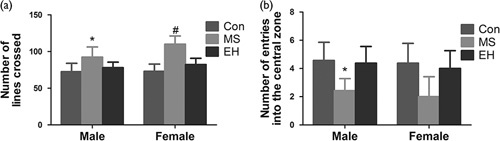
The effects of each group on behaviour in the open-field test include the number of lines crossed (a) and the number of entries into the central zone (b). Results are expressed as the mean±SDM (*Comparison among groups, P<0.05; #Intragroup comparison, P<0.05). Con, control; EH, early handling; MS, maternal separation.
The number of entries into the central zone in the open field is summarized in Fig. 3b. A significant effect was induced by the early life condition [F (2, 58)=20.112, P<0.01], but the sex of the rats did not affect the results [F (1, 58)=0.992, P=0.324]. Therefore, the interactions between the groups and the sexes within the groups were not significant [F (2, 58)=0.050, P=0.951].
Fear behaviour in the elevated plus-maze test
For the EPM test, the time that the rats spent in the open arm of the maze is summarized in Fig. 4a. A significant effect of the early life condition was found [F (2, 58)=24.667; P<0.01], but no differences were found between male and female rats [F (1, 58)=1.972; P=0.166]. The interaction between the early life condition and the sex of the rats was also not significant [F (2, 58)=0.122, P=0.885]. Post-hoc analysis showed that the rats in both the MS and EH groups spent significantly less time in the open arm of the maze.
Fig. 4.
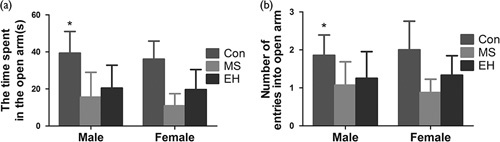
The effects of each group on behaviour in the elevated plus-maze test include the amount of time spent in the open arm of the maze (a) and the number of entries into the open arm of the maze (b). Results are expressed as the mean±SDM (*Comparison among groups, P<0.05; #Intragroup comparison, P<0.05). Con, control; EH, early handling; MS, maternal separation.
In Fig. 4b, the number of entries into the open arm of the elevated plus-maze is similar to the time spent in the open arm of the maze. A significant effect of the early life condition [F (2, 58)=13.535; *P<0.01] was found, whereas no significance was associated with the sex of the rats [F (2, 58)=0.004; P=0.952] or in the interaction between early life condition and the sex of the rats [F (2, 58)=0.459; P=0.634]. However, post-hoc analysis showed that the number of entries into the open arm of the maze by the Con rats was significantly greater than that of both the MS and the EH group rats.
Discussion
For early life stress, MS plays an important role in physiological and psychological development during individual ontogeny, which increases the risk of mental disorders in each life stage 3,6. Adolescence is the last stage of mature brain development. Thus, this study chose to examine adolescent rats to determine the different effects of early life on EH and MS in rats. Periodic MS had a clear impact on abnormal behaviour in adolescence pups. At the same time, pups that suffered from EH deficits also showed a disparate adolescence behavioural expression in the behaviour tests.
In the LDB test, both the MS and EH group rats spent less time and entered the light compartment of the LDB fewer times than the Con group rats, which indicated that both the EH and MS group rats had enhanced anxiety-like behaviours and decreased exploratory behaviour on the LDB test, which was consistent with several previous studies documenting adverse effects 12,24. Nevertheless, there was no significant difference between the EH and MS group rats, although the EH group rats spent more time in the light compartment compared with the MS group rats. The lack of changes present in the LDB test creates uncertainty about what impact the difference in exploratory behaviour has between the EH and the MS. However, in the MS group, female rats spent significantly less time in the light compartment, which suggests that periodic long MS might affect females more than males, but because of the lower anxiety level in the EH group compared with the MS group, the difference in behaviour between male and female rats was not significant.
The OFT data indicate that repeated MS (one daily period of 3 h from PND 1 to 21) increased spontaneous locomotor activity in pubescent rats. At the same time, female rats showed significantly higher movement compared with male rats in the MS group. However, there was no significant difference in locomotor activity in the OFT between the EH group compared with the Con group. Together, these results suggest that female rats are more susceptible to MS than male rats, but that neither female nor male rats were significantly impacted by EH. Similarly, Qiong et al. 25 reported that MS in rats (4 h from PND 1 to 21) resulted in a significant increase in locomotor activity in the OFT. Anderson and Teicher 26 also found that MS (3 h from PND 1 to 14) significantly increased locomotor activity in adult rats. However, Rana et al. 27 indicated that after repeated MS (3 h from PND 1 to 14), the Wister adult rats had significantly lower locomotor activity compared with rats presented with decreased MS (15 min from PND 1 to 14). Chang et al. 28 reported a similar conclusion to that of the study by Rana. We previously suggested that because individuals express anxiety through behaviour differently, any abnormal behaviour, whether expressed through increased or decreased locomotor activity, signifies increased anxiety 29.
Although there was no significant difference in locomotor activity, Markostamou et al. 30 showed that the EH group (15 min from PND 1 to 6) spent less time in the centre of the open field compared with the NMS or MS (3 h from PND 1 to 6) group. Also, in the study by Qiong et al. 25, the MS group pups spent them the most time in the central area during puberty, but did not as adult rats. However, in our present study, the results showed that there was no significant difference between the EH and the Con group and that the number of times that the MS group rats entered into the central zone was less than that in the other two groups. Different behavioural phenotypes have been reported depending on the various MS protocols used. Thus, in the OFT, we suggested that EH enhances the anxiety-like behaviour of pubescent rats, but does not increase locomotor behaviour. High anxiety levels caused by MS increased the locomotor activity in rats in the open-field region and avoidance behaviour in the central region. In addition, the negative effect of MS on female rats was more evident than that in male rats.
In the present study, anxiety-like behavioural data in the EPM test showed that both the EH group and the MS group showed enhanced anxiety-like behaviour compared with the Con group, especially in pubescent rats in the MS group. Although the MS group rats spent less time in the open arm of the maze, no significant differences were found between the MS group and the EH group. Also, the sex of the rats did not affect the result. In previous studies, the influence of MS on rats was similar. Shreya et al. 31 reported that early MS (3 h from PND 2 to 14) enhanced the fear behaviour of adult rats in the EPM test. Similarly, Markostamou et al. 30 found that MS for 3 h each day from PND 1 to 6 enhanced fear behaviour in adult rats. Trujillo reported a similar result that MS (4.5 h from PND 1 to 21) produced a greater fear level during the EPM test in juvenile rats 32.
However, similar to the separation of dams and pups for 15 min/day from PND 1 to 21 in this study, the influences of EH separation were different from that of other types of separation. No fear was observed in adult rats 20. Furthermore, the research of Oines et al. 32 (EH 10 min from PND 2 to 14) reported results similar to those of Markostamou and colleagues. In this study, we found that the reason for the differences in the test results was because adolescence is a critical interval in life. Adolescence is particularly susceptible to the onset of specific neuropsychiatric diseases 26. Repeated EH throughout lactation affected the anxiety-like behaviour of adolescence rats. Nevertheless, when the rats entered the juvenile stages of development and because of differences in individual development, this impact disappeared. This phenomenon may possibly be associated with the release of brain-derived neurotrophic factor (BDNF) after stress. BDNF, upon release into the brain, is distributed widely throughout the central nervous and peripheral nervous systems 30,33. BDNF can adjust the division, differentiation and maturity of nervous system growth 34. After suffering chronic stress, BDNF levels in different brain regions can change to offset the impact of neurons 35. Future studies will explore changes in the brain that are associated with BDNF after stressful events occur and with the developmental stages, and will focus on changes in brain processing in all areas of the brain.
It is interesting that after comparing the results of each behaviour test, we found that the impact from EH on individual behaviour was specific. The results in both the LDB and the EPM tests reported that EH has a significant impact on rats. This situation was similar to that of the MS group rats. Therefore, we suggested that in both the LDB and the EPM tests, young rats in the EH and MS groups responded robustly to new environments, indicating an increase in anxiety levels. However, in the OFT, because of the anxiety-level differences, the MS group rats showed significant abnormal behaviour, but the EH group rats did not 36,37. Thus, during puberty, repeated EH deficits caused the rats to respond robustly in the LDB and EPM tests, which indicated that EH deficits could increase anxiety levels in a short period, which was not as severe as that observed in the MS group.
To summarize, the different effects of MS and EH on anxiety in the present study could be induced by differences in the duration and frequency of separation. However, it is certain that high-frequency EH has a negative influence on pubescent rats. In addition, across laboratories, not only do the frequency and duration of MS periods vary considerably but also the time points at which the manipulation is performed during the preweaning period 4. Therefore, on the one hand, we can further refine the experimental design. The interaction between the duration, frequency and time point of separation at different ages is interesting, and clearly worthy of further investigation to discover more specific explanations. However, MS, as an animal model that induces anxiogenic state, is used in combination with other models to simulate multiple stressful life events. On the basis of these approaches, the effects of these multiple stressors on behaviours, HPA-axis activity and biochemical changes in the central nervous system can be investigated, which could provide more insight into the relevant mechanisms underlying the effects of early adversity on subsequent vulnerability to stressors.
Conclusion
This study found that repeated MS induced enhanced locomotor activity in the OFT and abated exploratory behaviour of pubescent rats in both the LDB and EPM tests. EH abated the exploratory behaviour of pubescent rats in the EPM test. These results indicated that MS significantly increased anxiety levels in individuals and that EH enhanced anxiety levels slightly, which was expressed by the abnormal behaviour of fear memory. At the same time, female rats were more prone to showing anxiety-like behaviour compared with male rats in the presence of high-intensity stimulation. Further research is needed to study the interaction between the duration and the frequency of separation at different ages.
Acknowledgements
This work was supported by the Research Award Foundation Programme for Outstanding Young Scientists in the Shandong Province (BS2014YY043), the Education teaching reform and research foundation of Weifang Medical University (2015Y024), the Science and Technology Foundation Programme of colleges and universities of the Shandong Province (J17KB096). The content of the article is solely the responsibility of the authors and does not represent the official views of the funding agencies and institutes.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Shengtao Jin and Yanan Zhao contributed equally to the writing of this article.
References
- 1.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioural consequences. Crit Rev Neurobiol 1998; 12:129–162. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012; 62:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 1997; 277:1659–1662. [DOI] [PubMed] [Google Scholar]
- 4.Weiss IC, Domeney AM, Moreau JL, Russig H, Feldon J. Dissociation between the effects of pre-weaning and/or post-weaning social isolation on prepulse inhibition and latent inhibition in adult Sprague–Dawley rats. Behav Brain Res 2001; 121:207–218. [DOI] [PubMed] [Google Scholar]
- 5.Paternain L, Martisova E, Campión J, Martínez JA, Ramírez MJ, Milagro FI. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav Brain Res 2016; 299:51–58. [DOI] [PubMed] [Google Scholar]
- 6.Moloney RD, Stilling RM, Dinan TG, Cryan JF. Early-life stress-induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterol Motil 2015; 27:1831–1836. [DOI] [PubMed] [Google Scholar]
- 7.Bouet V, Lecrux B, Tran G, Freret T. Effect of pre- versus post-weaning environmental disturbances on social behaviour in mice. Neurosci Lett 2011; 488:221–224. [DOI] [PubMed] [Google Scholar]
- 8.Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res 1998; 30:251–260. [DOI] [PubMed] [Google Scholar]
- 9.Garner B, Wood SJ, Pantelis C, van den Buuse M. Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: no further effect of post-pubertal chronic corticosterone treatment. Behav Brain Res 2007; 176:323–332. [DOI] [PubMed] [Google Scholar]
- 10.Xiongzhao Z, Ting L, Sufang P, Xiuling M, Xiaogang C, Xiuwu Z. Maternal deprivation-caused behavioral abnormalities in adult rats relate to a non-methylation-regulated D2 receptor levels in the nucleus accumbens. Behav Brain Res 2010; 209:281–288. [DOI] [PubMed] [Google Scholar]
- 11.Jiaojie H, Zhijun Z, Shanshan L, Guangjun X, Xiangrong Z, GaoJun T. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: a magnetic resonance study. Behav Brain Res 2011; 217:122–127. [DOI] [PubMed] [Google Scholar]
- 12.Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S, et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS ONE 2014; 9:e91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 1993; 18:195–200. [DOI] [PubMed] [Google Scholar]
- 14.de Kloet ER, Rots NY, van den Berg DT, Oitzl MS. Brain mineralocorticoid receptor function. Ann N Y Acad Sci 1994; 746:8–20. (Discussion 20–21, 64–27). [PubMed] [Google Scholar]
- 15.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol 2010; 25:237–258. [DOI] [PubMed] [Google Scholar]
- 16.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 2005; 30:939–946. [DOI] [PubMed] [Google Scholar]
- 17.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 1995; 69:83–88. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Hernández VS, Liu B, Medina MP, Nava-Kopp AT, Irles C, et al. Hypothalamic vasopressin system regulation by maternal separation: its impact on anxiety in rats. Neuroscience 2012; 215:135–148. [DOI] [PubMed] [Google Scholar]
- 19.Guohua W, Yufang C, Meifang G, Baofang L, Mingzi Z, Yupin C. Systematic correlation between spine plasticity and the anxiety/depression-like phenotype induced by corticosterone in mice. Neuroreport 2013; 24:682–687. [DOI] [PubMed] [Google Scholar]
- 20.Szymkowicz SM, Finnegan N, Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J Affect Disord 2013; 147:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macrì S, Chiarotti F, Würbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav Brain Res 2008; 191:227–234. [DOI] [PubMed] [Google Scholar]
- 22.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013; 70:27–34. [DOI] [PubMed] [Google Scholar]
- 23.Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 1998; 350:21–29. [DOI] [PubMed] [Google Scholar]
- 24.Trujillo V, Durando PE, Suárez MM. Maternal separation in early life modifies anxious behavior and Fos and glucocorticoid receptor expression in limbic neurons after chronic stress in rats: effects of tianeptine. Stress 2015; 19:91–103. [DOI] [PubMed] [Google Scholar]
- 25.Qiong W, Man L, Wei D, Feng S, Weiwen W. The different effects of maternal separation on spatial learning and reversal learning in rats. Behav Brain Res 2015; 280:16–23. [DOI] [PubMed] [Google Scholar]
- 26.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 2008; 31:183–191. [DOI] [PubMed] [Google Scholar]
- 27.Rana S, Pugh PC, Jackson N, Clinton SM, Kerman IA. Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neurosci Lett 2015; 584:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang S, Ling X, Jihua T, Gaohua W, Xueping Z, Xiaoping W. Blunted behavioral and molecular responses to chronic mild stress in adult rats with experience of infancy maternal separation. Tohoku J Exp Med 2015; 235:81–87. [DOI] [PubMed] [Google Scholar]
- 29.Rao RM, Sadananda M. Influence of state and/or trait anxieties of Wistar rats in an anxiety paradigm. Ann Neurosci 2016; 23:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markostamou I, Ioannidis A, Dandi E, Mandyla MA, Nousiopoulou E, Simeonidou C, et al. Maternal separation prior to neonatal hypoxia-ischemia: impact on emotional aspects of behavior and markers of synaptic plasticity in hippocampus. Int J Dev Neurosci 2016; 52:1–12. [DOI] [PubMed] [Google Scholar]
- 31.Shreya G, Banerjee KK, Vaidya VA, Kolthur-Seetharam U. Early stress history alters serum insulin-like growth factor-1 and impairs muscle mitochondrial function in adult male rats. J Neuroendocrinol 2016. 28. [DOI] [PubMed] [Google Scholar]
- 32.Oines E, Murison R, Mrdalj J, Gronli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behaviour after chronic social stress. Physiol Behav 2012; 105:1058–1066. [DOI] [PubMed] [Google Scholar]
- 33.Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry 2004; 55:708–714. [DOI] [PubMed] [Google Scholar]
- 34.Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience 2013; 239:173–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013; 239:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav 2012; 61:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress 2014; 17:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


