Abstract
Purpose of review
Despite over 60 years of progress in the field of since the first organ transplant, insufficient organ preservation capabilities still place profound constraints on transplantation. These constraints play multiple and compounding roles in the predominant limitations of the field: the severe shortages of transplant organs, short-term and long-term posttransplant outcomes and complications, the unmet global need for development of transplant infrastructures, and economic burdens that limit patient access to transplantation and contribute to increasing global healthcare costs. This review surveys ways that advancing preservation technologies can play a role in each of these areas, ultimately benefiting thousands if not millions of patients worldwide.
Recent findings
Preservation advances can create a wide range of benefits across many facets of organ transplantation, as well as related areas of transplant research. As these technologies mature, so will the policies around their use to maximize the benefits offered by organ preservation.
Summary
Organ preservation advances stand to increase local and global access to transplantation, improve transplant outcomes, and accelerate progress in related areas such as immune tolerance induction and xenotransplantation. This area holds the potential to save the healthcare system many billions of dollars and reduce costs across many aspects of transplantation. Novel preservation technologies, along with other technologies facilitated by preservation advances, could potentially save millions of lives in the coming years.
Keywords: cryopreservation, organ banking, perfusion, tissue preservation
INTRODUCTION: REALIZING THE FULL POTENTIAL OF ORGAN PRESERVATION
The goal of organ preservation is to maintain an organ outside the body in a state that is ideal for its intended application. For decades, the field of transplantation has focused on a relatively simplistic version of this goal: moving a deceased donor organ from the site of procurement to the site of transplantation with tolerable levels of ischemic injury. But the organ preservation concept itself is far more expansive, entailing many different conditions under which an organ must be maintained for various durations depending on the individual circumstances. The impact of organ preservation on transplantation can be equally expansive, if the full scope of the field is realized.
Beyond simply transporting organs from point A to point B, diverse goals within the field of transplantation can be accomplished by preservation technologies. First, transplantation can be profoundly improved by active intervention during and after organ recovery, such as assessment of organ function, rehabilitation of marginal transplant organs, organ immunomodulation, and treatment for a variety of transplant contraindications. Second, organs from deceased donors that are not transplanted could be efficiently used for other lifesaving purposes such as cell transplantation, biomedical research, and preclinical drug testing, each of which can require optimization of a variety of unique preservation considerations. Third, many new opportunities to expand access to transplantation or improve transplant outcomes can be created by organ banking, a sub-field of organ preservation that aims to achieve preservation durations constituting a true ‘shelf life’.
To achieve these goals, a family of organ preservation approaches should be pursued that encompasses ex-vivo perfusion at a variety of temperatures [1–3,4▪,5–10], cryopreservation [11–15], ‘high-subzero’ preservation regimes such as supercooling [16,17], pharmacological induction of a hypometabolic tissue state [17–20], and related innovations. These preservation regimes can be readily combined with each other and with other cytoprotective strategies [21–23] applied before and after organ recovery.
Historically, financial support for these preservation approaches has been fragmented and relatively modest. But a wave of interest in advancing organ preservation has begun to develop in recent years, with targeted funding and support from White House Office of Science and Technology Policy [24], US Department of Defense [25], US Multi-Agency Tissue Engineering working group [26], the Canadian Institutes of Health Research [27], and others. Growing recognition of the importance of preservation to transplantation is also reflected by the recent launch of the American Society for Transplantation's Organ and Tissue Preservation Community of Practice in partnership with the Organ Preservation Alliance [28].
This attention has been spurred by the increasing recognition that if pursued together and at scale, organ preservation technologies hold promise to dramatically improve organ transplantation and human health [29▪▪]. The social, economic, and policy implications are discussed in the following.
Box 1.
no caption available
ADDRESSING THE ORGAN SHORTAGE
Although the US transplant waitlist numbers roughly 115 000 people [30], this represents only a fraction of the true need for organ replacement. Only approximately 50 000 people are added to the US transplant waitlist each year, yet over 700 000 US deaths per year are attributable to end-stage organ disease [31,32]. Some estimates suggest that as many as 30% of deaths in the United States could be prevented by organ replacement with supply and technology constraints removed [33–35]. The unmet need is far greater worldwide; globally, deaths from organ impairment or from other causes theoretically addressable by organ transplantation number above 15 million per year [36].
Increasing organ utilization and expanding the donor pool
In the United States, only 0.3% of deaths result in organ donation [31,37]. Each of these donors can theoretically provide eight lifesaving organs, yet on average only two to three of these are currently transplanted [31]. Short preservation windows heavily constrain organ use, resulting directly in the discard of thousands of organs [31], placing constraints on transplant centers’ ability to respond to an organ offer [38], and impeding optimal donor–recipient matching [39] and organ assessment [40▪▪,41▪] (Table 1). Additionally, perfusion-based preservation platforms have enabled the rehabilitation of otherwise unsuitable organs by repairing a great variety of defects [10,42,43▪,44–52], even resulting in the successful transplantation of donor hearts after circulatory death [48,53,54,55▪,56▪▪]. This offers the promise of increasing organ utilization and dramatically widening the pool of organ donors, ameliorating the organ shortage. Although roughly 70% of hearts from organ donors in the United States currently go untransplanted [57,58], studies suggest that often a heart turned down in its allocation region would be accepted by a transplant center elsewhere [38,59]. Successfully transplanting just 10% of those hearts would provide organs for all of the current heart waitlist patients who do not receive a new organ in time [60].
Table 1.
Impacts on organ transplantation
| Impact on transplantation | Perfusion | High subzero | Cryobanking |
| Expanding the donor pool | x | ||
| Ex-vivo functional assessment | x | ||
| Ex-vivo immunomodulation | x | ||
| Ex-vivo functional enhancement | x | ||
| Increasing organ utilization | x | x | x |
| Improving transplant outcomes | x | x | x |
| Immune tolerance induction | x | x | x |
| Disease screening | x | x | x |
| Reduced procurement costs | x | x | x |
| Reduced postoperative complications | x | x | x |
| Increased use of marginal organs | x | x | x |
| Improved matching for disadvantaged patients | x | x | x |
| Geographic constraints | x | x | x |
| Increased hand, limb transplant | x | x | x |
| Donor–recipient matching | x | x | x |
| Increased organ quality | x | x | x |
| Reduced postoperative costs | x | x | x |
| Elective scheduling of surgery | x | x | |
| On-demand for acute conditions | x | ||
| Backup transplant organs | x | ||
| Fertility protection for recipients with cancer | x | ||
| Off-the-shelf research organs and tissues | x | ||
| Future transplant of today's marginal organs | x | ||
| Global organ matching | x | ||
| Xenotransplantation supply chain | x |
Some impact, including indirect, exists for almost all relationships between the aspects of transplantation shown and each preservation modality; however, the most direct and dramatic impacts are indicated with an ‘x.’
Reducing disparities in access to transplantation
By greatly increasing opportunities for donor–recipient matching, preservation advances can help level the playing field for historically hard-to-match populations: children, ethnic minorities, and patients in areas with the worst organ shortages who do not have the financial means to relocate. These disparities are often stark. For example, while 84% of the adults who die on the liver waitlist are offered at least one organ, only 45% of the children that die or are delisted receive such offers [61]. Similarly, ethnic minorities receive fewer transplants than their white counterparts despite making up a majority of the transplant waitlist [30,62▪]. Geographic differences in organ availability and need can result in three times more deaths on the liver waitlist in organ-poor areas, compared with organ-rich regions [63].
Expanding limb, face, and other vascularized tissue transplantation
Approximately 185 000 people per year undergo amputation [64], and an estimated two million people are living with limb loss (approximately half from traumatic injury) [65]. Vascularized composite allograft (VCA) transplantation can restore function [66] and self-image [67] after amputation, and a large fraction of amputees have expressed a desire for both upper limb and lower limb transplantation [68,69]. However, access to these procedures is bottlenecked by the immunogenicity of VCA and difficulty to find adequately matching donors and recipients within the short (ideally 4-h) window to transport tissue from the procurement site to the patient's transplant center [70]. Extending preservation times would dramatically expand options for donor–recipient matching, and, importantly, preservation advances could also enable immune tolerance induction approaches that have been successful in the context of live kidney donation [71–73] to be adapted to VCA transplantation [74,75].
Creating new organ supplies
Addressing the true need for transplantation necessitates development of organ sources beyond donation to increase the number of organs available. There have been significant investments in optimizing humanized animal (xenogeneic) [76–79] and bioengineered [80,81] organs for transplantation. In particular, because of advances in immune tolerance induction and gene editing [78,82], xenotransplantation has made a resurgence in recent years and is now showing success in primate studies [83▪] with clinical trials beginning as early as 2018 [78]. Many groups, such as the White House Office of Science and Technology Policy [24], Department of Defense [25], and US Multi-Agency Tissue Engineering working group [26] have noted that preservation constraints have already slowed progress toward nondonor sources of transplant organs, while also creating looming concerns for future patient access to life-saving transplants.
Enabling off-the-shelf transplantation for acute conditions
The prospect of organ banking offers the novel opportunity to treat patients with acute conditions, organ injury, or rapidly deteriorating health. Trauma, including motor vehicle accidents, represents the largest cause of early death in the United States and over 50 000 of these deaths per year are because of organ damage that might be treatable with on-demand organ transplantation [84,85]. In cases of acute liver failure, 20% of patients die waiting for a life-saving liver transplant [86]. Similarly, in 2016, over 100 000 people died from a heart attack [87], and the International Society for Heart and Lung Transplantation has speculated that many of these deaths could be prevented if a replacement heart were immediately available [88]. Even a product that provides limited graft lifespan could act as a first-of-its-kind bridge therapy for patients with no other option.
IMPROVING TRANSPLANT OUTCOMES
Over the past 25 years, US patients have gained over two million life-years because of organ transplantation [89], and organ transplant has become the gold-standard treatment for an extensive range of conditions [90–95]. Yet the success of transplantation is still limited by significant morbidity and mortality because of primary graft dysfunction and chronic rejection [96]. Roughly half of all hearts, kidneys, and livers are rejected within 10 years of transplantation, and as few as 25% of transplant lungs and intestines survive a decade after transplant [97]. This makes the long-term success of transplant operations a central concern for achieving this field's potential for addressing organ impairment. Many benefits for transplant efficacy can be created by preservation advances, outlined in the following.
Delaying and preventing graft rejection
Constraints on immunological matching contribute heavily to both acute and chronic graft rejection, limiting both immediate transplant success and long-term outcomes [98,99]. For example, even a single human leukocyte antigen (HLA) mismatch is associated with a 44% higher chance of kidney graft failure [62▪]. Increases in preservation time would enable national or world-wide matching, which could eliminate the need to accept a suboptimally matched organ while also enabling efficient HLA matching for all organs (rather than kidney only) [100].
Immune tolerance induction
Inducing donor-specific immune tolerance has the potential to increase postoperative survival for transplant patients from the current typical gain of 4.3 life-years [89] to decades [101], making organ transplantation a cure rather than a lifelong condition. It could also decrease or even eliminate the need to take immunosuppressive drugs, which are associated with cancer, infections, and other complications that can limit the length and quality of a transplant recipient's life [102]. For instance, heart transplant patients on high levels of immunosuppressive drugs develop non-Hodgkin's lymphoma at rates 120 times higher than the general population [103]. Significant progress is being made in tolerance induction through stem cell transplantation, but the approaches that have been developed require days to weeks, and the protocols that have shown clinical success at MGH, Stanford, and Northwestern are restricted to transplantation from living donors [71,73,104]. With increased organ preservation times, new possibilities would be created for recipients of organs from deceased donors (80% of organ transplants today) to undergo immune tolerance protocols, by beginning protocols before transplantation.
Providing flexibility for transplant procedures
Outcomes for the same procedure are typically better for elective surgeries than urgent ones [105,106▪,107], and even extending preservation times to days could provide tremendous flexibility in scheduling transplant operations. This can have compounding effects, for instance, reducing the need for transplant surgeons to remain on call day or night and allowing more freedom to train younger transplant surgeons and other personnel.
Increasing donor organ quality
Given that increases in ischemic time – even during organ recovery – increase the risk of graft loss [108,109], preservation protocols that minimize ischemic time and allow rehabilitation of allografts should improve patient outcomes. Recent advances in machine perfusion [6,110–115] have also opened the door to active intervention after organ recovery to improve organ function prior to implantation: there has already been success integrating perfusion platforms and gene editing techniques to enhance organs in animal transplant models [116▪,117] and in human lungs [118] that were not transplanted. Other perfusion-based approaches have included ‘immunocloaking [119],’ RNAi [120], and reversal of liver steatosis (’de-fatting’) [121].
Preventing infection and malignancy
Although transmission of infectious diseases and malignancies to organ recipients remains an extremely rare event (<1% of all transplants) [122], minimizing this risk remains a significant concern. Similarly, transmission of cancer from donor to recipient is uncommon, but occurs before cancer has been detected in the donor [123]. Increasing organ preservation time could enable more comprehensive testing for infections, better informing transplant decisions. Protocols could be expanded to include nucleic acid testing in combination with serologic tests and testing of organ preservation fluids, which under current time constraints have unclear efficacy [122]. With increased preservation times, positive results could be confirmed by subsequent testing, preventing deaths that occur when false positives prevent safe organs from reaching recipients in need. Preservation technologies could also provide a platform to resolve infections or administer prophylactic treatment in donated organs, avoiding complications and preventing otherwise healthy organs from being discarded. Early successes using this approach have recently been reported, where ex-vivo lung perfusion of antibiotics and antifungals decreased the microbial load of lungs [124,125▪▪].
Assessment of organ quality
Perfusion allows clinicians ample time to assess organ function and to determine suitability for transplantation [126,127▪▪,128,129]. For instance, metabolic profiling to predict the likelihood of primary graft dysfunction [40▪▪,130] could enable more informed decisions about an organ's suitability for transplant and its potential longevity.
FACILITATING GLOBAL ACCESS TO TRANSPLANTATION
Dramatic disparities currently exist in worldwide access to transplantation. For instance, although approximately 16% of the world's population resides in Africa, under 0.5% of the world's transplants are performed on the continent (Fig. 1) [131,132]. As of 2011, deceased donor transplantation was limited to roughly 40% of the World Health Organization's member countries with low-income countries performing less than 1% of all transplants [133]. And of the countries with transplantation capabilities, 28% perform limited or no deceased donor transplantation. This suggests that surgical skill and patient care infrastructure are not the principal bottlenecks in a country's ability to develop transplant infrastructure; rather, the logistics of organ procurement and transport play a major role. Without robust organ procurement organization and the rapid transport capabilities of jets and helicopters, many areas lack the time to get usable organs to patients in need. In addition to overcoming these barriers and lowering transplant costs, extension of organ preservation times could allow the intimidating requirements of transplantation to be addressed individually rather than developing colocalized infrastructure, while facilitating involvement of countries with developed transplant systems. Barriers are expected to be further lowered as tolerance induction protocols [71,73,104] making the need for lifelong immunosuppression unnecessary in many cases.
FIGURE 1.
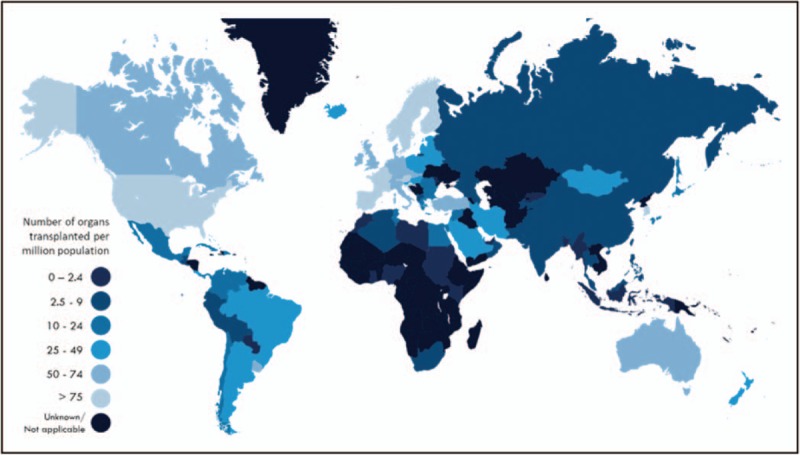
The World Health organization estimated that less than 10% of the global organ transplantation need is met. A small number of countries have high transplant rates per capita, while the majority of countries fail to meet their populations’ organ replacement needs. Reproduced with permissions from [29▪▪].
Overcoming initial low-transplant infrastructure density
The sites of organ recovery and implantation must be relatively colocalized, preventing transplants in areas where few such sites exist. Organ sharing across long distances and even across borders could ameliorate or eliminate these issues, creating tremendous synergy between countries’ parallel efforts to develop transplantation. For instance, cross border sharing may be especially valuable in sub-Saharan Africa, where countries with nascent transplant programs (e.g. Ghana, Nigeria, Kenya, and Zambia) do not often border each other [134–137].
Global propagation of transplant expertise
The project ‘Transplants Without Borders’ has provided proof-of-principal for bringing abdominal transplantation to developing regions through dedicated training programs, while maintaining success rates comparable with the United States (>90%) [138]. Currently programs of this nature require long-term deployment of transplant surgeons to other countries, limiting scalability and increasing costs. Saving organs after procurement would allow transplantation in batches with ‘tutor’ surgeons who only need to commit to days or weeks of service – greatly accelerating training and propagation of transplantation in areas wherever surgical expertise is needed.
Centralized testing and organ recovery
As noted above, organ preservation advances can facilitate access to donor–recipient matching assays, testing for transmissible diseases and malignancies, assessment of organ function, and emerging technologies such as siRNA treatment or gene editing for immunomodulation. With extended preservation windows, these methods could be centralized and scaled, greatly lowering costs and providing opportunities to bring cutting-edge advances to faraway, developing transplant infrastructures.
DELIVERING HEALTHCARE SAVINGS
The economic toll of organ impairment is immense, and diseases treatable by organ replacement disproportionately strain healthcare infrastructures. In 2014, more was spent by Medicare on renal disease treatment than all cancer treatments combined, despite cancer claiming 12 times as many lives as renal disease. The cost of end-stage organ disease is also tremendous at the global level, with more than $1 trillion spent over a decade globally on renal disease treatment alone [139]. In 2013, the two costliest diseases in the United States were diabetes and heart disease [140], both diseases for which transplantation is often the gold standard treatment.
Organ preservation advances can reduce the cost of transplantation itself as well as costs incurred by the treatment of posttransplant complications, and Table 2 provides examples of cost changes in heart transplantation that could be created by improved heart preservation technologies.
Table 2.
Current estimated heart transplant costs and examples of effects of organ preservation advances
| Stage of Tx | Average cost | Examples of cost changes |
| Pretransplant, procurement and transport | $145 300 | Eliminate the need for costly jet and helicopter flights to immediately transport organsAdditional testing (serology, HLA matching) enabled. Some testing can be centralized, lowering costs [121]Preservation technology itself will impose costs, varying widely according to model usedFewer cases where heart transplant surgeons and procurement teams are sent for organs that are subsequently turned down [37,58] |
| Hospital transplant admission | $979 700 | Elective scheduling, reducing the need for on-call surgical staff and operating roomsAdmission costs are a function of donor organ quality [141]; preservation technologies have already improved transplant outcomes, for example, lowering rates of primary graft dysfunction [127▪▪,142,143].Fewer complications should decrease the length of admission and decrease the costs of immediate postoperative care [127▪▪,142,143]Reduced need for retransplant in the case of graft failure |
| Posttransplant care | 257 300 (for first 6 months) | Reduce readmission for complications by increasing transplant efficacy [101]Reduce lifelong cancer incidence by facilitating immune tolerance induction and transmissible malignancy screeningReduce lifelong complications, for example, CMV, PTLDReduce need for immunosuppression and drugs for complications (e.g. $34 500 for first 6 months) [140] |
Current estimated heart transplant costs are discussed in [140].
Reducing organ procurement costs
Organ procurement and transport averages around $100 000 [141], with costs exacerbated by the need for round-the-clock ‘on call’ jet or helicopter transport. By increasing safe preservation durations from hours to days or longer, we can enable ground transport of organs in almost all cases and provide much needed flexibility for organ procurement organizations.
Reducing transplant and postoperative care costs
Approximately half of the costs associated with transplantation are incurred during hospital admission, including extended postoperative care [141]. Even short-term organ preservation would reduce the reliance on last-minute operating room bookings and on-call surgical staff, decreasing the currently high costs of transplant admission. Admission costs are additionally increased by transplant complications and even characteristics of the donor organ [142]. Current preservation technologies have already made progress towards improved transplant outcomes (see previous section), including a promising trend towards lower rates of primary graft dysfunction in recipients of perfused lungs [128,143,144]. Continued advances in preservation can further reduce complications and thus the need for expensive extended postoperative care.
Reducing lifelong complications
To prevent rejection, patients typically must begin immunosuppressant regimens after transplant. Immunosuppression leads to a number of serious complications including increased risk of cancer and viral infections, which requires long-term monitoring and drug prophylaxis to prevent [102]. Currently, the first 6 months of immunosuppression and additional prophylactic drugs average $22 000–$71 000 (depending on the organ) [141]. However, organ banking would enable immune tolerance induction and better donor–recipient matching, decreasing the need for immunosuppression and thus, lowering the costs of immunosuppression and monitoring while preventing cost of subsequent complications.
Transplantation as a cost-saver
For some organs, simply expanding access to transplantation can create substantial cost savings as well. For instance, the net savings of kidney transplantation as an alternative to dialysis has been estimated at nearly $500 000 per patient [145▪▪] while dramatically increasing quality of life [146,147]. The average lifetime costs for bilateral upper extremity prosthetics are about $1.5 million, with historical increases as technology advances, whereas bilateral transplant is currently under $500 000 for the first year, with costs likely to decrease as preservation technologies mature [148].
DESIGNING POLICIES FOR A CHANGING TRANSPLANT LANDSCAPE
Current allocation policies are, in large part, based on geographic structures designed to meet time limitations and specific logistical requirements of various organ types. Following donation, the allocation of organs usually proceeds in a sequential fashion to patients at programs in a defined local area surrounding the donor hospital to patients in a larger regional distribution, and then to patients nationally, if required. As distances and time increase, the potential for successful organ allocation decreases.
This necessary but artificial allocation hierarchy creates substantial logistical and medical complexities, resulting in a complex allocation policy. Current organ allocation policies are heavily time-dependent. For example, transplant centers have 1 h to acknowledge an organ offer and then one additional hour to accept or decline the offer before it goes to the next patient on the list. Policies governing these system operational issues will need to evolve to address new realities that result from enhanced organ preservation.
As new preservation technologies are incorporated into the donation and transplantation system, policy must be developed to guide selection of organs from the existing organ pool for enhanced preservation or for banking. Perhaps only organs not expected to be transplanted locally will be initially chosen for extended preservation or banking. The extra costs and system complexities of advanced technologies may not be appropriate, at least initially, for organs that are destined for local transplantation with current expected excellent outcomes. The present kidney allocation algorithm incorporates a measure of organ quality that drives distribution. Policies that incorporate an accurate assessment of the expected outcomes from a wide range of organ preservation technologies or organ modifications will add complexity into allocation decisions and into the modeling of expected outcomes.
System transparency and patient consent issues addressed by current policy will have to evolve to address the changing clinical environment. Allocation policy development will need to ensure that equitable access by all patient populations to an expanded pool of transplantable organs is maintained. Immunologic modification of organs must provide benefits broadly to the pool of potential recipients and not solely to a subgroup of patients.
To capture the potential benefits of enhanced preservation, Organ Procurement and Transplantation Network (OPTN) policies will need to address a wider spectrum of donor organ and recipient characteristics. Careful oversight will be critical, and this must be guided by appropriate policy that addresses organ modification procedures to ensure patient safety. The removal of time constraints from organ donation will allow a more thorough assessment of the risks of donor disease transmission and also enhance patient safety. Policy around living donation can change to decouple donation from the transplant operation. The living donor procedure can be scheduled to suit the donor's needs and not the immediate medical needs of the recipient.
Geographic access inequities, one of the biggest challenges facing the OPTN, will improve with adoption of new preservation and banking technologies. An enhanced supply of transplantable organs matched effectively to recipient-specific needs should more effectively address the disparity between the demand for organ transplantation and the number of available donor organs. As organ preservation and modification technologies mature, allocation policies focusing on recipient equity and outcomes and less on logistical considerations driven by specific organ needs will be possible.
CONCLUSION
In the last decade, a new generation of organ preservation technologies have shown promise to deliver a striking array of new capabilities for transplantation. There is reason for optimism that a family of synergistic research areas can deliver dramatic advances in preservation capabilities, with the potential to help address organ shortages, improve transplant outcomes, increase graft longevity, reduce long-term posttransplant complications, facilitate global proliferation of transplantation, reduce costs across many aspects of transplantation, and decrease the burden of end-stage organ disease on the economy. Ultimately, many aspects of organ allocation policy must evolve over the coming years and decades to keep pace with this progress.
Acknowledgements
The authors would like to acknowledge the efforts of Bradley Weegman, Eugene Sato, Peter Kilbride, Boris Schmalz, Valentina Morigi, Alessandro Tocchio, Rami El Assal, and Mitchell Rostad, who helped assemble some of the information presented here. We owe special thanks to Elling Eidbo, David Sachs, Martine Rothblatt, David Nelson, James Markmann, George Church, Luis Alvarez, Gerald Brandacher, Gloria Elliott, Korkut Uygun, Greg Fahy, Erik Woods, Kelvin Brockbank, Mike Taylor, Jason Acker, Mehmet Toner and Kevin Myer, who among many others have provided invaluable ideas and advice shaping the conclusions herein.
Financial support and sponsorship
None.
Conflicts of interest
J.K.L. is an officer of the Organ Preservation Alliance, a 501c3 nonprofit organization, dedicated to advancing organ and tissue preservation. A.M.W. and K.M.F. are employees of the same. S.G. is an officer of Sylvatica Biotech, a cryopreservation biotech company, and Ossium Health, a bone marrow banking company.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Wszola M, Kwiatkowski A, Diuwe F, et al. One-year results of a prospective, randomized trial comparing two machine perfusion devices used for kidney preservation. Transpl Int 2013; 26:1088–1096. [DOI] [PubMed] [Google Scholar]
- 2.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant 2010; 10:372–381. [DOI] [PubMed] [Google Scholar]
- 3.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 2009; 360:7–19. [DOI] [PubMed] [Google Scholar]
- 4▪.Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med 2017; 5:119–124. [DOI] [PubMed] [Google Scholar]; The authors analyzed records of nearly 100 recipients of lungs preserved by ex-vivo perfusion for over 12 h and found that the patient outcomes (length of hospital and intensive care unit stays, grade of primary graft dysfunction, and survival) were no different from lungs preserved for half as long. This paves the way for further increasing preservation times prior to transplantation, enabling even more flexibility in scheduling and matching, along with time for reconditioning and potential augmentation.
- 5.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012; 144:1200–1206. [DOI] [PubMed] [Google Scholar]
- 6.Berendsen TA, Bruinsma BG, Lee J, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res 2012; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Ji F, Zhang Z, et al. Combined liver-kidney perfusion enhances protective effects of normothermic perfusion on liver grafts from donation after cardiac death. Liver Transpl 2018; 24:67–79. [DOI] [PubMed] [Google Scholar]
- 8.Stratta RJ, Moore PS, Farney AC, et al. Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors. J Am Coll Surg 2007; 204:873–882. [DOI] [PubMed] [Google Scholar]
- 9.De Carlis R, Di Sandro S, Lauterio A, et al. Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl 2017; 23:166–173. [DOI] [PubMed] [Google Scholar]
- 10.Jiao B, Liu S, Liu H, et al. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS One 2013; 8:e81826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovatta O. Cryopreservation of testicular tissue in young cancer patients. Hum Reprod Update 2001; 7:378–383. [DOI] [PubMed] [Google Scholar]
- 12.Arav A, Friedman O, Natan Y, et al. Rat hindlimb cryopreservation and transplantation: a step toward ‘organ banking’. Am J Transplant 2017; 17:2820–2828. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, He B, Duan Y, et al. Cryopreservation and replantation of amputated rat hind limbs. Eur J Med Res 2014; 19:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahy GM, Wowk B, Wu J, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology 2004; 48:157–178. [DOI] [PubMed] [Google Scholar]
- 15.Fahy GM, Wowk B, Pagotan R, et al. Physical and biological aspects of renal vitrification. Organogenesis 2009; 5:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruinsma BG, Berendsen TA, Izamis ML, et al. Supercooling preservation and transplantation of the rat liver. Nat Protoc 2015; 10:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berendsen TA, Bruinsma BG, Puts CF, et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat Med 2014; 20:790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menze MA, Chakraborty N, Clavenna M, et al. Metabolic preconditioning of cells with AICAR-riboside: improved cryopreservation and cell-type specific impacts on energetics and proliferation. Cryobiology 2010; 61:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey KB, Storey JM. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv Clin Chem 2010; 52:77–108. [PubMed] [Google Scholar]
- 20.Storey KB. Regulation of hypometabolism: insights into epigenetic controls. J Exp Biol 2015; 218:150–159. [DOI] [PubMed] [Google Scholar]
- 21.Limkemann A, Lindell SL, Reichstetter H, et al. Donor gluconate rescues livers from uncontrolled donation after cardiac death. Surgery 2016; 159:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish D, Plant V, Lindell SL, et al. New low-volume resuscitation solutions containing PEG-20k. J Trauma Acute Care Surg 2015; 79:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma 1095-; 58:101–102. [DOI] [PubMed] [Google Scholar]
- 24. White House. Fact Sheet: Obama Administration announces key actions to reduce the organ waiting list. 2016. [Google Scholar]
- 25. Department of Defense. Cryopreservation for regenerative medical applications. 2015; pp. A15–A59. [Google Scholar]
- 26. Multi-Agency Tissue Engineering Sciences (MATES) Interagency Working Group. MATES: Advancing Tissue Science and Strategic Report. 2007. [Google Scholar]
- 27. [[Accessed 30 January 2018]]. Canadian National Transplant Research Program | ABOUT, 2014. Available at: https://www.cntrp.ca/about. [Google Scholar]
- 28.White House highlights AST's new initiative with Organ Preservation Alliance, 2016. Available at: https://www.myast.org/about-ast/white-house-highlights-asts-new-initiative-organ-preservation-alliance. [Google Scholar]
- 29▪▪.Giwa S, Lewis JK, Alvarez L, et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 2017; 35:530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]; This consensus article by about 40 leaders in transplantation and related fields is complementary to the present work, discussing the need for organ preservation technologies and the many benefits offered by advancing these technologies.
- 30. [[Accessed 3 December 2017]]. OPTN. Data Reports: National Data. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ [Google Scholar]
- 31.Israni AK, Zaun D, Bolch C, et al. OPTN/SRTR 2015 annual data report: deceased organ donation. Am J Transplant 2017; 17 Suppl 1:503–542. [DOI] [PubMed] [Google Scholar]
- 32.Hoyert DL, Xu J. Deaths: Preliminary Data for 2011. Natl Vital Stat Rep 2012; 61:1–51. [PubMed] [Google Scholar]
- 33.Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res 2006; 9:279–291. [DOI] [PubMed] [Google Scholar]
- 34.Lanza RP, Cibelli JB, West MD, et al. The ethical reasons for stem cell research. Science 2001; 292:1299. [DOI] [PubMed] [Google Scholar]
- 35.Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol 2002; 20:689–696. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. The top 10 causes of death. WHO ,2017. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/ [Accessed 15 December 2017] [Google Scholar]
- 37.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. National Vital Statisitcs Reports, vol. 65, Number 4 (06/30/2016); 2014. [PubMed] [Google Scholar]
- 38.Khush KK, Menza R, Nguyen J, et al. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail 2013; 6:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy MS, Varghese J, Venkataraman J, Rela M. Matching donor to recipient in liver transplantation: Relevance in clinical practice. World J Hepatol 2013; 5:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪▪.Faitot F, Besch C, Battini S, et al. Impact of real-time metabolomics in liver transplantation: graft evaluation and donor-recipient matching. J Hepatol 2017; 68:699–706. [DOI] [PubMed] [Google Scholar]; Analysis of biopsied liver samples showed that lactate and phosphocholine were significantly associated with early allograft dysfunction. Further refinement of these techniques could allow more advanced risk assessments for each transplant and decrease complications, thus improving patient outcomes.
- 41▪.Bruinsma BG, Sridharan GV, Weeder PD, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci Rep 2016; 6:22415. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors analyzed the metabolic profiles of both ‘transplantable’ and injured livers during sub-normothermic machine perfusion and found a distinguishable profile for injured livers. Moving forward, metabolic analyses could provide the most accurate assessment of organ condition, leading to more informed transplant criteria.
- 42.Jochmans I, O’Callaghan JM, Pirenne J, Ploeg RJ. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl Int 2015; 28:665–676. [DOI] [PubMed] [Google Scholar]
- 43▪.Pezzati D, Ghinolfi D, Balzano E, et al. Salvage of an octogenarian liver graft using normothermic perfusion: a case report. Transplant Proc 2017; 49:726–728. [DOI] [PubMed] [Google Scholar]; This report highlights the value of using ex-vivo organ perfusion to functionally evaluate grafts prior to transplantation. These assessments can result in utilization of life-saving grafts that would otherwise be discarded.
- 44.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of ‘orphan’ extended criteria donor livers. Am J Transplant 2015; 15:161–169. [DOI] [PubMed] [Google Scholar]
- 45.Bozso S, Freed D, Nagendran J. Successful transplantation of extended criteria lungs after prolonged ex vivo lung perfusion performed on a portable device. Transpl Int 2015; 28:248–250. [DOI] [PubMed] [Google Scholar]
- 46.Machuca TN, Hsin MK, Ott HC, et al. Injury-specific ex vivo treatment of the donor lung: pulmonary thrombolysis followed by successful lung transplantation. Am J Respir Crit Care Med 2013; 188:878–880. [DOI] [PubMed] [Google Scholar]
- 47.Christopoulos P, Faryal A, Dosani M, et al. A case of a living-related kidney transplantation after ex-vivo repair of the donor renal artery aneurysm. Hippokratia 2016; 20:90–92. [PMC free article] [PubMed] [Google Scholar]
- 48.Mownah OA, Khurram MA, Ray C, et al. Development of an ex vivo technique to achieve reanimation of hearts sourced from a porcine donation after circulatory death model. J Surg Res 2014; 189:326–334. [DOI] [PubMed] [Google Scholar]
- 49.Inci I, Ampollini L, Arni S, et al. Ex vivo reconditioning of marginal donor lungs injured by acid aspiration. J Hear Lung Transplant 2008; 27:1229–1236. [DOI] [PubMed] [Google Scholar]
- 50.Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo. Metab Eng 2009; 11:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nativ NI, Maguire TJ, Yarmush G, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation. Am J Transplant 2012; 12:3176–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Berendsen T, Izamis ML, et al. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant Proc 2013; 45:3209–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015; 385:2585–2591. [DOI] [PubMed] [Google Scholar]
- 54.Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med 2008; 359:709–714. [DOI] [PubMed] [Google Scholar]
- 55▪.García Sáez D, Bowles CT, Mohite PN, et al. Heart transplantation after donor circulatory death in patients bridged to transplant with implantable left ventricular assist devices. J Hear Lung Transplant 2016; 35:1255–1260. [DOI] [PubMed] [Google Scholar]; The two cases described here represent use of ex-vivo perfused DCD hearts to treat two patients with long-term left ventricular assist device support. Methods for using DCD organs in high-risk patients are vital to unlocking the estimated 15–23% increase in organ supply gained just through efficient use of DCD organs.
- 56▪▪.Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Hear Lung Transplant 2017; 36:1311–1318. [DOI] [PubMed] [Google Scholar]; Transplantation of DCD hearts is a major step in organ preservation as this represents the ability to rescue the very organ that caused the donor's death. In the 28 DCD transplants described here, the short-term outcomes (length of hospital stay, 90-day and 1-year survival, episodes of rejection and allograft function) of recipients of perfused DCD hearts were equivalent to recipients of matched DBD hearts, opening up an important new supply of organs.
- 57.Wigfield CH, Cypel M, Yeung J, et al. Successful emergent lung transplantation after remote ex vivo perfusion optimization and transportation of donor lungs. Am J Transplant 2012; 12:2838–2844. [DOI] [PubMed] [Google Scholar]
- 58.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2012 annual data report: deceased organ donation. Am J Transplant 2014; 14 Suppl 1:167–183. [DOI] [PubMed] [Google Scholar]
- 59.Shah MR, Starling RC, Schwartz Longacre L, Mehra MR. Working Group Participants. Heart transplantation research in the next decade–a goal to achieving evidence-based outcomes: National Heart, Lung, And Blood Institute Working Group. J Am Coll Cardiol 2012; 59:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 2015; 15 Suppl 2:1–34. [DOI] [PubMed] [Google Scholar]
- 61.Hsu EK, Shaffer ML, Gao L, et al. Analysis of liver offers to pediatric candidates on the transplant wait list. Gastroenterology 2017; 153:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Williams RC, Opelz G, McGarvey CJ, et al. The risk of transplant failure with HLA mismatch in first adult kidney allografts from deceased donors. Transplantation 2016; 100:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]; There has been disagreement about the importance of HLA matching in deceased kidney donation. This retrospective study analyzed nearly 200 000 transplants and found that there was a linear relationship between mismatches and the probability of allograft loss. This work highlights the value of the improved HLA matching that could be enabled by longer preservation times.
- 63.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation 2011; 91:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States. Vital Health Stat 1998; 13:1–119. [PubMed] [Google Scholar]
- 65.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to. Arch Phys Med Rehabil 2008; 89:422–429. [DOI] [PubMed] [Google Scholar]
- 66.Landin L, Bonastre J, Casado-Sanchez C, et al. Outcomes with respect to disabilities of the upper limb after hand allograft transplantation: a systematic review. Transpl Int 2012; 25:424–432. [DOI] [PubMed] [Google Scholar]
- 67.Salminger S, Sturma A, Roche AD, et al. Functional and psychosocial outcomes of hand transplantation compared with prosthetic fitting in below-elbow amputees: a multicenter cohort study. PLoS One 2016; 11:e0162507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majzoub RK, Cunningham M, Grossi F, et al. Investigation of risk acceptance in hand transplantation. J Hand Surg Am 2006; 31:295–302. [DOI] [PubMed] [Google Scholar]
- 69.Carty MJ, Duclos A, Talbot SG, et al. Attitudes regarding lower extremity allotransplantation among lower extremity amputees. Plast Reconstr Surg 2014; 134:1334–1342. [DOI] [PubMed] [Google Scholar]
- 70.Kueckelhaus M, Fischer S, Seyda M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl Int 2016; 29:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leventhal JR, Elliott MJ, Yolcu ES, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation 2015; 99:288–298. [DOI] [PubMed] [Google Scholar]
- 72.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 2008; 358:362–368. [DOI] [PubMed] [Google Scholar]
- 73.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ravindra KV, Xu H, Bozulic LD, et al. The need for inducing tolerance in vascularized composite allotransplantation. Clin Dev Immunol 2012; 2012:438078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fryer M, Grahammer J, Khalifian S, et al. Exploring cell-based tolerance strategies for hand and face transplantation. Expert Rev Clin Immunol 2015; 11:1189–1204. [DOI] [PubMed] [Google Scholar]
- 76.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol 2009; 21:81–86. [DOI] [PubMed] [Google Scholar]
- 77.Andrea B, Annegret W, Seebach D, Rieben R. Symposium on xenotransplantation, 189; 2013. doi:10.1111/xen.12155. [Google Scholar]
- 78.Pullen LC. Xenotransplantation: time to get excited? Am J Transplant 2017; 17:2995–2996. [DOI] [PubMed] [Google Scholar]
- 79.Reardon S. New life for pig-to-human transplants. Nature 2015; 527:152–154. [DOI] [PubMed] [Google Scholar]
- 80.Barkai U, Weir GC, Colton CK, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant 2013; 22:1463–1476. [DOI] [PubMed] [Google Scholar]
- 81.Yagi H, Fukumitsu K, Fukuda K, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 2013; 22:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fung RKF, Kerridge IH. Gene editing advance re-ignites debate on the merits and risks of animal to human transplantation. Intern Med J 2016; 46:1017–1022. [DOI] [PubMed] [Google Scholar]
- 83▪.Tena AA, Sachs DH, Mallard C, et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 2017; 101:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xenotransplantation synergizes with organ preservation approaches as it offers a virtually limitless supply of donor organs, but requires preservation for efficient transportation and storage of these organs and tissues. Through supplying human CD47, the authors were able to extend the graft lifespan of porcine skin grafted onto nonhuman primates. This is promising as the primate immune response is a critical barrier that must be overcome to enable successful xenotransplantation.
- 84. National Center for Health Statistics. Health, United States, 2016. Center for Disease Control; 2017. [Google Scholar]
- 85.Sobrino J, Shafi S. Timing and causes of death after injuries. Proc (Bayl Univ Med Cent) 2013; 26:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008; 92:761–794. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Writing Group Members, Mozaffarian D, Benjamin EJ, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 88.Nelson DP. Donor Management Research Consensus Conference. Int Soc Hear Lung Transplant Links e-Newsletters 2013. Available from: https://www.ishlt.org/ContentDocuments/2013Nov_Links.html. [Google Scholar]
- 89.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg 2015; 150:252–259. [DOI] [PubMed] [Google Scholar]
- 90.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2015 Annual Data Report: pancreas. Am J Transplant 2017; 17 Suppl 1:117–173. [DOI] [PubMed] [Google Scholar]
- 91.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: lung. Am J Transplant 2017; 17 Suppl 1:357–424. [DOI] [PubMed] [Google Scholar]
- 92.Colvin M, Colvin M, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: heart. Am J Transplant 2017; 17 Suppl 1:286–356. [DOI] [PubMed] [Google Scholar]
- 93.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: kidney. Am J Transplant 2017; 17 Suppl 1:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: liver. Am J Transplant 2017; 17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 95.Smith JM, Skeans MA, Horslen SP, et al. OPTN/SRTR 2015 Annual Data Report: intestine. Am J Transplant 2017; 17 Suppl 1:252–285. [DOI] [PubMed] [Google Scholar]
- 96.Lee E-M, Lee YE, Lee E, et al. Protective effect of heme oxygenase-1 on high glucose-induced pancreatic β-cell injury. Diabetes Metab J 2011; 35:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. OPTN/SRTR. Unadjusted graft and patient survival at 3 months, 1, 3, 5, & 10Y. OPTN/SRTR Annual Report - 2011 ADR Data Tables Section 1. 13 (2011). [Google Scholar]
- 98.Abboudi H, Macphee IA. Individualized immunosuppression in transplant patients: potential role of pharmacogenetics. Pharmgenomics Pers Med 2012; 5:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sypek M, Kausman J, Holt S, Hughes P. HLA epitope matching in kidney transplantation: an overview for the general nephrologist. Am J Kidney Dis 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 100.Ansari D, Bućin D, Nilsson J. Human leukocyte antigen matching in heart transplantation: systematic review and meta-analysis. Transpl Int 2014; 27:793–804. [DOI] [PubMed] [Google Scholar]
- 101.Kawai T, Sachs DH, Sykes M, Cosimi AB. Immune Tolerance Network. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2013; 368:1850–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351:2715–2729. [DOI] [PubMed] [Google Scholar]
- 103.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 2004; 4:222–230. [DOI] [PubMed] [Google Scholar]
- 104.Scandling JD, Busque S, Shizuru JA, et al. Induced immune tolerance for kidney transplantation. N Engl J Med 2011; 365:1359–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjo OH, Larsen S, Lunde OC, Nesbakken A. Short term outcome after emergency and elective surgery for colon cancer. Color Dis 2009; 11:733–739. [DOI] [PubMed] [Google Scholar]
- 106▪.Mullen MG, Michaels AD, Mehaffey JH, et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining ‘quality’ and reporting outcomes for urgent surgery. JAMA Surg 2017; 152:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]; Along with the increased flexibility offered by prolonged organ preservation durations is the distinct advantage of scheduling transplants as an elective procedure. This retrospective showed that elective surgeries have significantly lower rates of complications and mortality. Therefore, preservation durations that allow elective scheduling of transplants should improve patient outcomes even without the additional benefits discussed in the body of this review.
- 107.Silingardi R, Gennai S, Leone N, et al. Italian mbEVAR study group. Standard ‘off-the-shelf’ multibranched thoracoabdominal endograft in urgent and elective patients with single and staged procedures in a multicenter experience. J Vasc Surg 2017; 67:1005–1016. [DOI] [PubMed] [Google Scholar]
- 108.Jochmans I, Fieuws S, Tieken I, et al. The impact of hepatectomy time of the liver graft on posttransplant outcome: a Eurotransplant Cohort Study. Ann Surg 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 109.Coffey JC, Wanis KN, Monbaliu D, et al. The influence of functional warm ischemia time on DCD liver transplant recipients’ outcomes. Clin Transplant 2017; 31:e13068. [DOI] [PubMed] [Google Scholar]
- 110. [[Accessed 1 January 2016]]. Organ Recovery Systems. LifePort Kidney Transporter. Available at: http://www.organ-recovery.com/lifeport-kidney-transporter. [Google Scholar]
- 111. [[Accessed 1 January 2016]]. Organ Recovery Systems. LifePort Liver Transporter. Available at: http://www.organ-recovery.com/lifeport-liver-transporter. [Google Scholar]
- 112.Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl Int 2015; 28:634–642. [DOI] [PubMed] [Google Scholar]
- 113. [[Accessed 1 January 2016]]. Perfusix, 2016. Available at: http://www.perfusix.com/ [Google Scholar]
- 114. [[Accessed 14 December 2017]]. Functional Circulation, 2013. Available at: http://www.functionalcirculation.com/Site/Welcome.html. [Google Scholar]
- 115. [[Accessed 1 October 2018]]. Liver Assist. 2017. Available at: https://www.organ-assist.nl/products/liver-assist. [Google Scholar]
- 116▪.Machuca TN, Cypel M, Bonato R, et al. Safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy in a large animal lung transplant survival model. Hum Gene Ther 2017; 28:757–765. [DOI] [PubMed] [Google Scholar]; Ex-vivo adenoviral delivery to an organ in a large animal transplant model is a proof-of-principle that organ augmentation is possible and also that pro-inflammatory cytokine levels, linked to primary graft dysfunction, can be decreased.
- 117.Yeung JC, Wagnetz D, Cypel M, et al. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre and postlung transplantation in the pig. Mol Ther 2012; 20:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009; 1:4ra9. [DOI] [PubMed] [Google Scholar]
- 119.Brasile L. Orlando G. Immunocloaking. Regen Med Appl Organ Transplant 2014; 919–933. [Google Scholar]
- 120.Yang B, Hosgood SA, Nicholson ML. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation 2011; 91:501–507. [DOI] [PubMed] [Google Scholar]
- 121.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int 2015; 28:690–699. [DOI] [PubMed] [Google Scholar]
- 122.Fishman JA, Greenwald MA, Grossi PA. Transmission of infection with human allografts: essential considerations in donor screening. Clin Infect Dis 2012; 55:720–727. [DOI] [PubMed] [Google Scholar]
- 123.Desai R, Collett D, Watson CJ, et al. Cancer transmission from organ donors-unavoidable but low risk. Transplantation 2012; 94:1200–1207. [DOI] [PubMed] [Google Scholar]
- 124.Andreasson A, Karamanou DM, Perry JD, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant 2014; 33:910–916. [DOI] [PubMed] [Google Scholar]
- 125▪▪.Nakajima D, Cypel M, Bonato R, et al. Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant 2016; 16:1229–1237. [DOI] [PubMed] [Google Scholar]; Human donor lungs with various infections were treated with antibiotics during ex-vivo perfusion for 12 h and all of the infections resolved. This could increase donor organ supply by allowing transplantation of organs that would otherwise be discarded because of infection.
- 126.Sridharan GV, Bruinsma BG, Bale SS, et al. Metabolomic modularity analysis (MMA) to quantify human liver perfusion dynamics. Metabolites 2017; 7: pii: E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127▪▪.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 2016; 16:3235–3245. [DOI] [PubMed] [Google Scholar]; Livers that were declined for transplantation were treated by normothermic machine perfusion and successfully transplanted. This highlights the potential of organ preservation to increase organ supply by increasing use of marginal and declined organs.
- 128.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011; 364:1431–1440. [DOI] [PubMed] [Google Scholar]
- 129.Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int 2015; 28:657–664. [DOI] [PubMed] [Google Scholar]
- 130.Hsin MK, Zamel R, Cypel M, et al. Metabolic profile of ex vivo lung perfusate yields biomarkers for lung transplant outcomes. Ann Surg 2018; 267:196–197. [DOI] [PubMed] [Google Scholar]
- 131. [[Accessed: 21st September 2016]]. Global Observatory on Donation and Transplantation (GOTD). Transplant-observatory.org. (2015). Available at: http://www.transplant-observatory.org/summary/ [Google Scholar]
- 132. World Population Prospects: the 2015 Revision 2015. [Google Scholar]
- 133.White SL, Hirth R, Mahíllo B, et al. The global diffusion of organ transplantation: trends, drivers and policy implications. Bull World Health Organ 2014; 92:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. [[Accessed 30 January 2018]]. Korle Bu Teaching Hospital in Ghana. Department of Surgery. (2018). Available at: http://www.kbth.gov.gh/centres-and-departments/department-of-surgery.html. [Google Scholar]
- 135. [[Accessed 30 January 2018]]. Primus International Super Specialty Hospital, N. Best Kidney Transplant Hospital in Nigeria. 2017. Available at: http://www.primushospitalnigeria.com/kidney-transplant-dialysis.html. [Google Scholar]
- 136. Kunda S. Zambia: ‘UTH can now do organ transplants. News of Africa; 2018. [Google Scholar]
- 137.Manipal Hospitals. Manipal Hospitals - leaders in transplant innovation and expertise; 2018. [Google Scholar]
- 138.Perosa M, Branez L, Mota B, et al. Transplants without borders – a project for in locus training of new transplants centers in Brazil. Am J Transpl 2016; 16 suppl 3: [Google Scholar]
- 139.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385:1975–1982. [DOI] [PubMed] [Google Scholar]
- 140.Dieleman JL, Baral R, Birger M, et al. US spending on personal healthcare and public health, 1996-2013. JAMA 2016; 316:2627–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bentley TS, Phillips SJ. 2017 U.S. organ and tissue transplant cost estimates and discussion, 2017. [Google Scholar]
- 142.Serper M, Bittermann T, Rossi M, et al. Functional status, healthcare utilization, and the costs of liver transplantation. Am J Transplant 2017; 1–10. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 143.Luc JGY, Jackson K, Weinkauf JG, et al. Feasibility of lung transplantation from donation after circulatory death donors following portable ex vivo lung perfusion: a pilot study. Transplant Proc 2017; 49:1885–1892. [DOI] [PubMed] [Google Scholar]
- 144.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. J Hear Lung Transplant 2017; 36:744–753. [DOI] [PubMed] [Google Scholar]
- 145▪▪.Held PJ, McCormick F, Ojo A, Roberts JP. A cost-benefit analysis of government compensation of kidney donors. Am J Transplant 2016; 16:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kidney transplantion represents significant cost savings over a lifetime of expensive dialysis treatments. With the increased health and quality of life factored in, each additional transplant enabled by preservation technologies would save the American government/taxpayers millions of dollars.
- 146.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996; 50:235–242. [DOI] [PubMed] [Google Scholar]
- 147.Knoll G. Trends in kidney transplantation over the past decade. Drugs 2008; 68 Suppl 1:3–10. [DOI] [PubMed] [Google Scholar]
- 148.Shores JT, Malek V, Lee WPA, Brandacher G. Outcomes after hand and upper extremity transplantation. J Mater Sci Mater Med 2017; 28:72. [DOI] [PubMed] [Google Scholar]


