Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, diagnosis, histology, open lung biopsy, outcome, steroid treatment
Abstract
Objectives:
Approximately half of the patients undergoing lung biopsy for nonresolving acute respiratory distress syndrome exhibit another histologic pattern than diffuse alveolar damage, with some of the pathologies characterized by a potential response to corticosteroids. This study aimed to assess whether open lung biopsy performed in the ICU for nonresolving acute respiratory distress syndrome was able to identify steroid-sensitive diseases and whether patients with a steroid-sensitive pathology experienced different clinical courses and outcomes.
Design:
Retrospective analysis.
Setting:
One 22-bed mixed ICU within a tertiary medical center.
Patients:
Patients age greater than or equal to 16 years old who met the Berlin definition for acute respiratory distress syndrome and underwent open lung biopsy from January 2007 to January 2017.
Interventions:
None.
Measurements and Main Results:
During the study period, 695 patients diagnosed with acute respiratory distress syndrome were identified, 51 (7%) of whom underwent open lung biopsy. An alternative diagnosis to diffuse alveolar damage was found in 29 patients (57%), and a steroid-sensitive pathology was identified in 19 (37%). In-hospital and 180-day mortality rates were 55% and 61%, respectively. There was a significant difference in hospital mortality and 180-day mortality rates between patients with steroid-sensitive pathology and those with steroid-resistant pathology (37% vs 65%; p < 0.045 and 37% vs 75%; p < 0.007, respectively). We did not identify any variable that could reliably predict a steroid-sensitive histologic pattern before open lung biopsy.
Conclusions:
Open lung biopsy was able to identify a steroid-sensitive pathology in a significant proportion of nonresolving acute respiratory distress syndrome patients. These patients had a better outcome, with lower hospital mortality and 180-day mortality.
Acute respiratory distress syndrome (ARDS) is common in critically ill patients, while associated with high mortality and morbidity (1). The Berlin definition of ARDS proposes clinical and radiologic criteria for ARDS diagnosis and classification (2). The Berlin criteria have been widely used in clinical practice and as inclusion criteria in numerous clinical trials. However, they do not take into account pathologic findings for diagnosing ARDS.
Although diffuse alveolar damage (DAD) is considered the pathologic hallmark of ARDS, it is known, since its original description (3), that many patients with ARDS diagnosis do not suffer from DAD. A recent autopsy study by Thille et al (4) showed that among 356 patients meeting the Berlin ARDS definition, only 45% had DAD at autopsy. Similar results were found in patients undergoing open lung biopsy (OLB) for nonresolving ARDS (5–8). Pathologic findings other than DAD comprise interstitial lung disease, pneumonia, cancer, organizing pneumonia, alveolar hemorrhage, or drug reactions (5–8).
These conditions unlikely share the same pathophysiology and the same response to pharmaceutical treatment. The heterogeneity of ARDS patients included in therapeutic trials remains a challenge when interpreting trial results. In particular, the use of steroids in ARDS has been a matter of debate for over 20 years, with some trials (9, 10) showing a decreased mortality, in contrast to others (11). However, since none of these trials used lung biopsy–based histologic diagnosis, the underlying pathology remains largely unknown.
Our study primarily sought to assess whether OLB in nonresolving ARDS would identify pathologies other than DAD, with a particular focus on corticosteroid-sensitive pathologies. In addition, we aimed to evaluate whether the clinical characteristics and outcomes differed in patients with steroid-sensitive pathologies, compared with patients with other pathologies.
MATERIAL AND METHODS
The appropriate ethics committee (Comité d’Ethique Hospitalo-Facultaire, Saint-Luc, UCL N° 2017/21AOU/408) approved the reporting of our study results. Informed consent from the patients or their next of kin was waived due to the retrospective nature of the analysis.
Patients
The hospital records of all ARDS patients who underwent OLB from January 2007 to January 2017 in a 22-bed tertiary care mixed ICU were reviewed. The medical files of all patients with a discharge diagnosis code of 518.82 (according to the International Classification of Diseases, 9th Revision [ICD-9], “clinical modification suggesting ARDS”) and procedure code of 3,328 (OLB) or 3,402 (exploratory thoracotomy) were reviewed for possible inclusion in this study.
The inclusion criteria were as follows: 1) age greater than or equal to 16 years at the time of biopsy and 2) findings consistent with the Berlin ARDS definition (2). ARDS was characterized as mild/moderate/severe, as described in the Berlin definition, at the time of both diagnosis and biopsy (2).
OLB
OLB was conducted in the event of persistent respiratory failure, after ongoing lung infection was ruled out (via bronchoalveolar lavage [BAL] or via endotracheal aspirates) or when an alternative diagnosis was suspected, based on patient history, clinical and radiologic presentation. In most cases, OLB was performed at patient bedside in the ICU. In all cases, lung biopsy was performed using minithoracotomy. Two large lung samples were taken from two different lobes, with one chest tube inserted before suturing the chest.
Lung Pathology Grouping
All samples were examined by an experienced pathologist. In complex cases, a second pathologist was consulted, and discrepancies were resolved through consensus. The criteria for DAD diagnosis have been previously described (12). Other histologic patterns were defined in line with published guidelines (13–20). Patients were divided into a “corticosteroid-sensitive” and “corticosteroid-resistant” groups based on histologic findings, depending on published guidelines or commonly accepted expert opinions (13, 15, 17, 21–29). Importantly, the classification was based on histopathologic diagnosis and not on the presence or the absence of fibroproliferative response within the examined samples. Details regarding the exact methodology used for pathology classification are described in Appendix 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/D395).
Steroids Prescription Policy
When the risk is not deemed too high, empirical steroid therapy is systematically initiated right after OLB, while awaiting for definitive results of lung histology. Steroids are then usually discontinued if lung histology does not identify a steroid-sensitive pattern.
Data Collection
All hospital records were retrospectively reviewed. Study day 0 corresponded to the first day the patient met the diagnostic ARDS criteria. Demographic data and underlying conditions were recorded. Acute Physiology and Chronic Health Evaluation II score and Sequential Organ Failure Assessment (SOFA) score were calculated at ICU admission and lung biopsy days. In addition, laboratory data, ventilator settings, and respiratory variables were recorded for the first 7 days from ARDS diagnosis, on the day of lung biopsy, and for 7 days following corticosteroid introduction (if applicable) or lung biopsy (if no corticosteroids were given). Diagnostic procedures before lung biopsy (BAL and chest CT), adjuvant therapies, pathologic diagnosis, therapeutic alterations (new therapies or cessation of unnecessary therapy), as well as surgery-related complications were collected and recorded. Patients were followed up until hospital discharge, and it was recorded whether they were alive or dead at ICU discharge, at hospital discharge, and at 6-month follow-up.
Statistical Analyses
Statistical analyses were performed using the SPSS 21 software (SPSS software [IBM Corp. 2011. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp]), with graphs drawn using Graphpad Prism 7.5 (GraphPad Software, La Jolla, CA). Values were expressed as median (first–third quartiles) for continuous values and counts (per percent of group) for qualitative variables. The data were subjected to Kolmogorov-Smirnov normality test and Bartlett’s test for homogeneity of variance. We compared clinical and demographic variables between patients with and without a steroid-sensitive pathology, using the chi-square test (or Fisher exact test when appropriate) and unpaired t test (or the Wilcoxon rank-sum test according to statistical distribution) for quantitative data, respectively. Besides, nonlinear regression was employed to adjust the variations of biological and ventilatory variables over time in each group (from day 0 to day 6). The extra sum-of-squares F test was applied for comparison between curves. Furthermore, survival curves were generated using the Kaplan-Meier method, compared by log-rank test. All tests were two sided, with significance level set at 0.05.
RESULTS
From January 2007 to January 2017, 695 patients admitted in the ICU with the diagnosis of ARDS were retrieved from ICD-9 coding. Among them, a surgical lung biopsy was performed in 51 patients (7%). Ventilatory data were partially missing in four patients. These patients were included in the review but excluded from the analysis of ventilatory variables.
The patient characteristics at baseline and at OLB have been summarized in Table 1. Twenty-seven patients had immunocompromised status. The main immunocompromising conditions are listed in Table S1 (Supplemental Digital Content 2, http://links.lww.com/CCM/D396).
TABLE 1.
Characteristics at ICU Admission and at Open Lung Biopsy for 51 Nonresolving Acute Respiratory Distress Syndrome Patients, With or Without a Pathologic Diagnosis Suggesting Beneficial Corticosteroid Effects
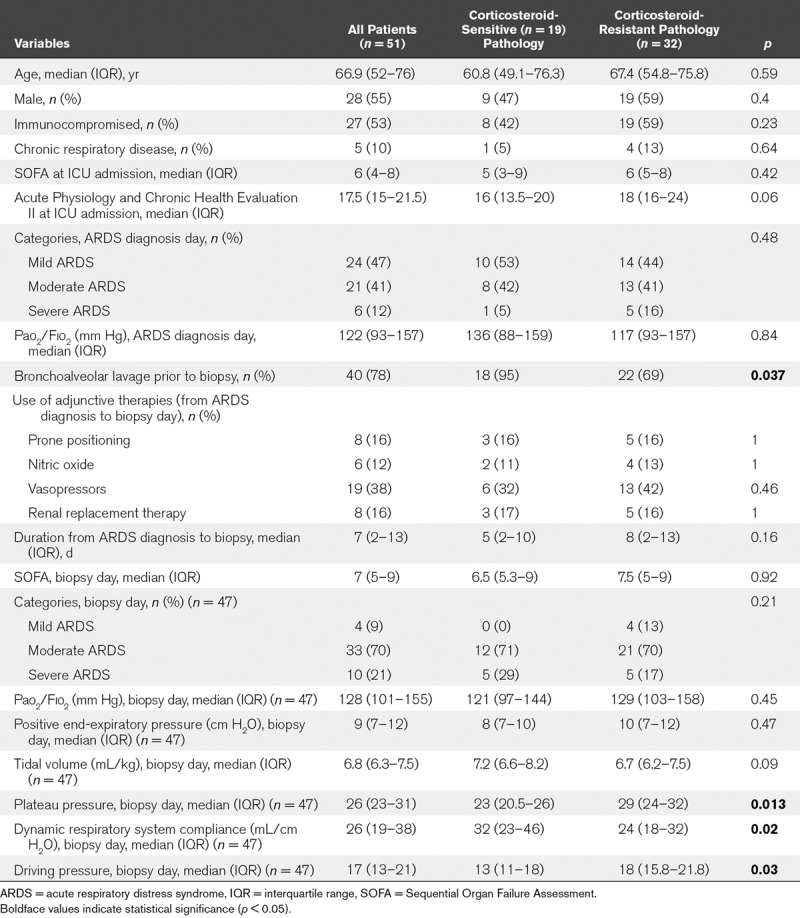
When patients with and without corticosteroid-sensitive pathology were compared at baseline, we found no significant differences in terms of organ support, ARDS severity, or SOFA score. At OLB time, plateau pressure and driving pressure were significantly lower and dynamic respiratory system compliance significantly higher in the steroid-sensitive group.
When we compared between both groups, the progression of physiologic and respiratory variables, from day 0 (day of ARDS diagnosis) to day 6, no significant difference was, however, found, as shown in Figure 1. The progression of biological variables during the same period is summarized in Figure S1 (Supplemental Digital Content 3, http://links.lww.com/CCM/D397; legend, Supplemental Digital Content 5, http://links.lww.com/CCM/D399).
Figure 1.
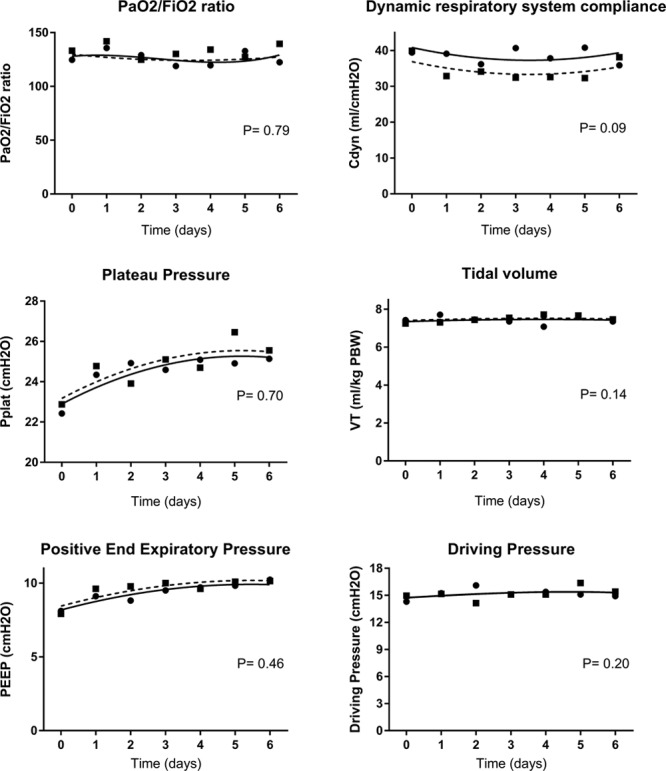
Time course of respiratory variables of nonresolving acute respiratory distress syndrome (ARDS) patients with (circles, continuous line) or without (squares, dashed line) “corticosteroid-sensitive” pathology. Day 0 is the day of ARDS diagnosis. Cdyn = dynamic respiratory system compliance, Plat = plateau pressure, PEEP = positive end-expiratory pressure, VT = tidal volume.
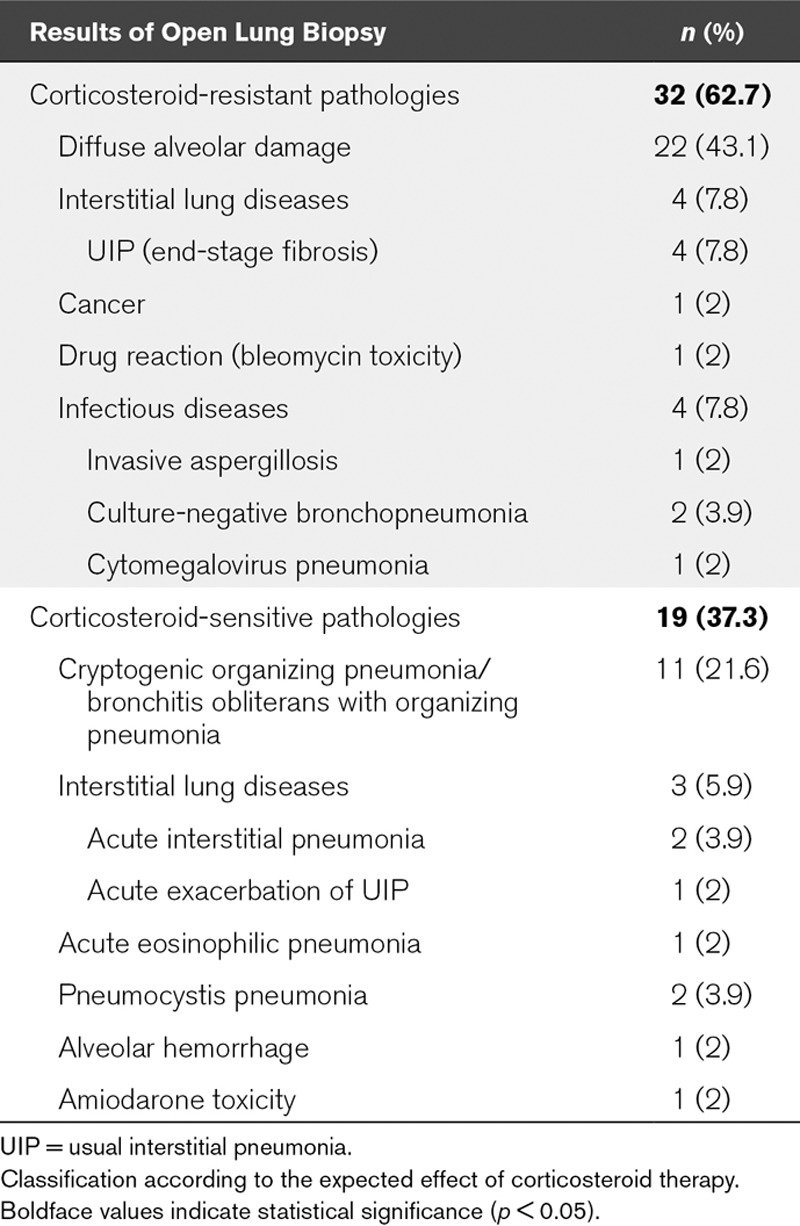
The OLB results have been summarized in Table 2. OLB revealed a diagnosis other than DAD in 29 patients (57%), with a corticosteroid-sensitive pathology identified in 19 (37%).
TABLE 2.
Pathologic Diagnosis of Patients With Nonresolving Acute Respiratory Distress Syndrome Undergoing Open Lung Biopsy (n = 51)
Based on biopsy results, therapy modifications were carried out on 38 patients (75%) (Table 3), with significantly more therapeutic changes in the steroid-sensitive group (89% vs 66%; p = 0.047). Thus, systemic corticotherapy was initiated in more patients from the steroid-sensitive groups (89% vs 58%; p < 0.001), with a trend toward a longer duration of steroid therapy (25.5 vs 9 d; p = 0.14). Besides, the two patients in whom steroids were not initiated in the steroid-sensitive group were already under systemic steroids at the time of OLB. OLB complications occurred in six patients (12%), although no death was directly attributable to OLB. Two patients developed pneumothorax with subcutaneous emphysema requiring chest tube insertion, three patients had persistent air leaks, and another patient developed acute cor pulmonale with hypotension and low cardiac output in the post-OLB hours.
TABLE 3.
Outcomes After Open Lung Biopsy for 51 Acute Respiratory Distress Syndrome Patients, With or Without a Pathologic Diagnosis Suggesting a Beneficial Effect From Corticosteroids
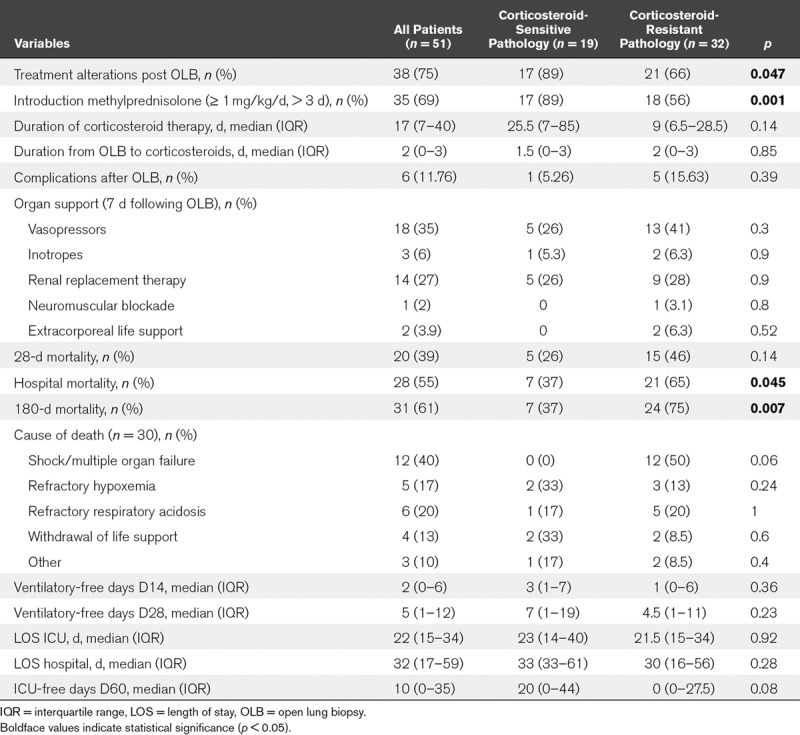
Overall in-hospital and 180-day mortality rates were 55 and 61%, with a significant difference between patients with and without steroid-sensitive pathology (37% vs 65%; p < 0.045 and 37% vs 75%; p < 0.007, respectively), which was confirmed by Kaplan-Meier analysis (Fig. 2) (log-rank p < 0.015). Conversely, there was no statistically significant between-group difference revealed in terms of mechanical ventilation duration or hospital length of stay.
Figure 2.
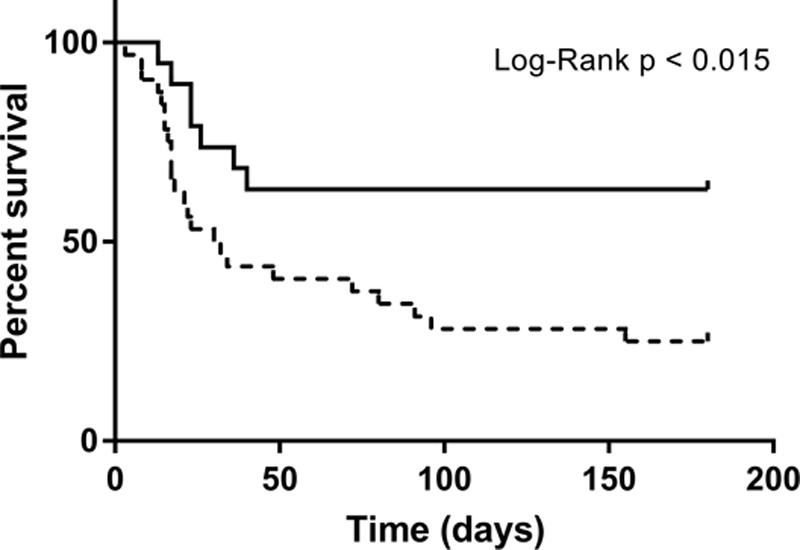
Kaplan-Meier survival analysis comparing nonresolving acute respiratory distress syndrome patients with (continuous line) or without (dashed line) “steroid-sensitive” pathology.
The evolution of respiratory variables after lung biopsy is summarized in Figure S2 (Supplemental Digital Content 4, http://links.lww.com/CCM/D398; legend, Supplemental Digital Content 5, http://links.lww.com/CCM/D399).
DISCUSSION
To our knowledge, this is the first study to address OLB’s role in identifying corticosteroid-sensitive pathologies in patients with nonresolving ARDS. Based on our study findings, corticosteroid-sensitive pathologies were commonly observed in patients undergoing OLB for nonresolving ARDS and patients with steroid-sensitive pathology presented a better outcome, with lower hospital and 180-day mortality.
The relationship between histology and clinical outcomes in nonresolving ARDS has been previously studied, yet with conflicting results. In the only prospective study so far, Papazian et al (8) reported, in 2007, a contributive result of OLB associated with a better survival. Interestingly, there was an unexpectedly low rate of DAD and high rate of cytomegalovirus pneumonia found in their series. Furthermore, the major medication changes consisted of adding ganciclovir for cytomegalovirus infection or glucocorticoids for fibroproliferative ARDS, and thus therapies whose beneficial effects are still controversial. Recently, major studies were mainly focused on identifying DAD in patients with nonresolving ARDS, and they revealed conflicting results as to the relationship between a specific DAD pattern and mortality (5, 6, 30, 31). From a clinical point of view, however, DAD identification may not add any relevant information, given the lack of specific therapy targeting this condition. Conversely, identifying steroid-sensitive pathologies is associated with important therapeutic implications.
In our series, DAD was found in 59% of patients, being the only pathologic finding in 43% of all patients. This was in accordance with recent series, sharing comparable criteria in view of performing OLB (5, 6). We identified a corticosteroid-sensitive pathology in 37% of our patients. In our series, the main alternatives to DAD diagnosis were cryptogenic organizing pneumonia (COP), interstitial lung diseases, and infections, in line with previously reported studies (5–7, 30). However, we found an unexpectedly high frequency of bronchitis obliterans with organizing pneumonia (BOOP)/COP in our series (21% of patients vs 4–11% in previously reported studies) (5–7). One of the possible explanations could refer to the high proportion of immunocompromised patients in our series, (53% of patients vs 24–38% in other OLB series) (5–7), reflecting a possible selection bias. Since organizing pneumoniae are shown to be frequently associated with immunocompromising conditions (27), the characteristics of our population could likely explain the high COP/BOOP frequency.
Importantly, in some patients in our series, despite a comprehensive diagnostic workup including BAL, OLB showed pathologic processes that were theoretically amenable to a bronchoscopic diagnosis (such as Pneumocystis pneumonia, diffuse alveolar hemorrhage, acute eosinophilic pneumonia). In those cases, OLB was performed either because BAL was of poor quality and considered inconclusive or because there was a discrepancy between the conditions diagnosed by the BAL and the clinical evolution under an ongoing, well-conducted treatment. In our opinion, this underlines the limits of bronchoscopy performed in ARDS patients, in whom high Fio2 and concerns about poor tolerance impose to limit the volume of the fluid used to perform BAL, potentially leading to decreased sensitivity or results interpretation issues. If BAL is commonly admitted as a powerful diagnosis tool in mechanically ventilated patients, to the best of our knowledge, its diagnostic yield has never been formally questioned in the context of ARDS.
One of our main study findings was that in-hospital and 180-day mortality rates were significantly lower in patients with a steroid-sensitive pathology (37% vs 65% and 37% vs 75%, respectively). Surprisingly, there was no statistically significant difference regarding ventilator-free days or regarding the evolution of the main respiratory variables (out of a statistically significant increase of dynamic compliance under steroid treatment, as shown in Fig S2, Supplemental Digital Content 4, http://links.lww.com/CCM/D398), possibly due to the limited number of patients in our series. We report an overall in-hospital mortality rate of 55% and 180-day mortality rate of 61%. Other studies involving patients with nonresolving ARDS found similarly high mortality rates (5–8, 30).
OLB safety in our study proved satisfactory with procedure-related complications in 12% of patients, without any death directly attributed to OLB. This is consistent with previous studies, yet lower than the overall surgical complication rate of 22% reported in a recent meta-analysis by Libby et al (32).
We were unable to identify any clinical, respiratory, or biological variable strongly related to a steroid-sensitive histologic pattern, out of a small difference in plateau pressure, driving pressure, and dynamic respiratory system compliance at the time of biopsy. However, there was a trend toward a shorter duration between ARDS diagnosis and OLB in the steroid-sensitive group (5 vs 8 d), possibly explaining the observed differences at the time of OLB.
Our findings highlight how challenging the interpretation of previous trials with respect to corticosteroid benefits in ARDS may prove to be. In a small randomized controlled trial, Meduri et al (9) revealed a marked reduction in ICU mortality in patients treated with systemic corticosteroids for persistent ARDS. In addition, a recent patient-level meta-analysis conducted by Meduri et al (33) suggested a beneficial impact of corticosteroid adjunction, resulting in decreased in-hospital mortality. Conversely, a multicenter randomized controlled trial conducted by the ARDS Network failed to show any difference in ICU or hospital mortality rates, nor in ICU stay length, despite a shorter duration of mechanical ventilation in the steroid group (11). Furthermore, in the steroid group, mortality was increased in patients enrolled later in the course of ARDS, suggesting that unproper use of steroids could be harmful. It should, however, be noted that none of these trials required a histologic diagnosis using lung biopsy. Thereby, it is not possible for us to exclude that the beneficial effects on survival or ventilatory endpoints observed in these studies could, at least to some extent, be attributed to the favorable progression of steroid-sensitive pathologies, upon systemic corticosteroid exposure.
Evidently, lung biopsy is not a practical tool to prospectively improve patient selection for clinical trials. Therefore, there is a need for new strategies to better define ARDS patient subsets that might benefit from specific treatments, such as corticosteroids. Based on the data from large randomized ARDS trials, Calfee et al (34) recently identified two ARDS subphenotypes with distinct natural histories, clinical and biomarker profiles, and differential response to therapy consisting of positive end-expiratory pressure and fluid management (35). It is unclear whether both subphenotypes were related to distinct histopathologic patterns, such as corticosteroid-sensitive pathologies. Studies using OLB to compare underlying histology between both phenotypes could prove useful. Another approach might be to use specific biomarkers. Some of the latter, such as N-Terminal-Procollagen III, could help identify a specific patient subset with an expected good response to corticosteroids (36).
We recognize that there are limitations to our study. First, it is a retrospective analysis, involving a single referral center, with a relatively small sample size of OLB. Second, classifying pathologies according to their expected response to corticosteroids proves challenging, and our classification may be subject to criticism. Most of these diseases are rare, with scarce evidence as to their optimal treatment, particularly in ventilated, critically ill patients. Furthermore, even in pathologies allegedly steroid sensitive, the benefits from corticosteroids depend upon several factors such as daily dose and initiation timing. Third, as our classification did not take into account the presence of fibroproliferative response, we cannot rule out that some potentially beneficial effects of steroids might have been missed or that steroids could have contributed to improve outcomes in the steroid-sensitive group through the inhibition of fibroproliferation. Thus, the retrospective design of the study cannot rule out the confounding effect of a higher proportion of corticosteroid therapy introduced in the steroid-sensitive group. Fourth, the decision to perform OLB was highly selective, restricted to patients with nonresolving ARDS or a high suspicion of an alternative diagnosis. We calculated that only 7% of all ARDS patients underwent lung biopsy (based on an estimated 695 patients diagnosed with ARDS, retrieved from ICD-9 coding). Although this selection bias could likely increase the probability of an alternative diagnosis, other studies investigating OLB in ARDS report similar biopsy rates (5–8).
CONCLUSIONS
In this retrospective review of prospectively collected data in a single academic center, a significant proportion of patients who underwent OLB for nonresolving ARDS suffered from a pathologic process that could likely benefit from corticosteroids. Patients with steroid-sensitive pathology displayed a better outcome, with lower hospital and 180-day mortality rates. No demographic, clinical, biological, or respiratory variables could reliably predict a steroid-sensitive pattern on lung biopsy. Other less invasive strategies able to identify treatment-specific ARDS subsets are not yet available in clinical routine. Thus, as OLB was safe, well tolerated, and able to add clinically relevant information in a significant proportion of patients, it could be considered in patients with nonresolving ARDS.
Supplementary Material
Footnotes
*See also p. 1017.
This work was performed at Université Catholique de Louvain (UCL), Cliniques Universitaires Saint-Luc, Brussels, Belgium.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force: Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967; 2:319–323. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767. [DOI] [PubMed] [Google Scholar]
- 5.Guerin C, Bayle F, Leray V, et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med 2015; 41:222–230. [DOI] [PubMed] [Google Scholar]
- 6.Kao KC, Hu HC, Chang CH, et al. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care 2015; 19:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SR, Karmpaliotis D, Ayas NT, et al. The role of open-lung biopsy in ARDS. Chest 2004; 125:197–202. [DOI] [PubMed] [Google Scholar]
- 8.Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 2007; 35:755–762. [DOI] [PubMed] [Google Scholar]
- 9.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. A randomized control trial. JAMA 1998; 280:159–165. [DOI] [PubMed] [Google Scholar]
- 10.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 2007; 131:954–963. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg KP, Hudson LD, Goodman RB, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354:1671–1684. [DOI] [PubMed] [Google Scholar]
- 12.Castro CY. ARDS and diffuse alveolar damage: A pathologist’s perspective. Semin Thorac Cardiovasc Surg 2006; 18:13–19. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society, European Respiratory Society: American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002; 165:277–304. [DOI] [PubMed] [Google Scholar]
- 14.Fend F, Prior C, Margreiter R, et al. Cytomegalovirus pneumonitis in heart-lung transplant recipients: Histopathology and clinicopathologic considerations. Hum Pathol 1990; 21:918–926. [DOI] [PubMed] [Google Scholar]
- 15.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 2010; 137:1164–1171. [DOI] [PubMed] [Google Scholar]
- 16.Luna MA, Bedrossian CW, Lichtiger B, et al. Interstitial pneumonitis associated with bleomycin therapy. Am J Clin Pathol 1972; 58:501–510. [DOI] [PubMed] [Google Scholar]
- 17.Martin SI, Fishman JA; AST Infectious Diseases Community of Practice: Pneumocystis pneumonia in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):272–279. [DOI] [PubMed] [Google Scholar]
- 18.Myers JL, Kennedy JI, Plumb VJ. Amiodarone lung: Pathologic findings in clinically toxic patients. Hum Pathol 1987; 18:349–354. [DOI] [PubMed] [Google Scholar]
- 19.Stergiopoulou T, Meletiadis J, Roilides E, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007; 127:349–355. [DOI] [PubMed] [Google Scholar]
- 20.Tazelaar HD, Linz LJ, Colby TV, et al. Acute eosinophilic pneumonia: Histopathologic findings in nine patients. Am J Respir Crit Care Med 1997; 155:296–302. [DOI] [PubMed] [Google Scholar]
- 21.de Prost N, Parrot A, Cuquemelle E, et al. Diffuse alveolar hemorrhage in immunocompetent patients: Etiologies and prognosis revisited. Respir Med 2012; 106:1021–1032. [DOI] [PubMed] [Google Scholar]
- 22.Maher TM, Whyte MK, Hoyles RK, et al. Development of a consensus statement for the definition, diagnosis, and treatment of acute exacerbations of idiopathic pulmonary fibrosis using the delphi technique. Adv Ther 2015; 32:929–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnell JA, Cannella AP, Injean P, et al. Adjunctive glucocorticoid therapy for non-HIV-related pneumocystis carinii pneumonia (NH-PCP). Am J Transplant 2014; 14:982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philit F, Etienne-Mastroïanni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med 2002; 166:1235–1239. [DOI] [PubMed] [Google Scholar]
- 25.Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J 2009; 16:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh GY, Kang EH, Chung MP, et al. Early intervention can improve clinical outcome of acute interstitial pneumonia. Chest 2006; 129:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: Clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011; 139:893–900. [DOI] [PubMed] [Google Scholar]
- 28.Moon SM, Kim T, Sung H, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother 2011; 55:4613–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother 2012; 18:793–806. [DOI] [PubMed] [Google Scholar]
- 30.Cardinal-Fernández P, Bajwa EK, Dominguez-Calvo A, et al. The presence of diffuse alveolar damage on open lung biopsy is associated with mortality in patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Chest 2016; 149:1155–1164. [DOI] [PubMed] [Google Scholar]
- 31.Kao KC, Chang CH, Hung CY, et al. Survival predictor in patients with acute respiratory distress syndrome and diffuse alveolar damage undergoing open lung biopsy. PLoS One 2017; 12:e0180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby LJ, Gelbman BD, Altorki NK, et al. Surgical lung biopsy in adult respiratory distress syndrome: A meta-analysis. Ann Thorac Surg 2014; 98:1254–1260. [DOI] [PubMed] [Google Scholar]
- 33.Meduri GU, Bridges L, Shih MC, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: Analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016; 42:829–840. [DOI] [PubMed] [Google Scholar]
- 34.Calfee CS, Delucchi K, Parsons PE, et al. ; NHLBI ARDS Network: Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Famous KR, Delucchi K, Ware LB, et al. ; ARDS Network: Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017; 195:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forel JM, Guervilly C, Hraiech S, et al. Type III procollagen is a reliable marker of ARDS-associated lung fibroproliferation. Intensive Care Med 2015; 41:1–11. [DOI] [PubMed] [Google Scholar]


