Abstract
Human papillomavirus (HPV) is required but not sufficient for cervical carcinoma (CxCa). Estradiol (E2) promotes CxCa development in K14E7 transgenic mice expressing the HPV16 E7 oncoprotein under the control of the keratin 14 (K14) promoter. E2 mainly works through estrogen receptor α (ERα). However, the role of ERα in human CxCa has been underappreciated largely because it is not expressed in carcinoma cells. We have shown that deletion of Esr1 (the ERα-coding gene) in the cervical stroma of K14E7 mice promotes regression of cervical intraepithelial neoplasia (CIN), the precursor lesion of CxCa. Here, we deleted Esr1 in the cervical epithelium but not stroma. We found that E2 induced cervical epithelial cell proliferation in epithelial ERα-deficient mice. We also found that E2 promoted the development of CIN and CxCa in epithelial ERα-deficient K14E7 mice, and all neoplastic epithelial cells were negative for ERα. In addition, proliferation indices were similar between ERα− and ERα+ CxCa. These results indicate that epithelial ERα is not necessary for E2-induced CIN and CxCa. Taken together, we conclude that stromal ERα, rather than epithelial ERα, mediates oncogenic E2 signaling in CxCa. Our results support stromal ERα signaling as a therapeutic target for the disease.
Keywords: cervical cancer, ERα, human papillomavirus (HPV), mouse model
Introduction
Cervical carcinoma (CxCa) caused by high-risk human papillomavirus (HPV) is the fourth leading cause of cancer death in women worldwide [1]. Among more than a dozen high-risk HPVs, HPV16 is responsible for 50% of CxCa. The HPV E6 and E7 oncoproteins are best known for their ability to inactivate p53 and pRb, respectively. Expression of E6 and E7 are not sufficient to cause CxCa in mice or to transform human cervical keratinocytes in vitro [2,3].
Long-term use of oral contraceptives and high parity are associated with increased risk of CxCa in HPV-infected women [4,5]. Exposure to diethylstilbestrol (synthetic estrogen) increases the risk of cervical intraepithelial neoplasia (CIN), the precursor lesion for CxCa [6]. Breast cancer patients who have used aromatase inhibitors are at a reduced risk of CIN compared to the nonuser group [7]. Aromatase is required for the biosynthesis of estradiol (E2), the most potent estrogen. Whilst these observations support estrogen as a risk factor, its mechanism in the pathogenesis of CxCa has been underexplored. In a transgenic mouse model expressing HPV16 E7 (K14E7), E2 promotes the development of CxCa preceded by CIN1, CIN2 and CIN3, which recapitulates human multistage cervical carcinogenesis [3]. Germline knockout of Esr1 (the murine ERα-coding gene) abrogates cervical carcinogenesis in K14E7 mice, demonstrating the requirement of estrogen receptor α (ERα), a ligand-dependent transcription factor [8]. However, its mechanism in human CxCa has been underappreciated. ERα is expressed in cancer-associated stroma, but not in cancer epithelial cells, suggesting that ERα in the stroma, rather than tumor cells, promotes CxCa [9]. In the present study, we show that specific deletion of epithelial Esr1 does not abrogate the development of CIN and CxCa. Our results support the notion that epithelial ERα plays little role in the cooperation of HPV with E2 in promoting CxCa.
Materials and Methods
Mouse strains are described in Table S1. Hormone treatment and procedures are described in Supplementary Information. All procedures for mice were carried out according to an animal protocol approved by the University of Houston Institutional Animal Care and Use Committee. Detailed procedures for tissue processing and histopathological analyses are described in Supplementary Information. Antibodies and immunohistochemistry (IHC) conditions are described in Table S2. One-sided Fisher’s exact tests were used for disease incidence and one-sided Wilcoxon rank sum test for epithelium thickness, cell proliferation, apoptosis and disease severity.
Results and Discussions
The cervical epithelium of Esr1ed/ed displays partial responses to E2
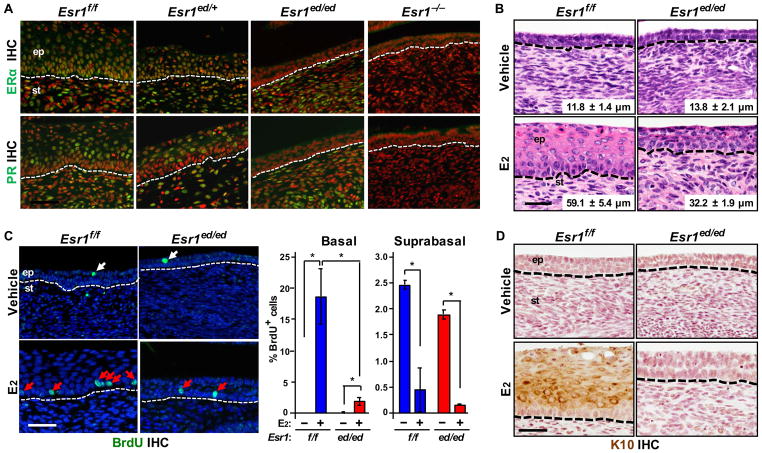
To study the role of epithelial ERα in the cervix we employed Wnt7a-Cre/Esr1f/f mice (referred to as Esr1ed/ed hereafter). ERα was specifically ablated in the cervical epithelia of Esr1ed/ed, confirmed by the absence of epithelial progesterone receptor (PR) (Figure 1A), a marker for ERα function [12]. E2 increased cervical epithelium thickness in Esr1f/f (P = 0.02) and Esr1ed/ed mice (P = 0.03) (Fig. 1B). Consistently, E2 increased BrdU incorporation (i.e., proliferation) in the basal layer of Esr1f/f and Esr1ed/ed cervical epithelium (Fig. 1C). However, thickness (P = 0.03) and basal cell proliferation (P = 0.04) decreased in E2-treated Esr1ed/ed compared to Esr1f/f mice(Figure 1B,1C). While proliferation indices of the suprabasal layer were much lower than the basal layer, E2 decreased BrdU incorporation in suprabasal cells in both genotypes (Figure 1C). Expression of K10 increased in E2-treated Esr1f/f, but not Esr1ed/ed mice (Figure 1D), indicating the requirement of epithelial ERα for differentiation of the cervical squamous epithelium. E2 increased neither epithelial thickness nor proliferation in the Esr1−/− cervix (Figure S1A,S1B). These results indicate that, while epithelial ERα is necessary for full E2 responses, stromal ERα also mediates E2-induced proliferation of cervical epithelial cells, consistent with previous results that stromal ERα is necessary and sufficient for E2-induced epithelial cell proliferation in the uterus and vagina [18].
Figure 1.
Epithelial ERα-deficient cervical epithelium partially responds to E2. (A) Specific ablation of ERα expression in the cervical epithelia. The female reproductive tracts were harvested from Esr1f/f and Esr1ed/ed mice at 8–10 weeks of age. Cervical sections were stained for ERα (green, upper panel) and PR (green, lower panel). Nuclei were stained with Hoechst 33258 (pseudocolored red). Esr1 null cervix (Esr1−/−) was used as negative control. Dotted lines separate epithelium (ep) from stroma (st). (B) Ovariectomized Esr1ed/ed mice were treated with vehicle or E2 for 7 days. Shown are representative images of H&E-stained cervical tissues. The thickness of epithelium is shown as mean ± SEM (n = 3–5). (C) Left panel: cervical sections described in (B) were stained for BrdU (green). Nuclei were stained with Hoechst 33258 (blue). BrdU+ basal and suprabasal cells are indicated by red and white arrows, respectively. Right panel: More than 200 cells per basal and suprabasal layer were counted. Results are shown as mean ± SEM (n = 3). *P < 0.05 (one-sided Wilcoxon rank sum test). (D) Staining for K10 (brown). Nuclei were counterstained with hematoxylin. Scale bar = 50 μm for (A), (C) and (D); 30 μm for (B)
ERα− cervical neoplastic diseases develop in K14E7/Esr1ed/ed mice
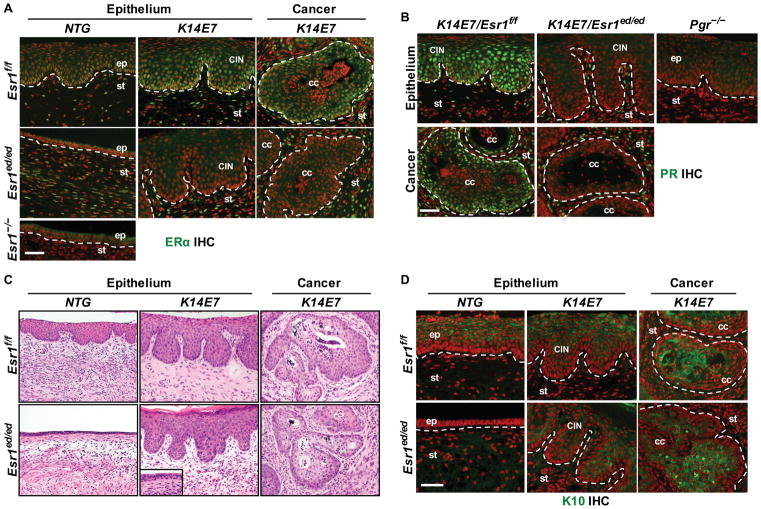
To determine whether epithelial ERα was required for E2-induced cervical neoplasia (CIN and CxCa) we treated K14E7/Esr1f/f, K14E7/Esr1ed/ed, nontransgenic (NTG)/Esr1f/f and NTG/Esr1ed/ed mice with E2 for 6 months [3]. All of fourteen K14E7/Esr1f/f, but none of six NTG/Esr1f/f, had CIN or CxCa (Table 1; P = 2.58×10−5). All of thirteen epithelial ERα-deficient K14E7/Esr1ed/ed, but none of ten NTG/Esr1ed/ed, displayed CIN or CxCa (Table 1; P = 8.74×10−7). Vaginal neoplastic diseases also developed in K14E7/Esr1f/f (100%) and K14E7/Esr1ed/ed mice (84.6%) (Table S3), which were not significantly different (P = 0.22). ERα was expressed in the stroma, but not cancerous and dysplastic epithelial cells in K14E7/Esr1ed/ed unlike K14E7/Esr1f/f mice (Figure 2A). PR was specifically undetectable in dysplastic and cancerous epithelium of K14E7/Esr1ed/ed (Figure 2B), confirming no expression of ERα. These results rule out the possibility that some epithelial cells escaped Esr1 deletion and became neoplastic. We concluded that epithelial ERα is not required for the development of CIN and CxCa. This is the first time to mimic ERα status of human CxCa, in which cancer cells are ERα − and stroma is ERα+ [9]. Expression of ERβ, the other nuclear ER, was undetectable in CIN and CxCa in K14E7/Esr1ed/ed mice, suggesting no compensatory overexpression of ERβ. ERβ was detected in the ovary of Esr2+/+ but not Esr2−/− mice, verifying specificity of the antibody (Figure S2A,S2B). We have demonstrated that deletion of stromal Esr1 promotes CIN regression in K14E7 mice [19]. Taken together, we conclude that stromal ERα is the major player in CIN and CxCa. FGF7, FGF9, HBEGF, IGF1, IL1A, CXCL1 and CXCL5 are upregulated by ERα in the cervical stroma [19–21]. It is plausible that secretory factors encoded by these stromal ERα target genes activate oncogenic signaling pathways in the epithelium through their cognate receptors on the plasma membrane of epithelial cells.
Table 1.
Summary of Worst Neoplastic Diseases in the Cervix¶
| Genotypes | Group size, n | No disease | Dysplasia only | Cancer & dysplasia | Incidence(%) | Multiplicity$ | Largest Cancer Size (mm2)$ | Total Invasion Area (mm2)$ | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| CIN1 | CIN2 | CIN3 | ||||||||
| NTG/Esr1f/f | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NTG/Esr1ed/ed | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K14E7/Esr1f/f | 14 | 0 | 0 | 1 | 2 | 11 | 78.6 | 2.79 ± 0.71 | 0.09 ± 0.03 | 0.17 ± 0.06 |
| K14E7/Esr1ed/ed | 13 | 0 | 1 | 4 | 4 | 4 | 30.8* | 0.38 ± 0.18** | 0.03 ± 0.02** | 0.04 ± 0.02** |
Mice were scored histopathologically for the worst disease present in the cervix of each mouse.
Mice without cancer are given ‘0’ and data is shown as mean ± SEM.
P = 0.02 compared to K14E7/Esr1f/f (Fisher’s exact test).
P < 0.02 compared to K14E7/Esr1f/f (Wilcoxon rank sum test).
Figure 2.
E2 promotes ERα − CxCa in K14E7/Esr1ed/ed mice. (A) Cervical sections of CxCa arising in K14E7/Esr1ed/ed mice stained for ERα (green). An Esr1−/− tissue section was used as negative control. Dotted lines separate stroma (st) from normal epithelium (ep), dysplastic epithelium (CIN), and cancer epithelium (cc). (B) PR staining (green). A Pgr−/− tissue section was used as negative control. (C) Representative H&E staining. Note that the nondiseased epithelia in K14E7/Esr1ed/ed mice were hypoplastic (inset). (D) K10 staining (green). In (A), (B) and (D), nuclei were stained with Hoechst 33258 (pseoudocolored red). Scale bar = 50 μm.
ERα+ and ERα− CxCa display similar differentiation status, biomarker expression and growth
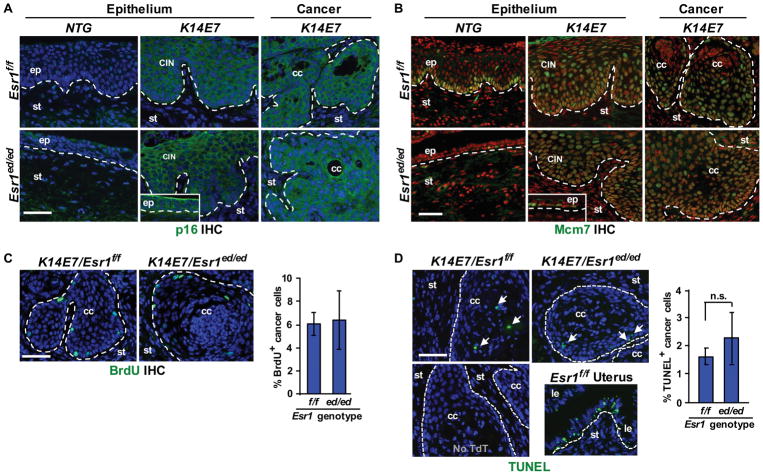
CINs and CxCa arising in K14E7/Esr1ed/ed and K14E7/Esr1f/f mice showed similar histology (Figure 2C). It was notable that non-diseased epithelium in K14E7/Esr1ed/ed mice was as hypoplastic as the entire epithelium of NTG/Esr1ed/ed mice (Figure 2C, inset). Expression of K10 in CIN and CxCa was similar between K14E7/Esr1ed/ed and K14E7/Esr1f/f (Figure 2D). Differentiation of cervical epithelial cells depends on epithelial ERα (see Figure 1D). These results indicate that neoplastic cells in K14E7/Esr1ed/ed mice acquired an ERα-independent differentiation program. Expression of p16 and MCM7, biomarkers for E7 function [22], was similarly upregulated in CIN and CxCa in K14E7/Esr1ed/ed and K14E7/Esr1f/f compared to NTG control (Figure 3A,3B). Rare MCM7+ and Ki67+ basal cells in NTG/Esr1ed/ed mice suggest that most epithelial cells are quiescent and epithelial ERα is required for proliferation of normal epithelial cells in response to long-term E2 treatment (Figure 3B, Figure S3). Proliferation of cancer cells was similar between K14E7/Esr1f/f and K14E7/Esr1ed/ed mice (Figure 3C). Apoptotic indices of carcinomas were also similar between the two genotypes (Figure 3D), indicating that ERα in cancer epithelial cells is not required for proliferation and survival of CxCa cells.
Figure 3.
Biomarker expression, proliferation, and apoptosis are similar between ERα+ and ERα − CxCa. (A) CxCa biomarker p16 (green) in cervical neoplastic diseases arising in K14E7/Esr1ed/ed mice. Nuclei were stained with Hoechst 33258 (blue). The inset shows p16 in the nondiseased epithelia of K14E7/Esr1ed/ed mice. Dotted lines separate stroma (st) from normal epithelium (ep), dysplastic epithelium (CIN) and cancer epithelium (cc). (B) Cervical sections stained for Mcm7 (green). Nuclei were stained with Hoechst 33258 (pseoudocolored red). The inset shows Mcm7 in the nondiseased epithelia of K14E7/Esr1ed/ed compared to NTG/Esr1ed/ed. (C) Left panel: CxCa sections were stained for BrdU (green). Nuclei were stained with Hoechst 33258 (blue). Right panel: results were quantified by analyzing more than 200 cells per cancer and shown as mean ± SEM (n = 3). (D) Apoptotic indices. Left panel: CxCa sections were subjected to TUNEL assay (green). TUNEL+ cells are indicated by arrows. Nuclei were stained with Hoechst 33258 (blue). A section that was not incubated with terminal deoxynucleotidyl transferase (TdT) was used as negative control (lower left panel). Uterus from E2-treated wt mice was used as positive control (lower right panel). Le, luminal epithelium. Right panel: results were quantified and shown as mean ± SEM (n = 3). n.s., not significant. Scale bars = 50 μm.
Epithelial ERα may play a role in an early stage of cervical carcinogenesis
CxCa burden (incidence, multiplicity and cancer size) was lower in K14E7/Esr1ed/ed than K14E7/Esr1f/f mice (Table 1). It is a caveat that ERα is not expressed in the normal cervical epithelium in Esr1ed/ed unlike Esr1f/f mice (see Figure 1A and 2A) and human cervix [9]. The entire epithelium was hypoplastic in NTG/Esr1ed/ed, but hyperplastic in NTG/Esr1f/f mice (see Figure 2C). This difference in baseline state of the epithelium may have contributed to the reduced cancer burden. An improved system allowing temporal deletion of epithelial Esr1 is required for better recapitulating the epithelial ERα status in human. Although most non-diseased cervical epithelia in K14E7/Esr1ed/ed mice were hypoplastic and undifferentiated (see Figure 2C,2D), CIN and CxCa in these mice were as differentiated and proliferative as those in K14E7/Esr1f/f (see Figures 2D and 3C). These results suggest that some ERα − epithelial cells acquired molecular changes mimicking the ERα pathway and were selected for during carcinogenesis. It appeared that E7 provided a selection advantage because a hyperplastic epithelium was absent in NTG/Esr1ed/ed mice (Figure 2C). It appeared that selection was a long-term process, because there was no hyperplastic epithelium in K14E7/Esr1ed/ed mice treated with E2 for 2 months (data not shown). Gene expression profiling of CxCa infers overactivation of ERα even if tumor cells do not express the receptor [23], suggesting transcriptional activation of ERα target genes through an ERα-independent mechanism. This transcriptome change may be due to genetic and/or epigenetic alterations caused by E7. HPV16 E7 inhibits DNA damage repair and induces genomic instability [24]. It also causes epigenetic reprogramming by inducing expression of KDM6A and KDM6B histone demethylases [25]. Taken together, we argue that epithelial ERα plays a positive role, if any, in the development of CIN1 but not its progression to higher grade disease.
Our results demonstrate that mouse cervical neoplastic diseases occur in the absence of epithelial ERα, recapitulating the human disease. The data described herein and published observations support the idea that oncogenic E2 signaling is mediated mainly by stromal ERα in the cervix [19,20]. Further studies are warranted to determine molecular mechanisms of stromal ERα and signaling pathways complementing the loss of epithelial ERα in CxCa.
Supplementary Material
E2 fails to increase the epithelium thickness and cell proliferation in the cervix of Esr1−/− mice
ERβ is not expressed in cervical neoplastic diseases. (A) Specificity of the anti-ERβ antibody is validated
Proliferative state of normal cervical epithelium in epithelial ERα+ and ERα − mice is different
Table S1. Description of Mouse Lines
Table S2. Antibodies and Conditions for IHC
Table S3. Summary of Worst Neoplastic Diseases in the Vagina
Acknowledgments
We thank Dr. Weihua for critically reading the manuscript. We also thank Drs. Korach and Warner for providing Esr2−/− tissues and the chicken anti-ERβ antibody, respectively. This work was supported in part by U.S. Public Health Service grant R01 CA188646 (S.H.C.) and the Cancer Prevention and Research Institute of Texas grant RP120617 and RP180275 (SHC).
Abbreviations
- HPV
human papillomavirus
- E2
estradiol
- ERα/β
estrogen receptor α/β
- Esr1/2
estrogen receptor 1/2
- CIN
cervical intraepithelial neoplasia
- CxCa
cervical cancer
Footnotes
Conflict of Interest: The authors have no conflict of interest.
Author Contributions
Conception and design: JS and SHC; development of methodology: JS, YP and SHC; acquisition, analysis and interpretation of data: JS, YP and SHC; writing, review and/or revision of the manuscript: JS, YP and SHC; study supervision: SHC.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.DiPaolo JA, Woodworth CD, Popescu NC, et al. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989;4:395–399. [PubMed] [Google Scholar]
- 3.Riley RR, Duensing S, Brake T, et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 4.Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 6.Hatch EE, Herbst AL, Hoover RN, et al. Incidence of squamous neoplasia of the cervix and vagina in women exposed prenatally to diethylstilbestrol (United States) Cancer Causes Control. 2001;12:837–845. doi: 10.1023/a:1012229112696. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh CJ, Hong MK, Chen PC, et al. Antiestrogen use reduces risk of cervical neoplasia in breast cancer patients: a population-based study. Oncotarget. 2017;8:29361–29369. doi: 10.18632/oncotarget.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SH, Wiedmeyer K, Shai A, et al. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–9934. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Boon JA, Pyeon D, Wang SS, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci U S A. 2015;112:E3255–3264. doi: 10.1073/pnas.1509322112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herber R, Liem A, Pitot H, et al. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daikoku T, Ogawa Y, Terakawa J, et al. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology. 2014;155:2718–2724. doi: 10.1210/en.2014-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta FF, Son J, Hewitt SC, et al. Distinct functions and regulation of epithelial progesterone receptor in the mouse cervix, vagina, and uterus. Oncotarget. 2016;7:17455–17467. doi: 10.18632/oncotarget.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winuthayanon W, Hewitt SC, Orvis GD, et al. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt SC, Kissling GE, Fieselman KE, et al. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J. 2010;24:4660–4667. doi: 10.1096/fj.10-163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder AK, Rodriguez KF, Hamilton KJ, et al. The absence of ER-beta results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology. 2013;154:2174–2187. doi: 10.1210/en.2012-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Valdivia R, Jeong J, Mukherjee A, et al. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48:106–113. doi: 10.1002/dvg.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elson DA, Riley RR, Lacey A, et al. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–1275. [PubMed] [Google Scholar]
- 18.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 19.Chung SH, Shin MK, Korach KS, et al. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm Cancer. 2013;4:50–59. doi: 10.1007/s12672-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung SH. Targeting female hormone receptors as cervical cancer therapy. Trends Endocrinol Metab. 2015;26:399–401. doi: 10.1016/j.tem.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spurgeon ME, den Boon JA, Horswill M, et al. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc Natl Acad Sci U S A. 2017;114:E9076–E9085. doi: 10.1073/pnas.1712018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brake T, Connor JP, Petereit DG, et al. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal S, Banks L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat Res. 2017;772:23–35. doi: 10.1016/j.mrrev.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin-Drubin ME, Crum CP, Munger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci U S A. 2011;108:2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
E2 fails to increase the epithelium thickness and cell proliferation in the cervix of Esr1−/− mice
ERβ is not expressed in cervical neoplastic diseases. (A) Specificity of the anti-ERβ antibody is validated
Proliferative state of normal cervical epithelium in epithelial ERα+ and ERα − mice is different
Table S1. Description of Mouse Lines
Table S2. Antibodies and Conditions for IHC
Table S3. Summary of Worst Neoplastic Diseases in the Vagina