Abstract
The worldwide switch to inactivated polio vaccines (IPVs) is a key component of the overall strategy to achieve and maintain global polio eradication. To this end, new IPV vaccine delivery systems may enhance patient convenience and compliance. In this work, we examine Nanopatch™ (a solid, polymer microprojection array) which offers potential advantages over standard needle/syringe administration including intradermal delivery and reduced antigen doses. Using trivalent IPV (tIPV) and a purpose-built evaporative dry-down system, candidate tIPV formulations were developed to stabilize tIPV during the drying process and on storage. Identifying conditions to minimize tIPV potency losses during rehydration and potency testing was a critical first step. Various classes and types of pharmaceutical excipients (∼50 total) were then evaluated to mitigate potency losses (measured through D-antigen ELISAs for IPV1, IPV2, and IPV3) during drying and storage. Various concentrations and combinations of stabilizing additives were optimized in terms of tIPV potency retention, and 2 candidate tIPV formulations containing cyclodextrin and a reducing agent (e.g., glutathione), maintained ≥80% D-antigen potency during drying and subsequent storage for 4 weeks at 4°C, and ≥60% potency for 3 weeks at room temperature with the majority of losses occurring within the first day of storage.
Keywords: poliovirus, vaccine, formulation, stability, delivery, Nanopatch™, microarray patch
Abbreviations used: WPV, wild poliovirus; tOPV, trivalent oral polio vaccine; bOPV, bivalent oral polio vaccine; VAPP, vaccine-associated paralytic poliomyelitis; cVDPV, circulating vaccine-derived poliovirus; IPV, inactivated polio vaccine; tIPV, trivalent inactivated polio vaccine; DPBS, Dulbecco's phosphate buffered saline; LCP, liquid crystal polymer; PS80, Polysorbate 80; BSA, bovine serum albumin; DTT, dithiothreitol; ELISA, enzyme-linked immunosorbent assay; PB, phosphate buffer
Introduction
In 1988, a global consortium of governmental and non-governmental entities began the Global Polio Eradication Initiative to vaccinate every child against polio. Since then, wild poliovirus (WPV) cases are at an all-time low (e.g., 74 reported cases globally of WPV type 1 in 2015) and WPV type 2 was declared eradicated in 2015.1 Immunization has traditionally been accomplished through the use of a live-attenuated trivalent oral polio vaccine (tOPV), with a global switch from tOPV to a bivalent OPV initiated in 2016. Despite the immense success with this vaccine, live-attenuated polio vaccines can cause vaccine-associated paralytic poliomyelitis (VAPP), and outbreaks of circulating vaccine-derived polioviruses (cVDPVs) have been reported. Therefore, to achieve complete worldwide polio eradication, both WPV and cVDPV must be eliminated.
To mitigate VAPP and cVDPV, OPV has been substituted with an inactivated polio vaccine (IPV) in routine immunization programs in >100 countries. While VAPP and cVDPV occurrences have greatly been reduced with IPV, the switch from OPV to IPV has been challenging due to multiple factors including supply shortages, higher production costs compared to OPV (see below), and administration (intramuscular) requiring a health care professional.1, 2, 3 In addition, IPV requires a cold-chain to maintain efficacy and potency during storage and transport with losses observed at elevated temperatures (≥25°C).4
Currently, several strategies are being examined to produce an improved IPV that is efficacious, inexpensive, safe to manufacture, and easier to administer. From the bulk vaccine manufacturing perspective, a key strategy is to develop IPV from the attenuated polio strains (e.g., Sabin-IPV). This strategy will avoid growing up large-scale wild-type polio strains for IPV manufacturing (e.g., Salk-IPV), and therefore provide additional safety during vaccine production.5, 6, 7 From the vaccine drug product perspective, one strategy is to avoid the cold-chain by formulating IPV in a dried state as a lyophilized powder,8 or to incorporate the dried vaccine into novel, less-invasive delivery dosage forms, which have potential advantages that not only include enhanced vaccine thermal stability for transportation and storage, but also potentially improved immunogenicity, antigen sparing, and reduced number of doses. For instance, Tzeng et al.9 encapsulated and vacuum-dried trivalent IPV (tIPV) in poly(lactic-co-glycolic acid)-based microspheres that enabled pulsatile release of IPV after a single intramuscular injection in a preclinical study in rats and therefore could potentially lower the number of doses required for a complete vaccination series. In addition to microspheres, dried IPV-coated microneedles/microprojections have been reported to elicit similar protection as the current liquid IPV formulation in multiple animal models.2, 10, 11
Microneedle/microprojection immunization technologies offer a variety of potential benefits compared with more traditional methods of vaccination including dose sparing, reduced associated costs, no required cold chain, and ease of administration.12, 13, 14, 15, 16 Among them, the Nanopatch™ is a microprojection vaccine delivery technology that has been successfully engineered and tested in preclinical studies for several vaccines.17, 18, 19, 20, 21, 22, 23, 24, 25, 26 The Nanopatch™ consists of a 1 cm2 silicon chip-like patch with a high-density (over 20,000 microprojections per cm2) array on the skin-facing surface.2, 27 When vaccine-coated devices are applied to the skin, the Nanopatch™ reproducibly targets the vaccine to thousands of antigen-presenting cells in both the viable epidermal and dermal layers of the skin.28 The combination of targeted vaccine delivery together with inflammation resulting from localized cell death may lead to improved immune responses over conventional needle-based intradermal delivery.2, 29 Vaxxas is currently well advanced in transitioning the Nanopatch™ technology away from a silicon patch to a polymer patch. This change is driven by the need to develop a technology which is low cost and scalable to commercial vaccine production volumes. The transition also leverages the historical and current activities and clinical manufacturing processes that have been established with the silicon Nanopatch™. Based on these considerations, IPV formulation development work described herein was undertaken using liquid crystal polymer (LCP)–based materials.
A key challenge in developing a microprojection-based IPV vaccine is ensuring antigen potency and stability not only during the drying process, but also during storage in the dried state when coated onto microneedles and during rehydration after patient administration. Therefore, the goal of the present study was to develop a laboratory scale down model of the Nanopatch™ microneedle drying/coating process and establish suitable analytical methods and stress conditions to screen pharmaceutical excipients with the goal of developing candidate tIPV formulations with minimized potency losses during drying and storage in a dried state. The identified candidate formulations are shown to be optimized to mitigate potency loss (measured through D-antigen ELISAs) of each of the 3 inactivated poliovirus serotypes (IPV type 1, 2 and 3) during drying and storage under conditions that simulates vaccine coating process of Nanopatch™.
Materials and Methods
Materials
Inactivated poliomyelitis vaccine type 1 (IPV1; Mahoney strain, containing 1250-3140 DU/mL D-antigen), inactivated poliomyelitis vaccine type 2 (IPV2; MEF strain, containing 430-1480 DU/mL D-antigen), and inactivated poliomyelitis vaccine type 3 (IPV3; Saukett strain, containing 520-2220 DU/mL D-antigen), were produced and supplied by Bilthoven Biologicals, Cyrus Poonawalla Group (Bilthoven, the Netherlands), in an M199 base medium. International IPV standards (a liquid trivalent blend of formaldehyde-inactivated monovalent pools of poliovirus type1, 2, and 3), were supplied by National Institute for Biological Standards and Control (Potters Bar, UK). Six millimeter diameter LCP discs and an in-house developed 96 well drying rig were provided by Vaxxas Pty Ltd. (Sydney, Australia). All other materials and reagents were purchased from commercial vendors.
Excipient Preparation
Concentrated (4×) stock excipients were dissolved in Dulbecco's Phosphate Buffered Saline (DPBS; Sigma-Aldrich, St. Louis, MO), and the pH was adjusted to 7.2 using HCl or NaOH. All solutions were sterilized by filtering through 0.22-μm PVDF membrane. The excipient stock solutions were stored at 4°C or room temperature (if precipitate was observed at 4°C) for up to 2 weeks, whereas unstable excipients (e.g., reducing agents) were prepared immediately before use.
tIPV Drying and Storage
To formulate tIPV, 7.5 μL of tIPV bulk solution (4.3, 1.1, and 3.6 DU of IPV1, IPV2, and IPV3, respectively, equivalent to ∼1/9 of a human dose) was mixed with 2.5 μL of 4× excipient or DPBS alone (no excipient control). Therefore, the final tIPV formulation contains 3 parts of M199 and one part of DPBS. Ten microliters of this mixture was then dispensed onto the center of each LCP disc in a 96 well plate. The solution was dried for 17-19 min under 14 L/min N2 flow in a custom drying rig and then sealed with thermo-stable adhesive film (VWR, Radnor, PA) and stored with desiccant. The LCP discs were used as surrogate for LCP-based microprojections to enable much faster experimental throughput at a laboratory scale.
tIPV Reconstitution and Recovery for Analytical Testing
To reconstitute dried tIPV samples and recover from the LCP discs, each disc was soaked in 200 μL of reconstitution buffer (DPBS with 0%-5% of BSA and 0%-1% PS80 (pH 7.2), filtered through 0.22-μm PVDF filter) and shaken at room temperature for 30 min at 200 rpm on a IKA HS 260 control shaker. The sample solution was then mixed 10 times using electronic multichannel pipette. The freshly reconstituted and recovered samples were immediately subjected to D-antigen assay without any additional storage.
Enzyme-Linked Immunosorbent (D-antigen Potency) Assay
Reconstituted vaccine samples were assayed for D-antigen potency (D-antigen units/mL) using a sandwich enzyme-linked immunosorbent assay (ELISA) optimized by Edens et al.11 Briefly, Nunc Maxisorp 96 well plates (Thermo Fisher Scientific, Waltham, MA) were coated with anti-IPV mAbs (Type 1; Novus Biologicals, Littleton, CA; type 2 and 3; Thermo Fisher Scientific) diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6. The plates were washed with PBST (0.01 M PBS, pH 7.2 with 0.05% Tween 20) and blocked with dilution buffer (0.01 M PBS with 0.5% gelatin and 0.25% Tween) before antigen (serially diluted IPV standards or formulated tIPV samples) incubation. Anti-IPV HRP–conjugated antibodies were then added and each plate was incubated for 1 h at 37°C. TMB peroxidase substrate (KPL Inc., Gaithersburg, MD) was then added, and the reaction was quenched with 1-M HCl. The absorbance of each well was measured at 450 nm (SpectraMax M5 microplate reader; Molecular Devices LLC, Sunnyvale, CA).
Vaccine Stability Evaluation Based on D-antigen ELISA Assay Results
To evaluate tIPV vaccine stability following drying, D-antigen potency results for each of the 3 IPV antigens (reconstituted and then recovered from LCP discs) were determined using the ELISA assay described above. The percent potency of dried vaccine was calculated by normalizing the D-antigen results of reconstituted, recovered dried samples to the values of a liquid stock vaccine stored at 4°C, which was considered to have 100% potency. Thus, the drying potency loss for each of the 3 antigens in the tIPV sample was calculated by subtracting the percent potency of freshly dried vaccine samples (recovered immediately after drying) from the liquid stock vaccine stored at 4°C (i.e., 100% − percent potency after drying = drying potency loss). Similarly, the potency loss during storage in the dried state was determined by subtracting the relative percent potency of the stored samples with the relative percent potency of the sample recovered immediately after drying (i.e., percent potency after drying − percent potency after storage = storage potency loss). Errors determinations for measured potency losses were calculated by propagation of error method using following equation
Results
Reconstitution Buffer Optimization and Initial Stability Evaluation of Dried tIPV Samples
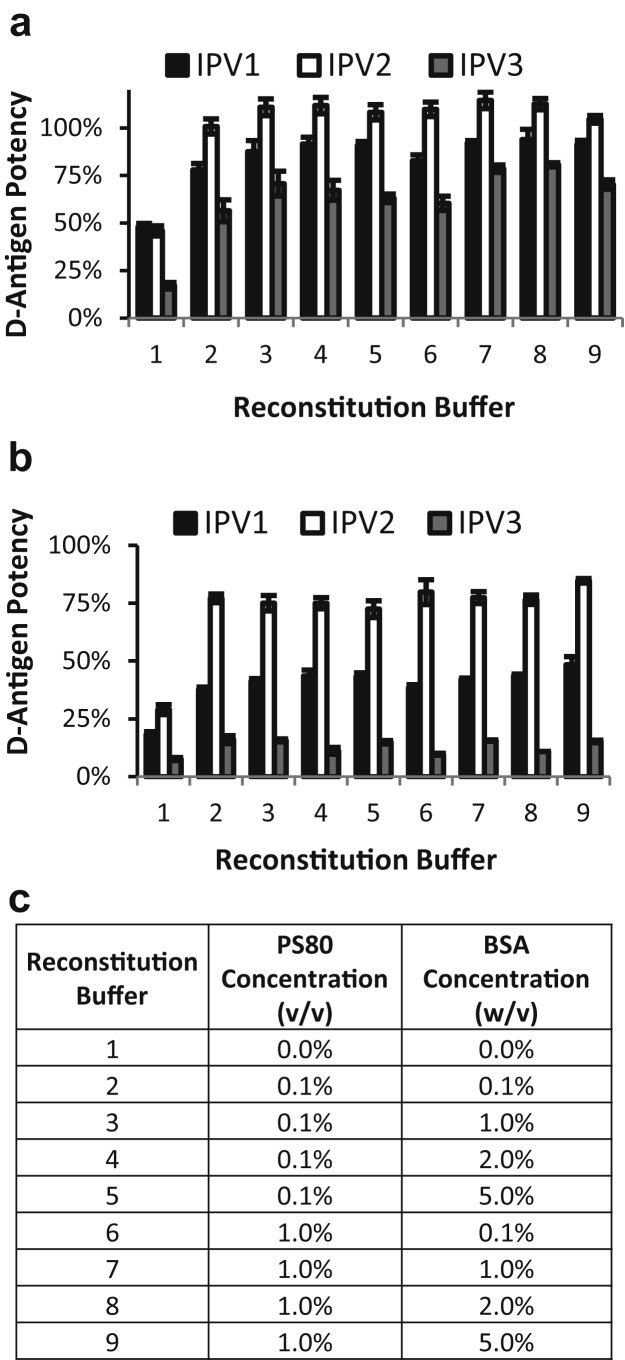
To assess relative potency loss of tIPV during drying via the D-antigen potency assay, tIPV solutions were dried on LCP discs in a 96 well plate format to simulate coating of the microprojection array technology (Nanopatch™) that is currently in development.21 On drying and reconstitution, however, DPBS buffer alone as the reconstitution medium was not sufficient to achieve expected D-antigen potency of the dried tIPV. As shown in Figure 1a, D-antigen potency with DPBS alone as the reconstitution buffer was ∼50% for IPV1 and IPV2, and only ∼15% for IPV3. We hypothesized that the apparent reduced potency may not only be due to losses due to drying, but some of the potency loss may be due to some of the viral particles adsorbing to the disc and container on reconstitution. To test this possibility, the addition of nonspecific blocking agents such as proteins and detergents (to mitigate this adsorption and therefore increase the amount of IPV recovered) were evaluated. The effects of various reconstitution buffers, comprising 0%-1% (v/v) polysorbate 80 (PS80), and 0%-5% (w/v) bovine serum albumin (BSA), in a DPBS base buffer, on D-antigen potency were tested on dried tIPV. The addition of 0.1% PS80% and 1% BSA to the reconstitution buffer greatly increased the measured potency of all 3 serotypes, in which the D-antigen potency of reconstituted tIPV improved as follows: IPV1 increased from 50% to 80%, IPV2 from 50% to 100%, and IPV3 from 15% to 70%. Increasing PS80 from 0.1% to 1%, or BSA from 1% to 5%, did not further improve the potency result of dried and then reconstituted tIPV. These results demonstrated that although some of the loss of potency was due to the drying of the viral particles, much of the potency loss observed during these initial drying experiments was actually mitigated by optimizing the reconstitution buffer used to rehydrate the sample and then transfer the samples into the D-antigen potency assay.
Figure 1.
Optimizing reconstitution buffer for dried tIPV followed by analysis in D-antigen potency assay. Trivalent IPV was dried on a liquid crystal polymer (LCP) discs, and various buffers containing different concentrations of PS80 and BSA were tested to reconstitute the dried tIPV samples followed by analysis in the D-antigen potency assay either (a) immediately after drying, or (b) after storage in dried state for 7 days at 4°C. (c) the key describing each of the solution conditions examined as reconstitution buffers. Each condition is shown as the relative D-antigen potency of each IPV serotype as compared with a liquid tIPV stock solution (100% potency), and error bars represent the SD (1 SD) from quadruplicate experiments.
The aim of this study was to develop potential clinical formulations of tIPV with the Nanopatch™ device with commercial tIPV bulks (supplied in M199 medium) without the requirement of further processing these bulks (e.g., diafiltration). To evaluate the effect of salt on IPV during drying, trivalent IPV mixture was dialyzed into 10-mM phosphate buffer (PB; pH 7.2) and tested during drying in comparison to samples with either 50 or 150 mM of NaCl (final concentration) added to PB before drying. Comparable yields were observed across the salt concentrations (∼50%-60% IPV1, ∼90%-100% IPV2, and ∼40%-50% IPV3 in an early version of reconstitution buffer containing DPBS with 0.1% BSA and 0.1% PS80; data not shown). Therefore, we did not observe negative effect of salt concentration on IPV drying yields in our nitrogen purge–based drying process. Based on these considerations, M199 containing IPV bulks were utilized for all subsequent formulation development studies.
In addition to testing the reconstitution buffer effects immediately after drying of the tIPV samples, the dried samples on LCP discs were also stored at 4°C for 7 days and then reconstituted in DPBS alone or in 0.1% PS80% and 1% BSA in DPBS. As shown in Figure 1b, the potency of IPV1 reconstituted with 0.1% PS80% and 1% BSA in DPBS decreased by ∼40% between drying and storage for 7 days (∼55% total loss after drying and 7 days storage at 4°C). The potency of IPV2 decreased by ∼20% between drying and storage for 7 days (∼20% total loss after drying and 7 days storage at 4°C). Finally, the potency of IPV3 decreased by ∼55% between drying and storage for 7 days (∼85% total loss after drying and 7 days storage at 4°C). These results indicate a substantial loss of potency for tIPV, particularly in IPV3, during both drying and storage.
Because of the low total protein in these tPIV-dried samples (estimated as ∼50 μg/mL in the bulk solution prior to drying), and the presence of protein and surfactants in the reconstitution buffer, biophysical methods could not be used to better understand the mechanisms of D-antigenicity loss in the IPV particles.8, 30 Preliminary results from a SDS-PAGE analysis of dried and stored tIPV samples, in which the major tIPV capsid proteins were silver stained and semi-quantified (see Supplemental Fig. S1), suggest that the total protein recovery in the freshly dried versus stability tIPV samples are similar, indicating that decreased D-antigen potency during storage is unlikely due to solely a physical loss of virus particles (similar results were also observed with monovalent IPV samples; data not shown).
Initial Excipient Screening Studies to Improve IPV3 Potency and Stability on Drying and Storage on LCP Discs
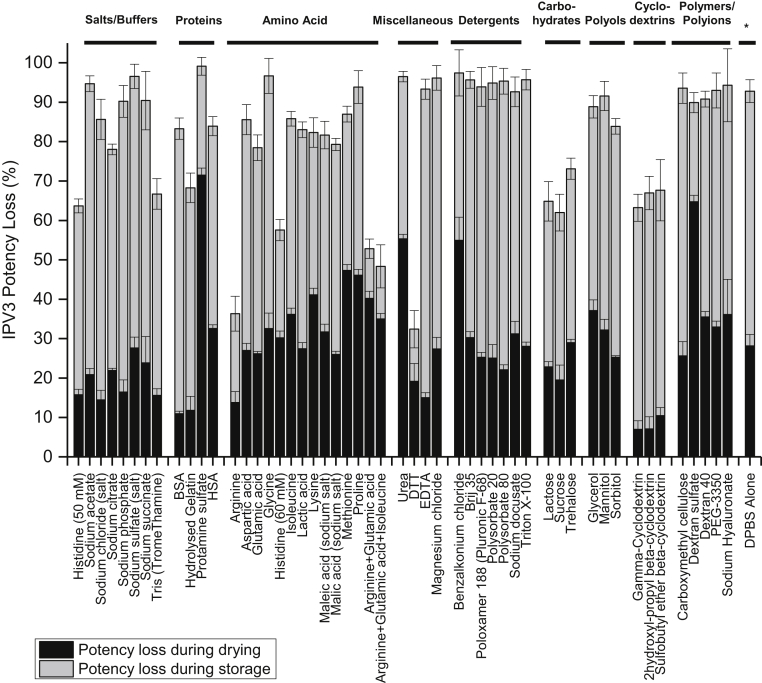
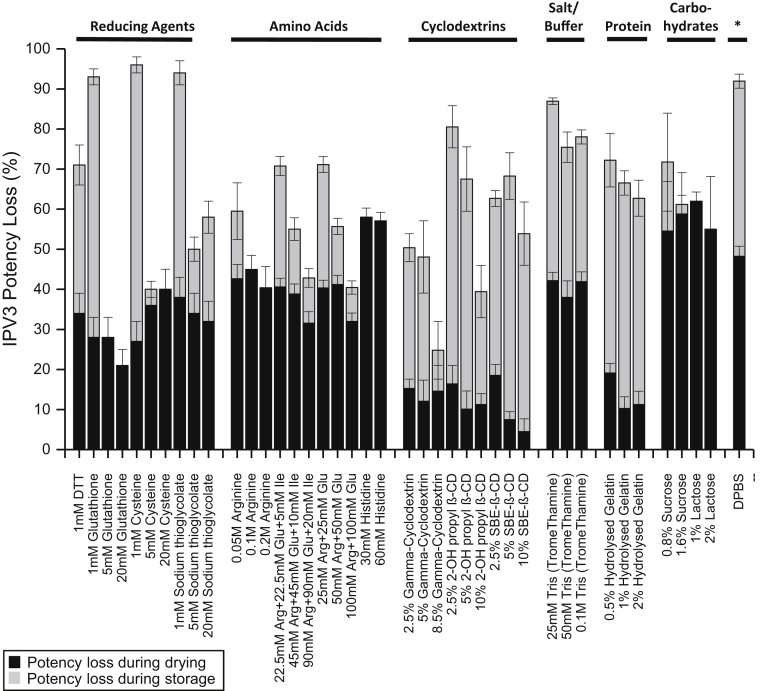
Based on the initial short-term stability study described above, IPV3 was the least stable IPV serotype after drying (∼30% loss) and storage (∼55% loss), for a total of ∼85% loss of D-antigen activity. Therefore, various pharmaceutical excipients and additives, mostly from the FDA inactive ingredient list for parenteral use with a few exceptions,31 were screened for their ability to stabilize each of the 3 IPV serotypes, but particularly to improve the stability of IPV3, in the trivalent mixture. Fifty-one different compounds in DPBS were individually mixed with tIPV, dried onto the LCP discs, and stored at 4°C for 7 days (with desiccant). Corresponding formulations alone (no tIPV) were also included in the study, and no background signal was observed from these solutions in the D-antigen ELISA assay (data not shown). The D-antigen potency values of each of the 3 serotypes were measured and then normalized to a Day 0 tIPV stock solution (non-dried, liquid solution) control sample. As shown in Figure 2 and Supplemental Table S1, out of the 51 compounds tested, the D-antigen potency of IPV3 was statistically higher in 32 formulations compared with the no excipient control (DPBS alone) as determined by a by 2-tailed unpaired equal variance Student t test (p <0.05). Some examples of stabilizing excipients identified in this initial screen included a reducing agent (dithiothreitol; DTT), individual amino acids (arginine and histidine), amino acid mixtures (arginine, glutamic acid, with or without isoleucine), 2 carbohydrates (sucrose and lactose), and 3 cyclodextrins (γ-cyclodextrin, 2-OH propyl β-cyclodextrin, SBE-β-cyclodextrin). The relative total D-antigen potency loss values for each of the 3 IPV serotypes in presence of each excipient and the statistical analysis results are summarized in Supplemental Table S1. The potency of the 3 IPV serotypes were observed to be statistically lower in the presence of most detergents (e.g., Triton X-100) compared with DPBS alone. This was not surprising since detergents appeared to interfere with the formation of the tIPV droplet on the LCP discs, and the dried tIPV formulated with detergents were likely prone to detachment from LCP discs during their preparation and storage.
Figure 2.
Effect of individual excipients on IPV3 relative potency losses after drying and storage of tIPV samples for 7 days at 4°C. Each condition is shown as a relative percentage D-antigen potency loss to a liquid tIPV stock solution (0% potency loss), and the black and gray bars denote D-antigen potency loss during drying and storage on LCP discs, respectively. Error bars represent the SD (1 SD) from quadruplicate experiments. Excipient samples were prepared in a M199/DPBS base buffer (see Materials and Methods section). Relative D-antigen potency stability values for IPV1, IPV2 and IPV3, and statistical analysis are shown in Supplemental Table S1.
Effect of Reducing Agents and Other Promising Stabilizing Excipients on Minimizing D-antigen Potency Loss of tIPV During Drying and Storage on LCP Discs
From the initial excipient screening experimental results described above, the commonly used laboratory reducing agent, DTT, was identified as one of the best stabilizers of IPV3; however, DTT is not a commonly used pharmaceutical excipient.32 Therefore, alternative, more pharmaceutically compatible reducing agents (i.e., sodium thioglycolate, cysteine, and reduced glutathione) were examined to determine if these excipients could also potentially stabilize IPV3 on drying and storage, similar to DTT. Each reducing agent was mixed with tIPV at 1, 5, or 20 mM, and the relative D-antigen potency of each serotype was measured immediately after drying or after 7 days storage at 4°C on LCP discs (Fig. 3). Interestingly, the ability of these alternative reducing agents to mitigate relative potency loss in each IPV serotype during drying was minimal; however, their stabilizing effects during storage for 7 days at 4°C in the dried state were dramatic compared with the DPBS control. A concentration-dependent stabilization was observed for each IPV serotype in the presence of each reducing agent, in which the relative D-antigen potency loss was notable at 1 mM, but at higher concentrations of 5- or 20-mM sodium thioglycolate, cysteine, and reduced glutathione, minimal to moderate relative potency losses were observed. Furthermore, reduced glutathione was found to be the best stabilizer among tested excipients examined in this experiment (∼20% IPV3 relative D-antigen potency loss, and essentially no loss in relative potency for IPV1 and IPV2 after drying and storage for 7 days on LCP discs. See Supplemental Tables S2-S4). To better understand these results, we compared the effect of oxidized versus reduced glutathione with IPV3 during drying and storage using a candidate formulation (described in more detail below). Oxidized glutathione (GSSG) was significantly less effective at mitigating IPV3 potency loss during storage compared with reduced glutathione (GSH; Supplemental Fig. S2).
Figure 3.
Concentration-dependent stabilization effect of individual excipients on IPV3 relative potency in tIPV samples after drying and storage for 7 days at 4°C. Each condition is shown as a relative percentage D-antigen potency loss compared with a liquid tIPV stock solution (0% potency loss), and the black and gray bars denote potency loss during drying and storage on LCP discs, respectively. Excipient samples were prepared in a M199/DPBS base buffer (see Materials and Methods section). Error bars represent the SD (1 SD) from triplicate experiments. Relative D-antigen potency loss values for IPV1, IPV2, and IPV3 are shown in Supplemental Tables S2-S4.
In addition to examining the stabilizing effects of reducing agents, multiple concentrations (2×, 1×, and 0.5× of the concentration used in the initial screening experiment; see Fig. 2 and Supplemental Table S1) of other promising categories and types of stabilizing excipients identified from the initial screening studies were tested with tIPV under similar conditions as described above with the reducing agents. Please note that the concentrations of sucrose, lactose, or histidine could not exceed 1× due to insufficient drying with tIPV or insolubility at higher concentrations. Owing to the vaccine coating process used for the Nanopatch™, loading of the discs requires balancing a number of factors including the loading capacity of the discs (volume and solids content) and drying times. As shown in Figure 3, IPV3 relative D-antigen potency loss during drying on LCP discs was the lowest in the presence of cyclodextrin or gelatin, whereas carbohydrates, amino acids, or high concentrations of a cyclodextrin-mitigated potency loss to a greater extent during storage in the dried state. Similarly, IPV1 relative potency loss during drying was the lowest in the presence of cyclodextrin or gelatin, whereas amino acids greatly mitigated potency loss during storage. For IPV2, almost all tested excipients mitigated potency loss during drying, and all excipients tested except carbohydrates mitigated potency loss to some extent during storage (Supplemental Tables S2 and S3).
Identifying Candidate tIPV Formulations and Further Stability Optimization
In the aforementioned studies, the lead stabilizing excipients appeared to mitigate relative potency losses of the tIPV antigens on LCP discs either during drying or during storage in the dried state, but were generally not beneficial for both processes. Therefore, we hypothesized that combinations of the lead excipients would further diminish relative D-antigen potency losses with tIPV antigens during both drying and on storage. Owing to the large number of promising stabilizing excipients, the experiments to evaluate combinations of stabilizers were divided into 2 steps. First, excipient combinations were screened to identify the optimal excipient(s) for drying on LCP discs, and then the optimal drying combination(s) were tested with one or multiple other lead excipients to stabilize tIPV during storage in the dried state.
Combinations of reducing agent (15-mM GSH and 1-mM cysteine), cyclodextrins (5% w/v SBE-β- cyclodextrin or 2.5% w/v γ-cyclodextrin), and 1% w/v gelatin (type A or hydrolyzed) were tested with tIPV to identify an optimal stabilizing agent/s for drying conditions for vaccine coating of the Nanopatch™ technology (Fig. 4a). Note that the concentration of GSH was lowered in this experiment from 20 to 15 mM to evaluate a lower dose.
Figure 4.
Effects of excipient combinations on the stability of IPV3 in tIPV samples during drying and storage in dried state on LCP discs. Each condition is shown as a relative D-antigen percentage potency loss to a liquid tIPV stock solution (0% potency loss) immediately after drying (a) or potency loss during drying and storage in dried state for 7 days at (b) 4°C or (c) 25°C, respectively. The black and gray bars denote potency loss during drying and storage, respectively. Excipients tested in a M199/DPBS base buffer (see Materials and Methods section): β-CD, 5% w/v SBE-β-cyclodextrin; Glut, 15-mM glutathione; His, 30-mM histidine; Lac, 2% w/v lactose; γ-CD, 2.5% w/v γ-cyclodextrin; Arg, 0.2-M arginine; Cys, 1 mM (in Fig. 4a) or 20 mM (in Fig. 4b, c) cysteine; Suc, 1.6% w/v sucrose. The concentration of gelatin type A and hydrolyzed gelatin was 1% w/v. Error bars represent the SD (1 SD) from triplicate experiments. Relative D-antigen potency loss values for IPV1, IPV2 and IPV3 are shown in Supplemental Table S5 (Fig. 4a) and S6 (Fig. 4b and 4c).
As expected, IPV3 relative potency loss values in the D-antigen assay, with each of the different lead excipient combinations, were notably lower than the DPBS control. Combinations of cyclodextrin and a reducing agent, in particular, appeared to mitigate relative potency loss to maximal extent, whereas relative D-antigen potency losses in formulation combinations containing reducing agents or reducing agent and gelatin were more limited. The IPV1 relative D-antigen potency losses followed the similar trends, and the drying losses for IPV2 were minimal at all tested conditions (Supplemental Table S5). From this study, the optimal stabilizing excipient combination to mitigate D-antigen potency losses during drying included cyclodextrin and a reducing agent; however, the type of cyclodextrin (SBE-β-cyclodextrin or γ-cyclodextrin) and reducing agent (GSH or cysteine) combinations that would be overall optimal for tIPV stability could not be delineated from these set of results.
In the second step, either of 2 cyclodextrins (5% SBE-β-cyclodextrin or 2.5% γ-cyclodextrin) were tested in combinations with one or multiple stabilizing excipients for assessing stability of tIPV during storage in dried state. Relative D-antigen potency losses were determined immediately after drying and after storage on LCP discs for 7 days at 4°C or 25°C (Fig. 4b and 4c). Comparing the stability profile of IPV3 with combinations of excipients in tIPV samples stored at 4°C, the cyclodextrin/reducing agent combinations, or the cyclodextrin/amino acid combinations, stabilized IPV3 better (8%-22% total relative D-antigen potency loss) than the cyclodextrin/carbohydrate combinations (31%-37% total relative D-antigen potency loss). Furthermore, although the total relative D-antigen potency loss of IPV3 with cyclodextrin/carbohydrate combinations in the tIPV samples was dramatically higher after storage at 25°C (82%-92% total relative D-antigen potency loss), the relative D-antigen potency loss of IPV3 with cyclodextrin/reducing agent combinations, or with cyclodextrin/amino acid combinations in tIPV samples stored at 25°C were only marginally higher compared with 4°C. From this study, the cyclodextrin/reducing agent combinations, or cyclodextrin/amino acid combinations, appeared to be the best combinations of excipients to mitigate relative potency losses of IPV3 in the tIPV samples during both drying and storage on the LCP discs. Similarly, the cyclodextrin/reducing agent combinations, or cyclodextrin/amino acid combinations, also appeared to be the best combinations of excipients to mitigate relative D-antigen potency losses of IPV1 and IPV2 in the tIPV samples during both drying and storage on the LCP discs (Supplemental Table S6). Interestingly, although 20-mM cysteine (alone or in combination with other excipients) mitigated relative potency losses for IPV3, this excipient appeared to destabilize (increase the observed relative potency loss) IPV1 during the drying process (Supplemental Table S6).
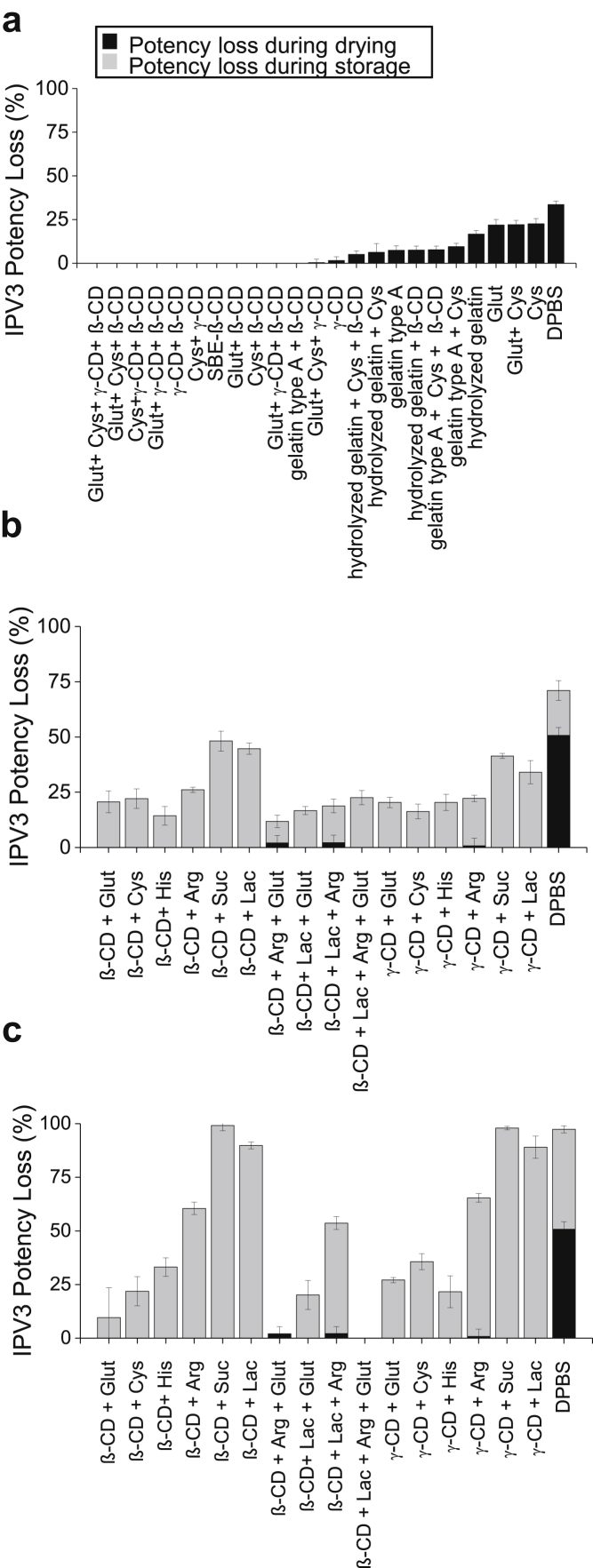
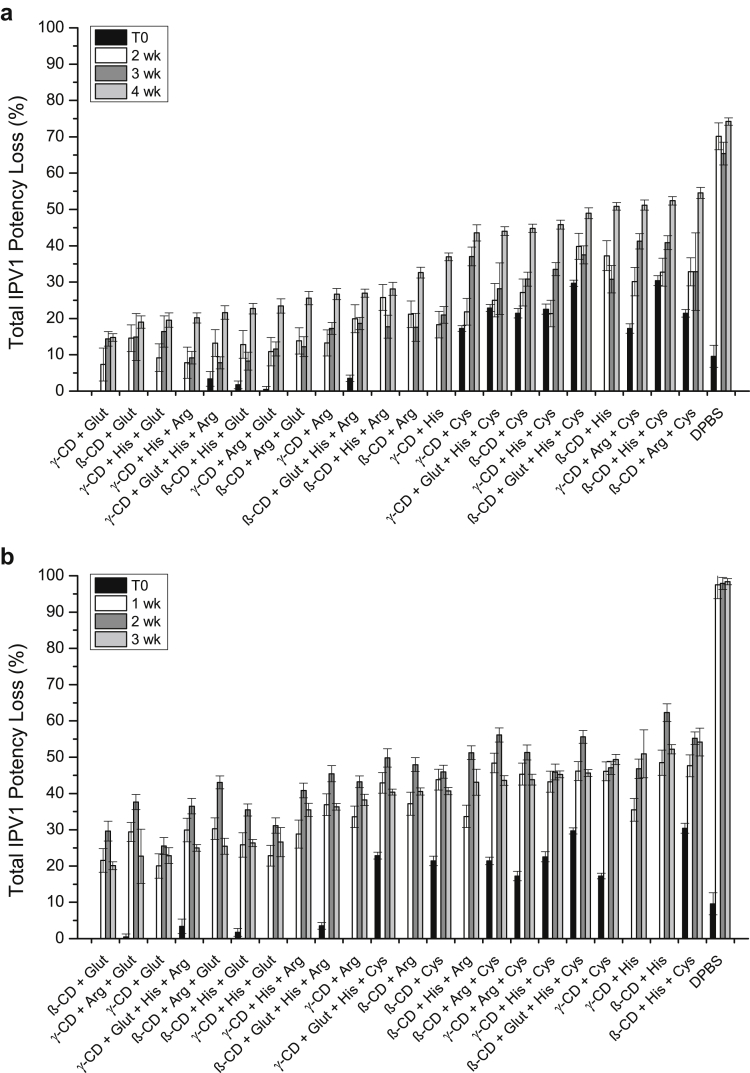
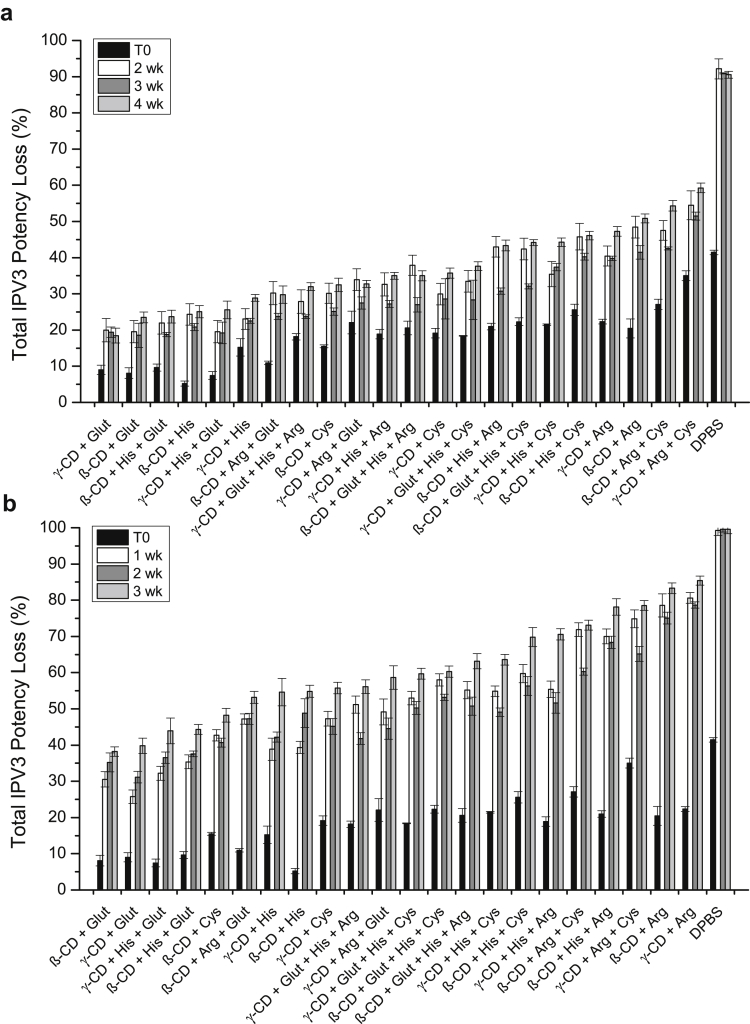
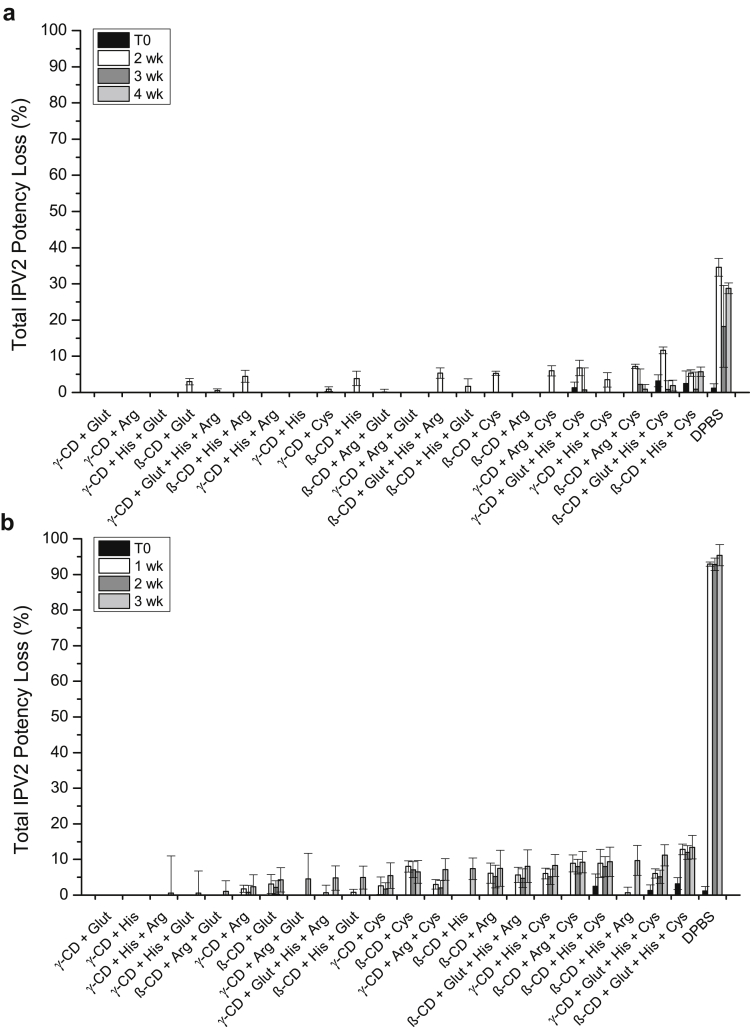
Since the stabilization effect of IPV3 in the dried tIPV samples was similar between the most promising combinations of excipients after 7 days of storage, the performance of different combinations of these excipients were tested for longer storage periods. In total, 22 different excipient combinations of cyclodextrins, reducing agents, and amino acids were tested for tIPV stability both for drying and storage stability in the dried state for 2-4 weeks at 4°C, and for 1-3 weeks at 25°C. Note that the concentration of SBE-β-cyclodextrin was lowered in this experiment from 5% to 4.5%, and arginine from 0.2 M to 0.15 M to ensure more proper drying with tIPV (data not shown). As shown in Figure 5, the loss of relative D-antigen potency of IPV3 in tIPV samples was lower in all tested excipient combinations compared with DPBS alone. After 4 weeks of storage at 4°C or 3 weeks at 25°C, the total relative potency loss (drying and storage) ranged from 18%-59% (Fig. 5a) and 38%-85% (Fig. 5b), respectively. The best excipient combination to mitigate relative D-antigen potency loss during drying and during storage in the dried state at both storage temperatures was 15-mM GSH and either cyclodextrin (4.5% SBE-β-cyclodextrin or 2.5% γ-cyclodextrin). In addition to stabilizing IPV3 in tIPV, these 2 combinations of excipients also minimized relative potency losses of IPV1 (<30%) and IPV2 (<10%) during drying and during storage in dried state on LCP discs when stored for 4 weeks. at 4°C or 3 weeks. at 25°C (Figs. 6 and 7).
Figure 7.
Effects of optimized excipient combinations on the stability of IPV1 in tIPV samples during drying and storage in dried state on LCP discs. Each optimized tIPV formulation was stored for either 2, 3, or 4 weeks at 4°C (a), or 1, 2, or 3 weeks at 25°C (b) and the measured relative D-antigen potency loss was determined compared with a liquid tIPV stock solution (0% potency loss). T0 represents relative potency loss immediately after drying. Excipients tested in a M199/DPBS base buffer (see Materials and Methods section): β-CD, 4.5% w/v SBE-β-cyclodextrin; Glut, 15-mM glutathione; His, 30-mM histidine; γ-CD, 2.5% w/v γ-cyclodextrin; Arg, 0.15-M arginine; Cys, 20-mM cysteine. Excipients were sorted from the lowest potency loss to the highest loss at the last tested time point. Error bars represent the SD (1 SD) from triplicate experiments.
Figure 5.
Effects of optimized excipient combinations on the stability of IPV3 in tIPV samples during drying and storage in dried state on LCP discs. Each optimized tIPV formulation was stored for either 2, 3, or 4 weeks at 4°C (a), or 1, 2, or 3 weeks at 25°C (b) and the measured relative D-antigen potency loss was determined compared with a liquid tIPV stock solution (0% potency loss). T0 represents relative potency loss immediately after drying. Excipients tested in a M199/DPBS base buffer (see methods section): β-CD, 4.5% w/v SBE-β-cyclodextrin; Glut, 15-mM glutathione; His, 30-mM histidine; γ-CD, 2.5% w/v γ-cyclodextrin; Arg, 0.15-M arginine; Cys, 20-mM cysteine. Excipients were sorted from the lowest potency loss to highest loss at the last tested time point. Error bars represent the SD (1 SD) from triplicate experiments.
Figure 6.
Effects of optimized excipient combinations on the stability of IPV2 in tIPV samples during drying and storage in dried state on LCP discs. Each optimized tIPV formulation was stored for either 2, 3, or 4 weeks at 4°C (a), or 1, 2, or 3 weeks at 25°C (b), and the measured relative D-antigen potency loss was determined compared with a liquid tIPV stock solution (0% potency loss). T0 represents relative potency loss immediately after drying. Excipients tested in a M199/DPBS base buffer (see methods section): β-CD, 4.5% w/v SBE-β-cyclodextrin; Glut, 15-mM glutathione; His, 30-mM histidine; γ-CD, 2.5% w/v γ-cyclodextrin; Arg, 0.15-M arginine; Cys, 20-mM cysteine. Excipients were sorted from the lowest potency loss to highest loss at the last tested time point. Error bars represent the SD (1 SD) from triplicate experiments.
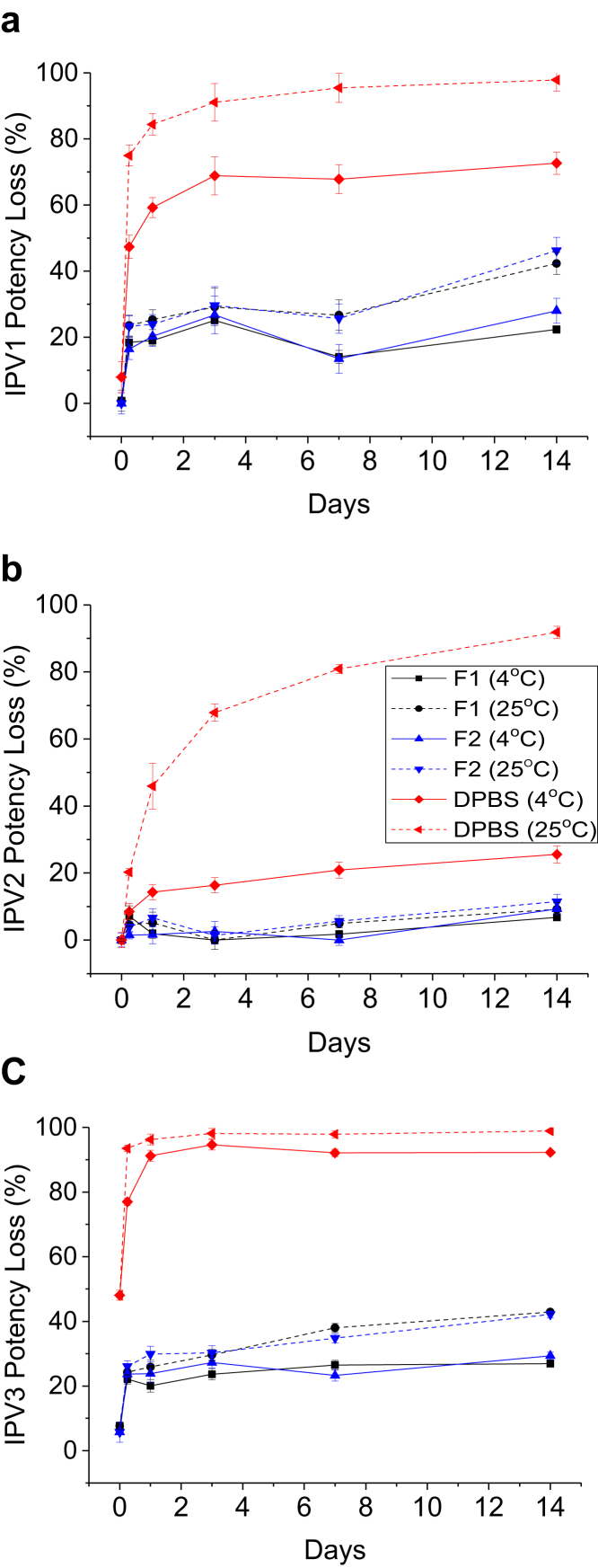
The results in Figure 5, Figure 6, Figure 7 also indicate that the largest loss of relative D-antigen potency occurred during the first few weeks of storage following drying. Therefore, to better understand the dynamics of relative potency loss of dried tIPV samples between these time points, the relative D-antigen potency of dried tIPV in 2 candidate formulations (F1: 4.5% SBE-β-cyclodextrin and 15-mM GSH in DPBS, pH 7.2, F2: 2.5% γ-cyclodextrin and 15-mM GSH in DPBS, pH 7.2) and DPBS alone was more closely monitored over the initial 2 weeks of storage after drying. As shown in Figure 8, the D-antigen relative potency loss of each IPV serotype in tIPV formulations was measured immediately after drying, and after 6 h, 1 day, 3 days, 7 days, and 14 days storage at 4°C or at 25°C. Immediately after drying, IPV1, IPV2, and IPV3 in the DPBS control lost 10, 0, and 50% relative potency, respectively. In either candidate formulation, relative D-antigen potency loss in IPV1, IPV2, and IPV3 was 0, 0, and 10%, respectively, after drying onto the LCP discs. During storage in the dried state at 4°C, relative D-antigen potency loss of dried tIPV in the DPBS control increased dramatically between time zero (immediately after drying) and the 6-h time point for both IPV1 and IPV3 (relative potency loss in IPV2 was minimal between these time points). Furthermore, relative potency loss continued at a lower rate between 6 h and 3 days, and then leveled out between 3-14 days. Similar trends were observed in the 2 candidate tIPV formulations, albeit at much lower relative D-antigen potency losses compared to the DPBS control (Fig. 8). As observed previously, relative potency losses of tIPV in the DPBS control was exacerbated at 25°C, however, the relative D-antigen potency losses in the candidate formulations were only marginally higher during storage at 25°C compared with 4°C. These results indicate much improved stability of the tIPV antigens at higher temperatures with the majority of relative D-antigen potency loss in the 3 IPV serotypes in the dried tIPV formulations occurring within the first few days of post-drying, particularly within the first 6 h of post-drying.
Figure 8.
Two-week tIPV stability study with 2 candidate formulations in an M199/DPBS base buffer. Trivalent IPV was dried and stored in 2 candidate formulations (F1 [black]: 4.5% SBE-β-cyclodextrin + 15-mM glutathione, and F2 (blue): 2.5% γ-cyclodextrin + 15-mM glutathione) or DPBS alone (red) at either 4°C (solid lines) or 25°C (dashed lines) for 6 h, and 1, 3, 7, or 14 days. Relative D-antigen potency loss was measured in each IPV serotype (a) IPV1, (b) IPV2, (c) IPV3 and is relative to a liquid tIPV stock solution (0% potency loss). T0 represents each IPV serotypes potency loss immediately after drying. Excipient samples were prepared in a M199/DPBS base buffer (see Materials and Methods section). Error bars represent the SD (1 SD) from triplicate experiments.
Discussion
In this study, candidate formulations were developed to stabilize tIPV during drying and storage (in the dried state) on an LCP disc, which simulates the vaccine coating process and storage of an in-development microprojection (Nanopatch™) vaccine delivery system. Two candidate formulations of tIPV, containing cyclodextrin (4.5% SBE-β-cyclodextrin or 2.5% γ-cyclodextrin) and a reducing agent (15-mM GSH) in a DPBS/M199 base buffer, maintained ≥90% D-antigen relative potency for each of the 3 IPV serotypes in tIPV during drying (100%, 100%, and 90% relative potency remaining for IPV1, IPV2, and IPV3, respectively); ≥80% D-antigen relative potency for each of the 3 IPV serotypes in tIPV in a dried state during 4 weeks storage at 4°C (80%, 100%, and 80% relative potency remaining for IPV1, IPV2, and IPV3, respectively). At higher temperature (25°C), these 2 candidate formulations maintained ≥60% potency during 3-week storage (70%, 100%, and 60% relative potency remaining for IPV1, IPV2, and IPV3, respectively).
These 2 candidate formulations stabilized tIPV during different environmental stresses (i.e., N2 drying process and storage in a dried state) exerted during the preparation and storage of a Nanopatch™ microprojection vaccine device. Similar to the more traditional lyophilization drying process, each of the 3 IPV antigens is exposed to numerous physicochemical stresses (e.g., dehydration, increases in excipient and antigen concentrations, potential pH shifts) during the initial N2 drying process onto the LCP discs. While sugars and polyols are generally used as either cryoprotectants or lyoprotectants to stabilize biological drugs and vaccines during lyophilization, cyclodextrins were identified as the best excipients to limit tIPV potency loss during N2 drying onto the LCP discs under the tested conditions (i.e., nitrogen-flow evaporation at room temperature). Cyclodextrins are a family of cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic central cavity.33 Owing to their cone-like shape, cyclodextrins are widely used as “molecular cages” to stabilize pharmaceuticals of suitable molecular size and shape. Cyclodextrins have also been shown to improve stability and solubility of macromolecules such as proteins through masking hydrophobic regions/amino acids and therefore lower the proteins aggregation propensity.34 Cyclodextrins have been used in many pharmaceutical drugs, including at least 5 FDA-approved small molecule drugs (VFEND®, Abilify®, Geodon®, Kyprolis™, and Nexterone). In addition to these drugs, cyclodextrins have recently been shown to improve the stability of IPV in the liquid state in which Chacornac et al.35 identified that β-cyclodextrin, γ-cyclodextrin, and cyclodextrin-derivatives mitigated (D-antigen) each of the 3 IPV serotypes titer loss induced by thimerosal, a common vaccine preservative.
While cyclodextrin mitigated tIPV relative D-antigen potency losses during drying onto the LCP discs, reduced GSH was shown to stabilize each serotype primarily during storage. In addition to this observation, a recent study by Abdelnabi et al.36 highlighted the benefit of this reducing agent in which tOPV in the liquid state was stabilized in a concentration-dependent manner in the presence of reduced GSH. Although the exact mechanism(s) by which reduced GSH mitigates tIPV relative D-antigen potency loss have not been fully elucidated, reduced GSH has been shown previously to interact directly with poliovirus capsid proteins, and both reduced and GSSG were able to improve the stability of the virus against thermal inactivation.37, 38 Ma et al.38 reported that 2 reducing agents (DTT and β-ME) did not protect poliovirus type 1 from heat inactivation in the liquid state and hypothesized that GSH's ability to stabilize the virus was due to direct (non-covalent) interaction and not a result of the molecule's antioxidant activity.37 The stabilizing effect of GSH during storage in the dried state largely depends on its reducing potential, as GSSG was substantially less effective at mitigating IPV3 potency loss compared with reduced GSH (Supplemental Fig. S2). In addition to reduced GSH, other reducing agents examined in this study (i.e., DTT, cysteine, sodium thioglycolate) substantially improved the stability of tIPV during storage in the dried state, suggesting that while these reducing agents may be potentially ineffective towards heat inactivation in the liquid state, these excipients are beneficial to mitigate relative potency losses for tIPV during storage in a dried state.
The overall goal of this formulation development work was to develop potential clinical formulations of tIPV with the Nanopatch™ device with commercial tIPV bulks (supplied in M199 medium) without the requirement of further processing these bulks (e.g., diafiltration). Thus, commercial tIPV bulks (supplied in M199 medium) were utilized in this work directly and added to the drying process by simple dilution. In addition, because of the low protein concentrations and the presence of BSA and PS-80 in the reconstitution buffer, biophysical methods could not be used to better understand the degradation pathways of IPV leading to D-antigenicity loss as function of processing and storage conditions. Preliminary SDS-PAGE analysis (Supplemental Fig. S1) suggested that decreased D-antigen potency observed on drying and subsequent storage of tIPV was unlikely solely due to loss of viral particles (total protein), but rather a loss of D-antigen epitopes, possibly through conformational changes, during drying or storage. This possibility could be tested using C-antigen ELISA assay in the future studies if such reagents become commercially available.
As reported by others for dried tIPV microsphere and microneedle/microprojection technology, the most significant D-antigen potency losses may occur during the initial drying process. For instance, there were over 80% potency losses for each of the 3 IPV serotypes on vacuum drying (a process that simulates drying process in microsphere technology).9 Similarly, over 80% potency losses for IPV1 and IPV2, and over 90% potency loss for IPV3, were observed previously on lyophilization.8 The addition of stabilizers has been shown to be able to greatly mitigate IPV potency loss during these types of drying processes. For instance, the addition of 8% gelatin before drying maintained over 80% potency for each of the 3 IPV serotypes during vacuum drying,9 and the addition of 10-8.5%-8.5% of sorbitol-MgCl2-MSG (monosodium glutamate) benefited both vacuum drying (maintained ∼70% potency for each of the 3 IPV serotypes) and lyophilization (maintained ∼90% potency for IPV1 and IPV2, and ∼70% potency for IPV3).8, 9 The sorbitol-MgCl2-MSG mixture also showed improved stability during subsequent storage.8, 10 For instance, minimal potency losses were observed for sorbitol-MgCl2-MSG formulated tIPV for up to 24-week storage at 25°C.8 In contrast during our study, gelatin, but not carbohydrates, polyols, MgCl2, or glutamic acid showed benefit in stabilizing tIPV during drying onto the LCP discs. Compared with these studies, some amino acids shown previously to stabilize IPV during storage in dried state (dried by other processes), also benefited tIPV stability during storage to some extent during Nanopatch™ drying process (Supplemental Tables S2-S4). However, in this work, we identified other stabilizers for tIPV (such as cyclodextrin and reduced GSH) during drying, and reduced GSH was also shown to be the best tIPV stabilizer during storage in the dried state after the drying process.
The combination of one cyclodextrin (4.5% SBE-β-cyclodextrin or 2.5% γ-cyclodextrin) and 15-mM GSH (equivalent to 0.46% (w/v)) was found to be the best combination of additives for candidate formulations for tIPV during N2 drying and subsequent storage on the LCP discs, respectively. The uniqueness of the combinations of these stabilizers may be due to different mechanisms of IPV antigen inactivation during dehydration versus storage in the solid state. For drying, a different drying process was applied in our study (drying under 14 min/L N2 flow at room temperature for 20 min on LCP discs) to simulate the coating of vaccine onto the microprojections Nanopatch™ versus other drying processes such as lyophilization and vacuum drying described previously.8, 9, 10
The stability profile of a vaccine over long-term storage is not defined by a certain percent loss (rate), but rather it must ensure that the potency at release (time zero) is clinically safe and the potency at expiry is clinically efficacious,39 especially since multiphasic kinetics with viral vaccines during long-term stability is not uncommon.40 Interestingly, since most of the tIPV potency loss observed over 14 days of storage occurred within approximately 0.5 day (Fig. 8), such a stability profile possibly indicates a leveling off of tIPV potency loss that could enable sufficient retention of potency during longer term stability. Preliminary stability data (n = 1, 3 discs) with IPV3 potency losses in the dried state (tIPV in the 15-mM GSH and 4.5% SBE-beta-cyclodextrin formulation on LCP discs) levels off at ∼20%-30% (i.e., ∼70%-80% D-antigen potency remaining vs. bulk liquid antigen also stored at 4°C) after 4, 12, and 52 weeks of storage at 4°C (data not shown). In addition, the maximal dose in these experiments was 1/9th of a full tIPV human dose per disc, which is a lower IPV concentration. Therefore, it will of interest to determine the long-term stability profile of tIPV (e.g., full human dose of all 3 serotypes) dried in these candidate formulations on Nanopatch™ microprojections as part of future work.
Conclusion
In summary, a combination of excipients were identified which greatly improve the stability of tIPV during N2 drying and subsequent storage in the dried state on LCP discs, a laboratory mimic of the Nanopatch™ coating and drying process. A combination of one cyclodextrin (4.5% SBE-β-cyclodextrin or 2.5% γ-cyclodextrin) and 15-mM GSH (equivalent to 0.46% [w/v]) provided the most improved stabilization of tIPV by protecting the antigens during the drying process and during storage, respectively. We also demonstrated that the reconstitution buffer used to rehydrate these samples for testing is another important consideration, and the presence of proteins (such as BSA) and non-ionic detergents (such as PS-80) in the reconstitution buffer greatly improved tIPV potency yields as measured by the D-antigen assay. While the results from these formulation development studies demonstrate the importance of identifying and optimizing stabilizing excipients for tIPV, the compatibility and performance of the 2 candidate formulations during the actual Nanopatch™ drying/coating manufacturing process at clinical manufacturing scale will need to be examined (including longer term stability studies and assessment of residual moisture on vaccine stability). Furthermore, although the improved tIPV stability in the 2 candidate formulations was demonstrated through a D-antigen ELISA test, the immunogenicity of dried tIPV samples in these 2 formulations in the Nanopatch™ microprojections also needs to be evaluated in an animal model41 to demonstrate efficacy during in vivo rehydration and intradermal delivery of the tIPV vaccine antigens. Such preclinical and process development studies are currently underway.
Acknowledgments
This work was supported financially by World Health Organization. The authors would like to acknowledge Bilthoven Biologicals, Cyrus Poonawalla Group, Netherlands for the supply of the 3 IPV bulk antigens, Cyrus Technology Pte Ltd (Singapore) for supplying the LCP discs, and Vaxxas staff/consultants including Christopher Flaim, Neil Harris, and David Muller. They also wish to acknowledge the assistance of Harshit Khasa and Kelly Schwinghamer during initial formulation experiments at KU.
Footnotes
This article contains supplementary material available from the authors by request or via the Internet at https://doi.org/10.1016/j.xphs.2018.01.027.
Supplementary Data
References
- 1.Hampton L.M. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine–worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(35):934–938. doi: 10.15585/mmwr.mm6535a3. [DOI] [PubMed] [Google Scholar]
- 2.Muller D.A. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci Rep. 2016;6:22094. doi: 10.1038/srep22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenfeld E., Modlin J., Chumakov K. Future of polio vaccines. Expert Rev Vaccines. 2009;8(7):899–905. doi: 10.1586/erv.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D., Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009;8(5):547–557. doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 5.Bakker W.A. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011;29(41):7188–7196. doi: 10.1016/j.vaccine.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 6.Minor P.D. Scientific consultation on the safety and containment of new poliovirus strains for vaccine production, clinical/regulatory testing and research. Report of a meeting held at NIBSC, Potters Bar, Hertfordshire, UK, 6/7th July 2016. Biologicals. 2017;48:92–100. doi: 10.1016/j.biologicals.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Westdijk J. Characterization and standardization of Sabin based inactivated polio vaccine: proposal for a new antigen unit for inactivated polio vaccines. Vaccine. 2011;29(18):3390–3397. doi: 10.1016/j.vaccine.2011.02.085. [DOI] [PubMed] [Google Scholar]
- 8.Kraan H. Development of thermostable lyophilized inactivated polio vaccine. Pharm Res. 2014;31(10):2618–2629. doi: 10.1007/s11095-014-1359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng S.Y. Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release. J Control Release. 2016;233:101–113. doi: 10.1016/j.jconrel.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraan H. Alternative delivery of a thermostable inactivated polio vaccine. Vaccine. 2015;33(17):2030–2037. doi: 10.1016/j.vaccine.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Edens C. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine. 2015;33(37):4683–4690. doi: 10.1016/j.vaccine.2015.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prausnitz M.R. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.-C., Park J.-H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edens C., Collins M.L., Ayers J., Rota P.A., Prausnitz M.R. Measles vaccination using a microneedle patch. Vaccine. 2013;31(34):3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone M., Monkare J., Bouwstra J.A., Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34(11):2223–2240. doi: 10.1007/s11095-017-2223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernando G.J., Zhang J., Ng H.I., Haigh O.L., Yukiko S.R., Kendall M.A. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J Control Release. 2016;237:35–41. doi: 10.1016/j.jconrel.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Pearson F.E., McNeilly C.L., Crichton M.L. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One. 2013;8(7):e67888. doi: 10.1371/journal.pone.0067888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prow T.W., Chen X., Prow N.A. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 20.Pearson F.E., Muller D.A., Roalfe L., Zancolli M., Goldblatt D., Kendall M.A. Functional anti-polysaccharide IgG titres induced by unadjuvanted pneumococcal-conjugate vaccine when delivered by microprojection-based skin patch. Vaccine. 2015;33(48):6675–6683. doi: 10.1016/j.vaccine.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 21.Crichton M.L., Muller D.A., Depelsenaire A.C.I. The changing shape of vaccination: improving immune responses through geometrical variations of a microdevice for immunization. Sci Rep. 2016;6:27217. doi: 10.1038/srep27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crichton M.L., Archer-Jones C., Meliga S. Characterising the material properties at the interface between skin and a skin vaccination microprojection device. Acta Biomater. 2016;36:186–194. doi: 10.1016/j.actbio.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 23.McNeilly C.L., Crichton M.L., Primiero C.A., Frazer I.H., Roberts M.S., Kendall M.A.F. Microprojection arrays to immunise at mucosal surfaces. J Control Release. 2014;196:252–260. doi: 10.1016/j.jconrel.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Chen X., Fernando, Germain J.P., Crichton M.L. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Raphael A.P., Prow T.W., Crichton M.L., Chen X., Fernando G.J., Kendall M.A. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6(16):1785–1793. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 26.Raphael A.P., Crichton M.L., Falconer R.J. Formulations for microprojection/microneedle vaccine delivery: structure, strength and release profiles. J Control Release. 2016;225:40–52. doi: 10.1016/j.jconrel.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins D., Jenkins D., Corrie S., Flaim C., Kendall M. High density and high aspect ratio solid micro-nanoprojection arrays for targeted skin vaccine delivery and specific antibody extraction. RSC Adv. 2012;2(8):3490–3495. [Google Scholar]
- 28.Crichton M.L., Ansaldo A., Chen X., Prow T.W., Fernando G.J.P., Kendall Mark A.F. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials. 2010;31(16):4562–4572. doi: 10.1016/j.biomaterials.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Depelsenaire A.C.I., Meliga S.C., McNeilly C.L. Co-localization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol. 2014;134(9):2361–2370. doi: 10.1038/jid.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi W., Zeng Y., Orgel S. Preformulation study of highly purified inactivated polio vaccine, serotype 3. J Pharm Sci. 2014;103(1):140–151. doi: 10.1002/jps.23801. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration . FDA; Silver Spring, MD: 2017. Inactive ingredient search for approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed September 1, 2017. [Google Scholar]
- 32.Kamerzell T.J., Esfandiary R., Joshi S.B., Middaugh C.R., Volkin D.B. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63(13):1118–1159. doi: 10.1016/j.addr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Aachmann F.L., Otzen D.E., Larsen K.L., Wimmer R. Structural background of cyclodextrin–protein interactions. Protein Engineering. Des Selection. 2003;16(12):905–912. doi: 10.1093/protein/gzg137. [DOI] [PubMed] [Google Scholar]
- 34.Serno T., Geidobler R., Winter G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv Drug Deliv Rev. 2011;63(13):1086–1106. doi: 10.1016/j.addr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Chacornac I, Francon A, Vacus P. Vaccine composition comprising ipv and cyclodextrins. 2016, Google Patents.
- 36.Abdelnabi R., Delang L., Neyts J. Glutathione is a highly efficient thermostabilizer of poliovirus Sabin strains. Vaccine. 2017;35(10):1370–1372. doi: 10.1016/j.vaccine.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 37.Thibaut H.J., van der Linden L., Jiang P. Binding of glutathione to enterovirus capsids is essential for virion morphogenesis. PLoS Pathog. 2014;10(4):e1004039. doi: 10.1371/journal.ppat.1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma H.C., Liu Y., Wang C. An interaction between glutathione and the capsid is required for the morphogenesis of C-cluster enteroviruses. PLoS Pathog. 2014;10(4):e1004052. doi: 10.1371/journal.ppat.1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumru O.S., Joshi S.B., Smith D.E., Middaugh C.R., Prusik T., Volkin D.B. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Schofield T.L. Vaccine stability study design and analysis to support product licensure. Biologicals. 2009;37(6):387–396. doi: 10.1016/j.biologicals.2009.08.009. discussion 421-3. [DOI] [PubMed] [Google Scholar]
- 41.Muller D.A., Fernando G.J.P., Owens N.S. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci Rep. 2017;7(1):12644. doi: 10.1038/s41598-017-13011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.