Abstract
Objective
To examine the independent and synergistic effects of gestational diabetes mellitus (GDM) and low socioeconomic status (SES) on neurodevelopment and attention-deficit/hyperactivity disorder (ADHD) outcomes.
Design
Cohort study.
Setting
Flushing, New York.
Participants
A total of 212 preschool children as a part of the ongoing cohort study.
Main Exposures
Gestational diabetes mellitus and low SES.
Main Outcome Measures
Primary outcomes are ADHD diagnosis based on Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria at age 6 years and several well-validated measures of neurobehavioral outcomes, cognitive functioning, ADHD symptoms, and temperament at age 4 years. Secondary outcomes are parent and teacher reports of behavioral and emotional problems at age 6 years. Neurobehavioral measures in relation to GDM and low SES were examined using generalized estimating equations and multivariate logistic regression analyses.
Results
Both maternal GDM and low SES were associated with an approximately 2-fold increased risk for ADHD at age 6 years. However, the risk by GDM was greater among lower SES families than among higher SES families. Children exposed to both GDM and low SES demonstrated compromised neurobehavioral functioning, including lower IQ, poorer language, and impoverished behavioral and emotional functioning. A test of additive interaction found that the risk for ADHD increased over 14-fold (P=.006) when children were exposed to both GDM and low SES. Neither children exposed to maternal GDM alone nor those exposed to low SES alone had a notable increased risk for ADHD.
Conclusions
Maternal GDM and low SES, especially in combination, heighten the risk for childhood ADHD. Long-term prevention efforts should be directed at mothers with GDM to avoid suboptimal neurobehavioral development and mitigate the risk for ADHD among their offspring.
Gestational diabetes mellitus (GDM) typically develops in the second and third trimesters and is defined as glucose intolerance with onset or first recognition duringpregnancy.1 Approximately7% of all pregnancies are complicated by GDM—more than 135 000 cases per year.2 The prevalence of GDM has been rising for over 20 years, particularly among ethnic minorities and individuals with low socioeconomic status (SES),3,4 as have lifestyle changes that heighten risk including greater consumption of saturated fats, sugar, and processed foods, and sedentary working environments.
The development of GDM coincides with a period of rapid fetal brain development.5–9 Yet, the long-term neurobehavioral consequences have remained relatively unexplored. Results from the few studies that have examined this show deficits in fine and gross motor function,10–13 lower verbal IQ,14 language impairment,15 greater inattention and hyperactivity,13,16 and poorer general cognitive function.17 The postpartum environment may also play a part in neurobehavioral consequences. Children in low SES households consistently demonstrated compromised neurological, cognitive function scores, and attention-deficit/hyperactivity disorder (ADHD).18 Explanations for these associations include limited access to health care resources,19,20 compromised quality of living,21–23 suboptimal lifestyle,24 poor nutrition,25,26 limited exposure to intellectual stimuli,27,28 and greater psychosocial stress.29 Furthermore, the effect of low SES is more detrimental for children born with low birth weight (LBW)30 and children of mothers with prenatal substance abuse31–33 relative to children born without such risk factors.
This study examines the risk for ADHD as a neurobehavioral consequence of GDM by comparing offspring of mothers with and without GDM in an economically diverse sample. We hypothesize that (1) offspring exposed to mother’s GDM will have greater inattention and hyperactivity scores as preschoolers and will be at greater risk for developing ADHD 2 years later; and (2) there will be poorer neurodevelopmental outcomes and greatest risk for ADHD among offspring exposed to both maternal GDM and lower SES.
METHODS
PROCEDURES AND PARTICIPANTS
The ADHD Rating Scale–IV (ADHD RS–IV)34,35 was distributed to parents of 3- and 4-year-old children in preschools surrounding Queens College, Flushing, New York, for this longitudinal study of preschoolers at risk for ADHD. Teachers were contacted after we received signed parental consents. Participants were recruited an approximately 2:1 ratio of “at-risk” to “typically developing” children. At-risk children had at least 6 inattention or 6 hyperactive and impulsive symptoms as rated by parents and/or teachers. Typically developing children had fewer than 3 symptoms in each domain. Children and parents were required to be English speaking. Children with an IQ below 80, pervasive developmental disorder, diagnosed neurological disorder, or who took systemic medication for medical conditions (including ADHD) were excluded. Four nonbiological children were excluded, resulting in 212 children in the current study. This study was approved by the Queens College institutional review board.
RISK FACTORS: GDM AND SES STATUS
History of GDM was obtained from mothers through face-to-face interview about the child’s developmental history by a trained interviewer blind to the child’s clinical data. Family SES was measured by the Socioeconomic Prestige Index, a widely used measure of social position36 with a theoretical range of 0 to 100. The cut-off point to create low and high SES was the families’ mean score of 55.4.
ADHD SYMPTOMS AND DIAGNOSIS
The ADHD RS–IV
Parents and teachers completed the ADHD RS–IV34,35 at baseline, which consists of the 9 inattention and 9 hyperactive/impulsive behaviors in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) rated on a 4-point scale. Collapsing across items, the mean (SD) ADHD RS–IV teacher and parent totals were 21.39 (16.58) and 21.10 (13.05), respectively. The ADHD RS–IV provides a reliable and valid measure of ADHD symptoms in children,37 including preschoolers.34,35
ADHD Diagnosis
The Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (Kiddie-SADS-PL)38 is a reliable, semistructured child psychiatric interview used to assess child psychiatric conditions as outlined in the DSM-IV. Interviews were repeated annually with the parent by a trained interviewer, either a PhD-level psychologist or a well-trained doctoral student (D.J.M. and J.M.H.), who was blind to the GDM and SES status. The presence or absence of diagnoses was determined by the clinician based on all available clinical information and was systematically reviewed at a consensus meeting led by a licensed psychologist (J.M.H.).
CHILD NEUROPSYCHOLOGICAL FUNCTIONING, TEMPERAMENT, AND BEHAVIORAL/EMOTIONAL FUNCTIONING
Neuropsychological functioning at baseline (ages 3–4 years) across 5 neuropsychological domains was measured by Developmental Neuropsychological Assessment (NEPSY)39: attention/executive functioning (ability to plan ahead and to inhibit impulsive responses); language; memory; sensorimotor (fine motor coordination); and visuospatial processing (ability to accurately perceive and reconstruct 2- and 3-dimensional designs). These domain scores have good stability over time (r=0.68–0.90).
The Wechsler Preschool and Primary Scale of Intelligence–Third Edition (WPPSI-III), which was administered at baseline, is a standardized, norm-referenced intelligence test for use with young children (ages 2.5–7 years). It provides a full-scale IQ score as well as separate verbal and performance IQ scores and a general language composite.
CHILD TEMPERAMENT (AGES 3–4 YEARS)
The 29-item Temperament Assessment Battery for Children Revised (TABC-R)40 has good internal consistency (Cronbach α, 0.86–0.95). Items are rated on a scale from 1 (hardly ever) to 7 (almost always) and generate 4 dimensions: inhibition, negative emotionality, activity level, and lack of task persistence.
BEHAVIORAL AND EMOTIONAL FUNCTIONING AT FOLLOW-UP (AGE 6 YEARS)
The Behavior Assessment System for Children–2 (BASC-2) Parent and Teacher Rating Scales are instruments that measure clinical and adaptive dimensions of behavior by parents and teachers, respectively.41 Clinical scales measure hyperactivity, aggression, anxiety, depression, somatization, atypicality, withdrawal, and attention problems. The composite scale measures the behavioral symptoms index. Adaptive scales measure adaptability and functional communication.
POTENTIAL CONFOUNDERS
The ages of the mother and child, sex, race/ethnicity, and LBW were considered a priori demographic confounders. In addition, self-reports of maternal and paternal ADHD symptoms, derived from the Conners’ Adult ADHD Rating Scale,42 maternal alcohol use, and smoking during pregnancy, and the risk-group status (at-risk vs typically developing) were considered as confounders.
DATA ANALYSIS
Regression analysis, applying generalized estimating equations (GEE),43,44 was used to estimate the effect of GDM on children’s ADHD symptoms and neurobehavioral functioning, rated by both parents and teachers. The GEE allows the use of information from multiple informants and provides regression coefficients and their standard errors, taking the correlation between ratings from multiple informants into account.45 We used the “unstructured” correlation as the covariance structure, which uses robust estimators of variances to protect against misspecifications of the covariance structure and to ensure that the P values are not biased. At-risk and typically developing children were combined to maximize the distribution of ADHD severity.
For binary diagnostic outcomes of ADHD, χ2 analysis evaluated the magnitude of the risk (odds ratio [OR]) for ADHD by GDM and family SES. If significant, the Breslow-Day test of homogeneity of OR examined a differential magnitude of risk for ADHD by GDM as a function of family SES. This was followed by logistic regression analysis, stratified on family SES. Finally, children were grouped as (1) neither GDM nor low SES (reference group), (2) only GDM, (3) only low SES, and (4) both GDM and low SES. Neuropsychological functioning, and temperament at baseline as potential early markers for ADHD were compared in these 4 groups. Pairwise comparisons were conducted with the Holm correction46 for multiple comparisons. Potential confounders were adjusted in the analysis.
To evaluate interactive effects of GDM and low SES on child neurobehavioral development, the presence or absence of additive interaction was tested.47–52 The increased risk for ADHD among children exposed to only GDM, only low SES, and both GDM and low SES was calculated relative to the risk among the reference group. Additive interaction (ie, synergy) exists when the risk of having both risk factors exceeds the sum of the risks for GDM and low SES. The presence of an additive interaction can be examined using an index: attributable proportion (AP) due to interaction; AP exceeding 0 indicates that the increased risk is due to the joint exposure to the 2 risk factors. The 95% CI was calculated based on the Hosmer-Lemeshow CI estimation of interaction.53
RESULTS
DEMOGRAPHICS
Children entered the study at age 3 or 4 years (mean age, 4.1) years. Mean ages for fathers and mothers were 33.6 and 31.3 years, respectively. Most families were “middle class,” with a mean SES score of 55.5.35 Girls comprised 26.5% of the children, and 12.4% had LBW (<2500 g). Participants were racially and ethnically diverse with 58.9% white, 12.3% black, 10.4% Asian, and 18.4% mixed race; 31% had at least 1 Hispanic parent.
Twenty-one mothers (10.0%) had GDM while pregnant with the study child. There was no difference in children’s age, family SES, LBW, race/ethnicity, sex, or history of mother’s smoking during pregnancy by GDM status. However, children exposed to mothers’ GDM compared with children unexposed had older mothers (30.8 vs 36.1 years) and fathers (33.1 vs 36.8 years). Mothers with GDM had higher ADHD symptoms (P=.02), and less alcohol use during pregnancy (P=.05) than mothers without GDM (Table 1).
Table 1.
Characteristics in Children of Mothers With and Without GDM During Pregnancy
| Variable | Total (n=212) | GDM Absent (n=191) | GDM Present (n=21) | Statistic
|
||
|---|---|---|---|---|---|---|
| F or χ2 | P Value | |||||
| Age, ya | ||||||
| Child | 4.31 (0.47) [3.05–5.00] | 4.31 (0.47) [3.05–5.00] | 4.35 (0.48) [3.50–4.97] | F1,210=0.14 | .71 | |
| Father | 33.61 (6.56) [19–61] | 33.12 (6.88) [19–62] | 36.80 (5.22) [28–46] | F1,206=5.85 | .02 | |
| Mother | 31.28 (6.12) [17–44] | 30.77 (5.95) [17–44] | 36.10 (5.65) [24–44] | F1,209=14.71 | <.001 | |
| Family SESa | 55.39 (15.10) [20.0–88.5] | 55.11 (15.33) [20.0–88.5] | 58.17 (12.63) [33.0–83.0] | F1,210=0.77 | .38 | |
| Mother’s ADHDa symptomsa,b | 46.0 (8.25) [26–90] | 45.56 (7.51) [26–75] | 50.0 (12.37) [31–90] | F1,206=5.59 | .02 | |
| Father’s ADHD symptomsa | 49.23 (6.64) [29–78] | 49.19 (6.22) [29–78] | 49.74 (9.33) [37–74] | F1,209=0.09 | .77 | |
| Race/ethnicity, No. (%) | ||||||
| White | 125 (59.0) | 110 (57.6) | 15 (71.4) |
|
.64c | |
| Black | 26 (12.3) | 24 (12.6) | 2 (9.5) | |||
| Asian | 22 (10.4) | 21 (11.0) | 1 (4.8) | |||
| Mixed/other | 39 (18.4) | 36 (18.8) | 3 (14.3) | |||
| Female, No. (%) | 56 (26.4) | 52 (27.2) | 4 (19.0) |
|
.60c | |
| Mother’s alcohol use in pregnancy, No. (%) | 45 (23.0) | 44 (25.0) | 1 (5.0) |
|
.05c | |
| Mother’s tobacco use in pregnancy, No. (%) | 36 (17.1) | 34 (17.9) | 2 (9.5) |
|
.54c | |
| Low birth weight, No. (%) | 26 (12.4) | 21 (11.2) | 5 (23.8) |
|
.15c | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; GDM, gestational diabetes mellitus; SES, socioeconomic status.
Data are given as mean (SD) [range].
t Test scores from the Conners Adult ADHD Rating Scale.
Fisher exact test was used.
ADHD SYMPTOM SCORES AND DIAGNOSIS BY GDM AND LOW SES
The mean inattention score at baseline for offspring exposed to mother’s GDM was significantly higher than for offspring unexposed (12.25 vs 9.50; P=.05), but there was no difference in hyperactivity/impulsivity scores between the groups (12.58 vs 11.29; P=.36). Offspring of low SES families, relative to high SES families, had greater inattention (11.96 vs 9.79; P=.01) and hyperactivity/impulsivity (13.23 vs 10.65; P=.01) scores.
Results showed no difference in the risk for ADHD at baseline (OR, 1.58; 95% CI, 0.77–3.27; P=.22), but a 2-fold increased risk at age 6 years (OR, 2.20; 95% CI, 1.00–4.82; P=.05) among the offspring exposed to GDM compared with those unexposed. There was an approximately 2-fold increased risk for ADHD at baseline (OR, 1.87; 95% CI, 1.21–2.89; P=.005) and at age 6 years (OR, 2.41; 95% CI, 1.53–3.79; P<.001) among the offspring from low SES families.
DIFFERENTIAL RISK OF ADHD BY GDM AMONG THE HIGHER AND LOWER FAMILY SES
The Breslow-Day tests of homogeneity of the OR showed a differential effect of GDM on ADHD at age 6 years as a function of SES (low vs high) OR (P=.01). Subsequent stratified logistic regression models confirmed this; high SES families had a negligible increased risk for ADHD associated with GDM (OR, 0.96; 95% CI, 0.71–1.30; P=.79), whereas low SES families had a 7-fold increased risk for ADHD (OR, 7.0; 95% CI, 1.58–31.48; P=.004).
NEUROPSYCHOLOGICAL DEVELOPMENT AND TEMPERAMENT CHARACTERISTICS AT AGE 3 TO 4 YEARS AMONG THE 4 GROUPS
Neuropsychological indicators showed significant differences in language (P<.001), visuospatial (P=.001), and memory domain scores (P=.005) among the 4 groups of offspring exposed to both GDM and low SES, only GDM, only SES, and neither. For more global WPPSI-III measures, verbal IQ (P <.001), full-scale IQ (P<.001), and the general language composite scores (P<.001) were significantly different. Across measures, group 4 was consistently the lowest (Table 2). We also found differences in temperament. Except for negative emotion (P=.19), offspring exposed to both GDM and low SES had the highest scores for all other domains: lack of inhibition (P<.001), activity level (P=.04), lack of persistence (P<.001), and impulsivity (P=.003).
Table 2.
Neuropsychological Functioning and Temperament at Age 3 to 4 Years as a Function of Maternal GDM and SES
| Child Outcomes | Groupa
|
Wald | P Value | Significant Pairwise Comparisonb | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Neuropsychological functioning, NEPSY | |||||||
| Attention/executive functioning | 100.5 (1.4) | 105.2 (3.3) | 99.7 (1.5) | 101.5 (3.2) | 2.6 | .46 | NA |
| Language | 103.6 (1.1) | 110.5 (3.3) | 99.2 (1.4) | 95.6 (2.9) | 18.4 | <.001 | 1, 2 > 3, 4 |
| Sensorimotor | 93.1 (1.5) | 93.6 (4.3) | 95.6 (1.5) | 91.5 (4.0) | 2.0 | .56 | NA |
| Visuospatial | 107.7 (1.3) | 104.2 (3.7) | 107.7 (1.4) | 98.0 (2.2) | 16.9 | .001 | 1, 2, 3 > 4 |
| Memory | 101.1 (3.8) | 96.6 (1.4) | 90.7 (1.6) | 89.4 (3.6) | 12.7 | .005 | 1, 2 > 3, 1 > 4 |
| Cognitive function, WPPSI-III | |||||||
| Verbal IQ | 113.6 (4.2) | 110.5 (1.5) | 100.2 (1.6) | 92.3 (2.7) | 32.5 | <.001 | 1, 2 > 3 > 4 |
| Performance IQ | 111.9 (3.8) | 109.8 (1.5) | 107.8 (1.5) | 100.6 (4.0) | 5.4 | .14 | NA |
| Full-scale IQ | 113.6 (3.5) | 109.2 (1.4) | 104.7 (1.4) | 97.0 (2.8) | 20.1 | <.001 | 1, 2 > 3 > 4 |
| General language composite | 112.9 (3.9) | 108.8 (1.4) | 102.1 (1.6) | 94.2 (2.7) | 32.4 | <.001 | 1, 2 > 3 > 4 |
| Temperament, TABC-R | |||||||
| Lack of inhibition | 43.7 (1.1) | 43.1 (3.4) | 43.9 (1.2) | 58.4 (2.1) | 34.8 | <.001 | 1, 2, 3 < 4 |
| Negative emotion | 49.5 (1.0) | 46.2 (3.6) | 50.2 (1.2) | 56.5 (3.8) | 4.1 | .25 | NA |
| Activity level | 53.2 (0.9) | 50.7 (2.6) | 54.6 (0.9) | 58.6 (2.0) | 8.3 | .04 | 1 < 4 |
| Lack of persistence | 50.1 (0.9) | 48.1 (2.8) | 50.6 (1.0) | 60.6 (2.3) | 19.7 | <.001 | 1, 2, 3 < 4 |
| Impulsivity | 50.5 (0.8) | 47.4 (2.8) | 51.3 (0.9) | 59.4 (2.5) | 14.1 | <.003 | 1, 2, 3 < 4 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; GDM, gestational diabetes mellitus; NA, not applicable; NEPSY, Developmental Neuropsychological Assessment; SES, socioeconomic status; TABC-R, Temperament Assessment Battery for Children–Revised; WPPSI-III, Wechsler Preschool and Primary Scale of Intelligence–Third Edition.
Data are given as means (SDs). Group 1, children exposed to neither mother’s GDM nor low SES (n=97); group 2, children exposed to mother’s GDM but not low SES (n=12); group 3, children exposed to low SES but not mother’s GDM (n=94); and group 4, children exposed to both mother’s GDM and low SES (n=9).
The α for P value for pair-wise comparisons was adjusted for multiple comparisons using sequential Bonferroni (Holm) method. Age of mother, mother’s alcohol use and smoking during pregnancy, age, sex, race/ethnicity, and birthweight of the child, and maternal ADHD symptoms, paternal ADHD symptoms, and risk-group status (at-risk vs typically developing) are included in the generalized estimating equations model as covariates.
BEHAVIORAL AND EMOTIONAL FUNCTIONING AT AGE 6 YEARS
Table 3 shows group differences in adaptability (P=.04) and functional communication (P=.009). Inspection of clinical subscales revealed significant differences in depression (P = .03), atypicality (P = .01), withdrawal (P=.05), and attention problems (P=.004). Across all areas, the group with both GDM and low SES consistently had the highest problem scores.
Table 3.
The Behavior Assessment System for Children-2 at Ages 3 to 4 Years Among Groups of Children Formed by Mother’s GDM Status and Family SESa
| Symptom | Groupb
|
Wald | P Value | Significant Pairwise Comparison | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Adaptive scalesc | |||||||
| Adaptability | 50.7 (0.6) | 49.6 (2.5) | 49.5 (0.9) | 45.1 (1.9) | 8.04 | .04 | 1 > 4 |
| Functional communication | 51.8 (0.8) | 52.8 (1.9) | 48.9 (0.8) | 44.4 (2.4) | 14.39 | .002 | 1 > 2, 3 > 4 |
| Clinical scalesd | |||||||
| Hyperactivity | 56.4 (0.9) | 52.3 (2.9) | 56.2 (1.0) | 58.3 (2.5) | 4.58 | .20 | NA |
| Aggression | 50.6 (3.2) | 51.6 (2.9) | 54.9 (1.1) | 50.6 (3.2) | 2.45 | .49 | NA |
| Anxiety | 51.4 (1.0) | 53.5 (2.6) | 51.1 (0.9) | 51.3 (2.9) | 1.22 | .75 | NA |
| Depression | 52.0 (1.1) | 57.7 (2.2) | 54.6 (1.2) | 58.2 (2.9) | 8.99 | .03 | NA |
| Somatization | 48.9 (0.6) | 51.4 (3.0) | 50.8 (0.8) | 51.7 (2.8) | 4.10 | .25 | NA |
| Atypicality | 51.9 (1.1) | 53.8 (3.5) | 55.8 (1.4) | 58.9 (2.7) | 11.36 | .01 | 1 < 4 |
| Withdrawal | 49.6 (0.8) | 47.0 (2.3) | 47.9 (0.9) | 54.3 (2.6) | 7.75 | .05 | 1, 2, 3 < 4 |
| Attention problems | 52.4 (0.7) | 51.0 (1.9) | 54.4 (0.8) | 58.4 (1.8) | 13.55 | .004 | 1, 2, 3 < 4 |
Abbreviations: GDM, gestational diabetes mellitus; NA, not applicable; SES, socioeconomic status.
The α values for P value for pair-wise comparisons were adjusted for multiple comparisons using sequential Bonferroni (Holm) method. The age of the child and of the mother at the child’s birth, child’s race/ethnicity, sex, low birth weight, maternal smoking during pregnancy, maternal alcohol use during pregnancy, maternal and paternal attention-deficit/hyperactivity disorder symptoms, and risk-group status (at-risk vs typically developing) were adjusted in all analyses.
Data are given as means (SDs). Group 1, children exposed to neither mother’s GDM nor low SES (n=97); group 2, children exposed to mother’s GDM but not low SES (n=12); group 3, children exposed to low SES but not mother’s GDM (n=94); and group 4, children exposed to both mother’s GDM and low SES (n=9).
Higher scores denote greater adaptive skills.
Higher scores denote greater clinical symptoms.
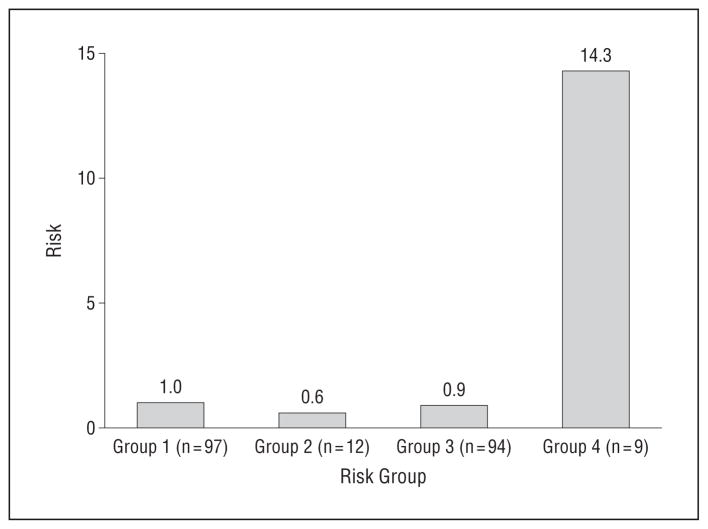
EVIDENCE FOR ADDITIVE INTERACTION OF GDM AND LOW SES ON THE RISK OF ADHD AT AGE 6 YEARS
The Figure shows a clear additive interaction52 between the mother’s GDM and low SES on the risk for ADHD at age 6 years, showing a synergistically increased risk of ADHD by joint effects of GDM and low SES. Specifically, relative to the reference group of children, neither children exposed to only GDM (OR, 0.60; 95% CI, 0.30–1.17; P=.13) nor children exposed to only low SES (OR, 0.90; 95% CI, 0.47–1.65; P=.70) exhibited a notably increased risk. However, children exposed to both GDM and low SES had more than a 14-fold increased risk for ADHD (OR, 14.31; 95% CI, 2.14–95.88; P=.006). This is substantial, considering the sum of risks by GDM alone53 and by low SES alone was only 1.50. A formal test of additive interaction showed the synergy indicator, AP, was 0.82 (95% CI, 0.77–0.87) suggesting that 82% of the increased risk for ADHD is attributable to the joint effects of the mother’s GDM and low SES, and additive interaction was significant as confirmed by the 95% CI not including 0.53,54
Figure.
Risk of attention-deficit/hyperactivity disorder at age 6 years by mother’s gestational diabetes mellitus (GDM) and family socioeconomic status (SES). Group 1, children exposed to neither mother’s GDM nor low SES; group 2, children exposed to mother’s GDM, but not low SES; group 3, children exposed to low SES, but not mother’s GDM; and group 4, children exposed to both mother’s GDM and low SES. Group 1: odds ratio (OR), 1 (reference group); group 2: OR, 0.60 (95% CI, 0.30–1.17); P =.13; group 3: OR, 1.54 (95% CI, 0.47–1.65); P =.70; group 4: OR, 14.31 (95% CI, 2.14–95.88); P =.006; attributable proportion to synergy, 0.82 (95% CI, 0.77–0.87).
COMMENT
The current study examined whether maternal GDM and low SES, alone and in combination, heighten risk for ADHD symptoms and diagnosis, cognitive and neuropsychological dysfunction, and emotional/behavioral problems in offspring. Our data are consistent with, and expand, the results of prior studies, providing the following 4 main findings. First, both GDM and low SES have negative effects on ADHD symptoms and diagnosis. Second, the magnitude of risk between GDM and ADHD differs significantly by family SES. Third, children exposed to both GDM and low SES demonstrated a wide range of compromised neurobehavioral functions. Fourth, the risk for ADHD increased synergistically when the children were exposed to both GDM and low SES. Neither children exposed to only lower SES nor children exposed to only mother’s GDM had a notable increase in the risk for ADHD at age 6 years.
To our knowledge, this is the first study to examine the joint effects of prenatal in utero exposure to GDM and postnatal family SES on various neurobehavioral functions and ADHD diagnosis among preschool-age children. It is also the first to document a synergistic increase in the risk for ADHD among offspring exposed to GDM and low SES.
The 1970s saw improved glucose control during pregnancy, and pregnant women were routinely monitored for elevated glucose levels to prevent adverse obstetric (eg, preeclampsia, excessive weight gain, hypertension, and cesarean delivery) and neonatal outcomes (eg, neonatal mortality, jaundice, nerve palsy, macrosomia, shoulder dystocia, and bone fractures). While these changes improved obstetric and neonatal outcomes, they were not designed to prevent potential adverse effects on central nervous system dysregulation of offspring. As this study clearly demonstrates, especially within low SES households, the risk of GDM for both mother and infant may extend far beyond birth. Few studies have focused on the role of GDM and the relationship between complications during pregnancy and birth and later neurocognitive dysfunction. Among them are studies from the 1960s and 1970s55 that examined the association between GDM and IQ, although results were inconclusive.56–58 Recently Ornoy et al13 and Ornoy16 demonstrated that the consequences of GDM affect a much wider array of neuropsychological domains related to children’s attention and learning problems than was previously thought.
Although the mechanism of synergistic influences of GDM and low SES on ADHD and other suboptimal neurobehavioral indicators remains unknown, practitioners in internal medicine, endocrinology, obstetrics, and pediatrics should be alerted so that there can be multiple points of intervention to prevent the development of neuropsychological dysfunction in offspring. For example, a woman with a family history of diabetes mellitus (DM) or those living in low SES households could be advised to work with a nutritionist, even before conception, so that through routine monitoring and dietary modification she could reduce the likelihood of GDM if and when she does get pregnant.
Of women who had GDM, 20% to 50% are at risk for developing DM in subsequent pregnancies59,60 and type II DM within 5 to 10 years postdelivery.2,61 Obstetricians should encourage these women to work with an endocrinologist and a nutritionist to prevent type II DM after pregnancy, especially if she is likely to become pregnant again. Mothers-to-be should be informed that elevated glucose levels during the critical period of fetal brain development must be avoided to prevent the diversion of fetal resources from supporting brain development to supporting pancreatic function. Pediatricians should be informed if a patient’s mother had GDM for closer monitoring for potential therapeutic services. All of these potential interventions are critical for those with lower SES.
Our study has several methodological strengths. First, both dimensional measures of behavioral problems related to ADHD and DSM IV–based ADHD diagnoses were established by semistructured psychiatric interviews by interviewers blind to mother’s GDM status and family SES. Second, to maximize available multiple informant data, we used an analytic strategy (GEE) that reduces informant bias by taking into account correlations between multiple informant reports.62,63 Third, an examination of additive interaction between GDM and low SES, rather than multiplicative interaction, on ADHD at age 6 years allowed us to actually estimate the degree of synergy. However, we have separately tested a model with a multiplicative interaction term (GDM×low SES). As expected, the interaction term was significant on an increased risk for ADHD at age 6 ( ; P <.001). Fourth, we are in the early phase of a prospective follow-up study evaluating the risk factors for ADHD in children. Thus, we will eventually be able to identify early patterns and sequence of the disorder.
Our study also has limitations. First, GDM status is based on mothers’ retrospective reports, which can be affected by recall bias, although several studies have validated mothers’ reports of their pregnancy complications and birth outcomes.64,65 Second, it might be the glucose level during the critical window of fetal brain development that is predictive of the risk for developmental problems, including ADHD. A measure of the serum glucose level would have strengthened our findings. Third, although measures of parental hyperactivity and inattention were adjusted in our analyses, the family history of ADHD was not available. However, mothers with GDM had greater ADHD symptoms than mothers without GDM. While both mothers’ and fathers’ ADHD symptoms have been statistically adjusted in all analysis, we should be cautious in interpreting our results.
Despite these limitations, this study demonstrates that children of mothers with GDM raised in lower SES households are at far greater risk for developing ADHD and showing signs of suboptimal neurocognitive and behavioral development. Since ADHD is a disorder with high heritability, efforts to prevent exposure to environmental risks through patient education may help to reduce the nongenetic modifiable risk for ADHD and other developmental problems. It remains unclear, however, whether GDM increases the risk for ADHD in particular or is a nonspecific risk factor for a spectrum of neurodevelopmental and psychiatric disorders. Nevertheless, it is clear that developing a refined understanding is urgently needed among expectant mothers regarding certain risk-prone behaviors (overeating, poor diet, and smoking during pregnancy) to mitigate the long-term human and economic costs.
Acknowledgments
Funding/Support: This work was supported by grants K01MH080062 (to Dr Nomura) and R01MH046448 (to Dr Halperin).
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Bipasha Basu, MA, Taneka Wellington, Khushmand Rajendran, MSW, PhD, Beatrice Bleier, and Rachel Lifshitz provided invaluable assistance.
Author Contributions: Dr Nomura had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Nomura, Grossman, and Halperin. Acquisition of data: Nomura, Marks, Grossman, Loudon, and Halperin. Analysis and interpretation of data: Nomura, Yoon, Stone, and Halperin. Drafting of the manuscript: Nomura and Yoon. Critical revision of the manuscript for important intellectual content: Nomura, Marks, Grossman, Loudon, Stone, and Halperin. Statistical analysis: Nomura. Obtained funding: Nomura and Halperin. Administrative, technical, and material support: Nomura, Grossman, Yoon, Loudon, and Halperin. Study supervision: Nomura, Marks, Stone, and Halperin.
References
- 1.Metzger BE, Coustan DR Organizing Committee. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998;21(suppl 2):B161–B167. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Accessed November 16, 2011]. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. [Google Scholar]
- 3.Berkowitz GS, Lapinski RH, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992;135(9):965–973. doi: 10.1093/oxfordjournals.aje.a116408. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 5.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy, I: an autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46(3):283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy, II: patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. Bibl Anat. 1986;28(28):1–26. [PubMed] [Google Scholar]
- 8.Henrichs J, Schenk JJ, Schmidt HG, et al. Fetal size in mid- and late pregnancy is related to infant alertness: the generation R study. Dev Psychobiol. 2009;51(2):119–130. doi: 10.1002/dev.20351. [DOI] [PubMed] [Google Scholar]
- 9.Jokhi RP, Whitby EH. Magnetic resonance imaging of the fetus. Dev Med Child Neurol. 2011;53(1):18–28. doi: 10.1111/j.1469-8749.2010.03813.x. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo TA, Dooley SL, Metzger BE, Cho NH, Ogata ES, Silverman BL. Prenatal and perinatal influences on long-term psychomotor development in offspring of diabetic mothers. Am J Obstet Gynecol. 1995;173(6):1753–1758. doi: 10.1016/0002-9378(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 11.Ratzon N, Greenbaum C, Dulitzky M, Ornoy A. Comparison of the motor development of school-age children born to mothers with and without diabetes mellitus. Phys Occup Ther Pediatr. 2000;20(1):43–57. [PubMed] [Google Scholar]
- 12.Ornoy A, Ratzon N, Greenbaum C, Peretz E, Soriano D, Dulitzky M. Neurobehaviour of school age children born to diabetic mothers. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F94–F99. doi: 10.1136/fn.79.2.f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab. 2001;14(suppl 1):681–689. doi: 10.1515/jpem.2001.14.s1.681. [DOI] [PubMed] [Google Scholar]
- 14.Ornoy A, Wolf A, Ratzon N, Greenbaum C, Dulitzky M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F10–F14. doi: 10.1136/fn.81.1.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dionne G, Boivin M, Séguin JR, Pérusse D, Tremblay RE. Gestational diabetes hinders language development in offspring. Pediatrics. 2008;122(5):e1073–e1079. doi: 10.1542/peds.2007-3028. [DOI] [PubMed] [Google Scholar]
- 16.Ornoy A. Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr Endocrinol Rev. 2005;3(2):104–113. [PubMed] [Google Scholar]
- 17.Veena SR, Krishnaveni GV, Srinivasan K, et al. Childhood cognitive ability: relationship to gestational diabetes mellitus in India. Diabetologia. 2010;53(10):2134–2138. doi: 10.1007/s00125-010-1847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clin Proc. 2004;79(9):1124–1131. [PubMed] [Google Scholar]
- 19.Winkleby M, Cubbin C, Ahn D. Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health. 2006;96(12):2145–2153. doi: 10.2105/AJPH.2004.060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubikaytis T, Larivaara M, Kuznetsova O, Hemminki E. Inequalities in health and health service utilisation among reproductive age women in St. Petersburg, Russia: a cross-sectional study. BMC Health Serv Res. 2010;10:307. doi: 10.1186/1472-6963-10-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govil SR, Weidner G, Merritt-Worden T, Ornish D. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: the Multisite Cardiac Lifestyle Intervention Program. Am J Public Health. 2009;99(7):1263–1270. doi: 10.2105/AJPH.2007.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hijmans CT, Fijnvandraat K, Oosterlaan J, Heijboer H, Peters M, Grootenhuis MA. Double disadvantage: a case control study on health-related quality of life in children with sickle cell disease. Health Qual Life Outcomes. 2010;8:121. doi: 10.1186/1477-7525-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahana E, Pappa E, Niakas D. The impact of ethnicity, place of residence and socioeconomic status on health-related quality of life: results from a Greek health survey. Int J Public Health. 2010;55(5):391–400. doi: 10.1007/s00038-010-0171-2. [DOI] [PubMed] [Google Scholar]
- 24.Ross NA, Gilmour H, Dasgupta K. 14-year diabetes incidence: the role of socioeconomic status. Health Rep. 2010;21(3):19–28. [PubMed] [Google Scholar]
- 25.Wilson TA, Adolph AL, Butte NF. Nutrient adequacy and diet quality in non-overweight and overweight Hispanic children of low socioeconomic status: the Viva la Familia Study. J Am Diet Assoc. 2009;109(6):1012–1021. doi: 10.1016/j.jada.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raffensperger S, Kuczmarski MF, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Effect of race and predictors of socioeconomic status on diet quality in the HANDLS Study sample. J Natl Med Assoc. 2010;102(10):923–930. doi: 10.1016/s0027-9684(15)30711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo AM, Angst D, Knafl KA, Hadley E, Smith C. Parents sharing information with their children about genetic conditions. J Pediatr Health Care. 2005;19(5):267–275. doi: 10.1016/j.pedhc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. J Int Neuropsychol Soc. 2011;17(1):120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- 29.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58(8):1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 30.Nomura Y, Halperin JM, Newcorn JH, et al. The risk for impaired learning-related abilities in childhood and educational attainment among adults born near-term. J Pediatr Psychol. 2009;34(4):406–418. doi: 10.1093/jpepsy/jsn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C. Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001;43(10):668–675. doi: 10.1017/s0012162201001219. [DOI] [PubMed] [Google Scholar]
- 32.Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett. 2003;140–141:171–181. doi: 10.1016/s0378-4274(02)00505-2. [DOI] [PubMed] [Google Scholar]
- 33.Ornoy A, Daka L, Goldzweig G, et al. Neurodevelopmental and psychological assessment of adolescents born to drug-addicted parents: effects of SES and adoption. Child Abuse Negl. 2010;34(5):354–368. doi: 10.1016/j.chiabu.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 34.DuPaul GJ, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent ratings of attention-deficit/hyperactivity disorder symptoms: factor structure and normative data. J Psychopathol Behav Assess. 1998;20:83–102. [Google Scholar]
- 35.DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. J Am Acad Child Adolesc Psychiatry. 2001;40(5):508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: how the new measures measure up. Sociol Methodol. 1994;24:1–72. [Google Scholar]
- 37.Faries DE, Yalcin I, Harder D, Heiligenstein JH. Validation of the ADHD Rating Scale as a clinical administered and scored instrument. J Atten Disord. 2001;5:107–115. [Google Scholar]
- 38.Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. [Accessed November 16, 2011];Kiddie-SADS Present and Lifetime Version. 1996 http://www.wpic.pitt.edu/research/AssessmentTools/ChildAdolescent/ksads-pl.pdf.
- 39.Korkman M, Kirk U, Kemp S. NEPSY-II Clinical and Interpretive Manual. San Antonio, TX: Psychological Corp; 2007. [Google Scholar]
- 40.Martin RP, Bridger RC. Temperamental Assessment Battery for Children, Revised: A Tool for the Assessment of Temperamental Traits and Types of Young Children. Brandon, VT: Clinical Psychology Publishing Co; 1998. [Google Scholar]
- 41.Reynolds CR, Kamphaus RW. Behavioral Assessment System for Children (BASC-2) 2. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 42.Conners CK, Erhardt D, Sparrow E. Conners Adult ADHD Rating Scale. New York, NY: Multihealth Systems Inc; 1999. [Google Scholar]
- 43.Diggle PJ, Liang K-Y, Seger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 44.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 45.Horton NJ, Laird NM, Murphy JM, Monson RR, Sobol AM, Leighton AH. Multiple informants: mortality associated with psychiatric disorders in the Stirling County Study. Am J Epidemiol. 2001;154(7):649–656. doi: 10.1093/aje/154.7.649. [DOI] [PubMed] [Google Scholar]
- 46.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 47.Saracci R. Interaction and synergism. Am J Epidemiol. 1980;112(4):465–466. doi: 10.1093/oxfordjournals.aje.a113014. [DOI] [PubMed] [Google Scholar]
- 48.Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 49.Rothman KJ. Epidemiology: An Introduction. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 50.Darroch J. Biologic synergism and parallelism. Am J Epidemiol. 1997;145(7):661–668. doi: 10.1093/oxfordjournals.aje.a009164. [DOI] [PubMed] [Google Scholar]
- 51.Agresti A. Categorical Data Analysis. 2. New York, NY: John Wiley & Sons; 2002. p. 120. [Google Scholar]
- 52.Rothman KJ. Estimation versus detection in the assessment of synergy. Am J Epidemiol. 1978;108(1):9–11. [PubMed] [Google Scholar]
- 53.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 55.Weintrob N, Karp M, Hod M. Short- and long-range complications in offspring of diabetic mothers. J Diabetes Complications. 1996;10(5):294–301. doi: 10.1016/1056-8727(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 56.Churchill JA, Berendes HW, Nemore J. Neuropsychological deficits in children of diabetic mothers: a report from the Collaborative Study of Cerebral Palsy. Am J Obstet Gynecol. 1969;105(2):257–268. doi: 10.1016/0002-9378(69)90067-2. [DOI] [PubMed] [Google Scholar]
- 57.Stehbens JA, Baker GL, Kitchell M. Outcomes at ages 1, 3, and 5 of children born to diabetic women. Am J Obstet Gynecol. 1977;127(4):408–413. doi: 10.1016/0002-9378(77)90499-9. [DOI] [PubMed] [Google Scholar]
- 58.Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325(13):911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- 59.Major CA, deVeciana M, Weeks J, Morgan MA. Recurrence of gestational diabetes: who is at risk? Am J Obstet Gynecol. 1998;179(4):1038–1042. doi: 10.1016/s0002-9378(98)70211-x. [DOI] [PubMed] [Google Scholar]
- 60.Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol. 2010;203(5):467e1–e6. doi: 10.1016/j.ajog.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 61.O’Sullivan JB. Body weight and subsequent diabetes mellitus. JAMA. 1982;248(8):949–952. [PubMed] [Google Scholar]
- 62.Richters J, Pellegrini D. Depressed mothers’ judgments about their children: an examination of the depression-distortion hypothesis. Child Dev. 1989;60(5):1068–1075. doi: 10.1111/j.1467-8624.1989.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 63.Chilcoat HD, Breslau N. Does psychiatric history bias mothers’ reports? an application of a new analytic approach. J Am Acad Child Adolesc Psychiatry. 1997;36(7):971–979. doi: 10.1097/00004583-199707000-00020. [DOI] [PubMed] [Google Scholar]
- 64.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. Am J Epidemiol. 1997;145(1):58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- 65.Elliott JP, Desch C, Istwan NB, Rhea D, Collins AM, Stanziano GJ. The reliability of patient-reported pregnancy outcome data. Popul Health Manag. 2010;13(1):27–32. doi: 10.1089/pop.2009.0008. [DOI] [PubMed] [Google Scholar]