Abstract
Mitochondria are important organelles referred to as cellular powerhouses for their unique properties of cellular energy production. With many pathologic conditions and aging, mitochondrial function declines, and there is a reduction in the production of adenosine triphosphate. The energy carrying molecule generated by cellular respiration and by pentose phosphate pathway, an alternative pathway of glucose metabolism. D-ribose is a naturally occurring monosaccharide found in the cells and particularly in the mitochondria is essential in energy production. Without sufficient energy, cells cannot maintain integrity and function. Supplemental D-ribose has been shown to improve cellular processes when there is mitochondrial dysfunction. When individuals take supplemental D-ribose, it can bypass part of the pentose pathway to produce D-ribose-5-phosphate for the production of energy. In this article, we review how energy is produced by cellular respiration, the pentose pathway, and the use of supplemental D-ribose.
Keywords: Adenosine Triphosphate, Bioenergetics, D-ribose, Mitochondria
INTRODUCTION
Mitochondria are among the most important organelles in cells. They function as the cell powerhouse because 99% of adenosine triphosphate (ATP) is produced within mitochondria, and ATP is the main energy source for intracellular metabolic pathways (1–3). Mitochondrial dysfunction can produce extreme fatigue and other symptoms that are common complaints among patients, especially those individuals with heart failure. The reduction in mitochondrial function at the cellular level is often associated with loss of both the electrical and chemical transmembrane potential, the alteration of the electron transport chain function, and diminished transport of mitochondrial metabolites needed for cellular function (2,4,5).
The mitochondria are considered to have developed from an ancient synergy in which a nucleated cell was engulfed by an aerobic prokaryote. In this endosymbiotic relationship, the host eukaryote gradually transformed into a mitochondrion using oxygen to produce energy (6). Mitochondria also contain their own deoxyribonucleic acid (DNA) and transcriptional and translational mechanisms. Certain diseases are now being associated with mitochondrial DNA (mtDNA) defects that contribute to underlying energetic factors (7,8). D-ribose is a naturally occurring monosaccharide within the pentose pathway that assists with ATP production. It is a 5-carbon chain (also called aldopentose) and is a key component of DNA, ribonucleic acid (RNA), acetyl coenzyme A, and ATP (9). Cells produce D-ribose through the pentose phosphate pathway (PPP) that is essential for ATP production. In many diseases or conditions, ATP synthesis is reduced, thus supplementation with D-ribose may provide a solution to impaired cellular bioenergetics (10). In this article, we review how energy is produced by cellular respiration, the pentose pathway, and the use of supplemental D-ribose.
Mitochondria
Mitochondria are highly dynamic double membrane-bound organelles (cellular components) found in the cytoplasm of most eukaryotic cells, those cells that contain a nucleus (11–14). The primary function of mitochondria is to provide chemical energy required for cellular biosynthesis through their vibrant abilities to convert energy from nutrient molecules and store this energy in phosphate bonds within a molecule known as ATP (15–17).
Synthesis of ATP (also known as bioenergetics) within mitochondria is essential for producing the energy needed for normal cellular processes. In addition to energy generation, mitochondria have a role in other mechanisms including cellular metabolism, calcium signaling, and cell death (18,19). Mitochondria also contain some DNA material, although the majority of genomic data reside within cell nuclei (17). The quantity of mitochondria needed is dependent upon energy demands. For example, cells requiring more energy, such as those found in skeletal muscles, possess greater quantities of mitochondria (20).
Mitochondria are oval shaped structures that vary in size and distribution to meet cellular requirements (21). Each mitochondrion has a double membrane composed of proteins and phospholipids (22,23). The double membranes of the mitochondrion form four distinct components within the organelle: (1) a smooth contour outer membrane; (2) an intermembrane space; (3) an inner membrane; and (4) a matrix. The outer membrane creates a physical border between the mitochondrion and the rest of the cell (24). This membrane is formed by a single phospholipid bilayer containing proteins called porins that enable permeability for free passage of other proteins such as ATP, ions, and nutrient molecules (16). The intermembrane space is the region between the outer and inner membranes. The matrix is enclosed within the intermembrane and contains a host of proteins and enzymes involved in ATP synthesis and genetic material. The inner membrane is a highly complex structure consisting of numerous infoldings that are organized into sophisticated layers known as cristae. In contrast to the outer membrane, the inner membrane does not contain porins and is highly impermeable to most molecules. As a result, ions and molecules require specialized transporters for selective membrane passage (25,26).
Cellular Respiration
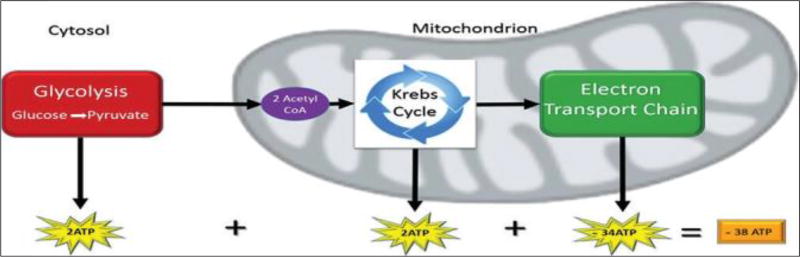
Cellular respiration is a series of biochemical reactions within mitochondria that results in ATP production (27). Adenosine triphosphate is generated through a highly organized system embedded within the inner membrane. Cellular respiration involves three processes: (1) glycolysis; (2) the citric acid cycle, also known as the Krebs cycle; and (3) the electron transport chain, also referred to as oxidative phosphorylation (Figure 1) (28).
Figure 1.
The stages of cellular respiration include glycolysis, pyruvate oxidation, Krebs cycle, and electron transport chain.
Glycolysis is the anaerobic pathway within the cell cytoplasm in which glucose, a six carbon sugar, is converted into two molecules made of three carbons called pyruvate. From this pathway, one glucose molecule yields two ATP molecules (29). Following glycolysis, pyruvate enters the mitochondrion, and enzymatic systems in the mitochondrial matrix convert pyruvate into two carbon molecules called acetyl-CoA(28).
Acetyl-CoA then enters the citric acid cycle and undergoes a series of biochemical reactions with enzymes that yield carbon dioxide and electron carrier molecules known as nicotinamide adenine dinucleotide (NADH) and flavinadenine dinucleotide (FADH2). There are two additional ATP molecules produced for each glucose molecule undergoing glycolysis. The majority of ATP is generated from the last phase of cellular respiration, an aerobic pathway known as the electron transport chain (30).
The electron transport chain consists of a group of complex proteins, referred to as protein complexes I to IV that are housed within the mitochondrial inner membrane (31). Hydrogen electrons from NADH and FADH2 molecules pass through the transport chain from one complex to another creating a proton gradient across the membrane. Energy transfer from electrons is used to pump protons across the membrane space throughout the entire cristae surface. Once the concentration gradient of electrons becomes higher in the membrane space, protons migrate to the area of lower concentration in the mitochondrial matrix via an enzyme known as ATP synthase (32). This enzyme catalyzes the production of ATP by the phosphorylation of adenosine diphosphate (ADP). The electron transport chain pathway generates approximately 34 additional ATP molecules. Thus, cellular respiration yields approximately 38 ATP molecules from one glucose molecule (Figure 1) (33).
D-ribose
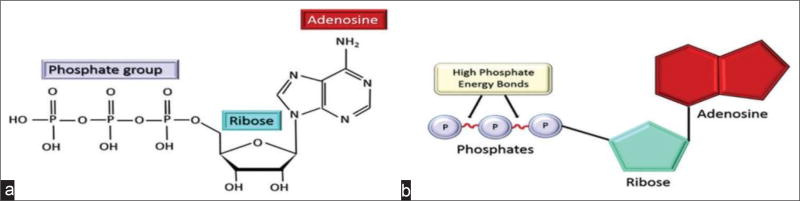
D-ribose is an energy producing substrate of the ATP molecule and is often called the “molecular currency” because of its role in intracellular energy transfer. Adenosine triphosphate consists of phosphate, ribose, and adenosine groups that are connected through two high-energy phosphoanhydride bonds within the molecule. (Figure 2) (34).
Figure 2.
(a) Chemical structure of adenosine triphosphate (ATP); (b) The ATP molecule is composed of an adenosine ring and a ribose sugar with three phosphate groups. From the high energy bonds among the phosphate group, ATP is produced.
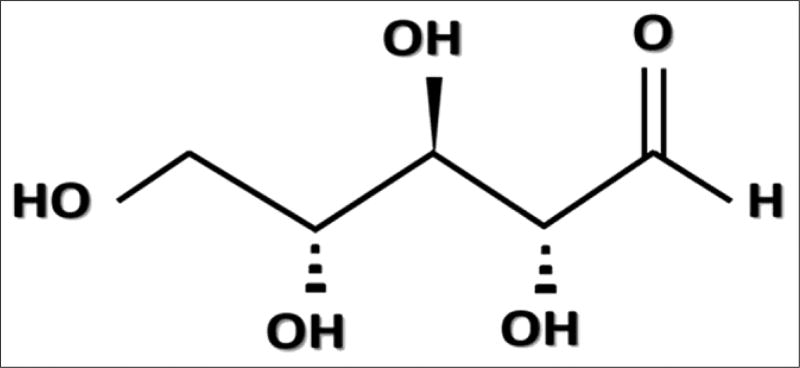
As a pentose sugar, D-ribose has five carbons in its ring structure; the chemical structure (Figure 3), and the molecular weight is 150.13 g/mol (35).
Figure 3.
An image of the chemical structure of D-Ribose which is a pentose monosaccharide (simple sugar).
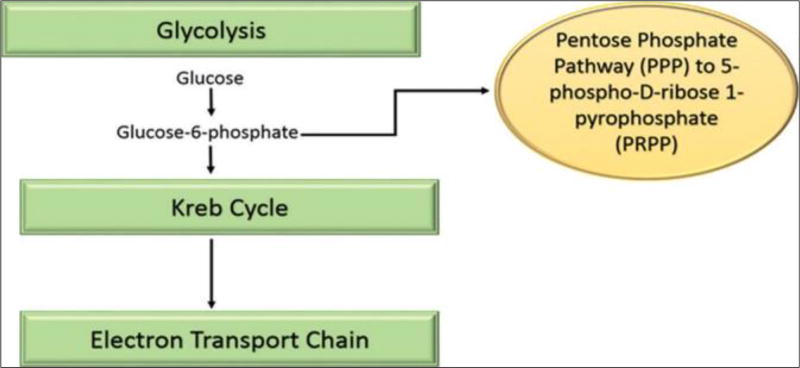
The ATP molecule is able to store and transport chemical energy within cells and is essential for synthesis of nucleus acids such as DNA and RNA. Ribose is a naturally occurring five-carbon sugar produced in the body through the PPP, a metabolic pathway parallel to glycolysis that generates nicotinamide adenine dinucleotide phosphate (NADPH), pentoses, and ribose 5-phosphate. The PPP is a slow process that requires an enzyme called glucose-6-phosphate dehydrogenase (G-6-PDH) that is often in short supply within the cells (Figure 4). This enzyme can have limited expression in the myocardial cells with cardiac disease leading to significant delay in the production of ribose (36).
Figure 4.
The relationship of the pentose phosphate pathway to stages of cellular respiration.
There are two main pathways — the de novo and salvage—for the synthesis of nucleotides. Using the 5-phosphoribo-syl-1-pyrophosphate (PRPP), the de novo pathway enzymes build purine and pyrimidine nucleotides from the beginning of the process with ribose. This pathway is much slower than the salvage pathway in which preformed ribose allows the cells to quickly and efficiently recycle the ATP end products. The mitochondria use the ATP metabolites to form new ATP for energy production. Consequently, ribose is crucial for both the de novo and salvage pathways (37–39).
Supplemental D-ribose
In certain pathologic conditions such as heart failure, cellular energy deficiency exists in myocardial mitochondria. The reduction in ATP production is directly correlated with the decreased supply of D-ribose in the mitochondria. This may be related to the limited expression of the G-6-PDH enzyme in myocardium that can significantly interrupt the production of ribose. Several studies have shown that augmenting D-ribose following myocardial ischemia improves mitochondrial function by increasing myocardial ATP production (40).
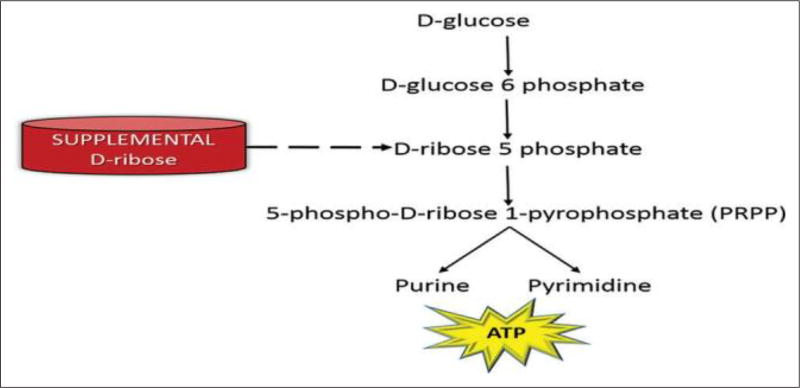
Administering supplemental D-ribose circumvents the enzymatic step to assist with replenishing ATP levels in cells. In purine metabolism, D-glucose is transformed to both D-ribose-6-phosphate and to D-ribose-5-phosphate and then altered to 5-phospho-D-ribose 1-pyrophsophate (PRPP) for the synthesis of purine and pyrimidine (Figure 5). In other words, supplemental D-ribose bypasses the rate-controlling PPP (slower pathway) and provides an alternate source of PRPP for ATP production.
Figure 5.
Supplemental D-ribose bypasses the upper part of the pentose pathway and is an alternative source for 5-phospho-D-ribose 1-pyrophosphate (PRPP).
D-ribose has been used both orally and intravenously in patients for many different pathologic conditions such as chronic fatigue syndrome (41), fibromyalgia (42), and myocardial dysfunction (40). It is often used to improve athletic performance and reduce symptoms of cramping, pain, and stiffness following exercise (41). Under different pathologic conditions, ATP, ADP, and adenosine monophosphate are degraded and not available for energy production. Supplemental D-ribose has been shown to enhance recovery of ATP levels and reduce cellular injury in humans and animals (9,43). A study by Pliml et al. found that patients with severe coronary artery disease who consumed D-ribose for 3 days had improved myocardial tolerance to ischemia. They hypothesized that supplemental D-ribose increased ATP metabolism and assisted with restoring cardiac energy metabolism (44). Another group of investigators found that daily oral D-ribose significantly improved left a trial function in congestive heart failure patients. They demonstrated that supplemental D-ribose not only improved diastolic performance but also improved the patient’s physical activity function and quality of life.
Supplemental D-ribose can be purchased in a dry powder form, and the recommended dose ranges from 5 to 15 grams per day and not by body mass units (42). The powder is mixed in a non-carbonated drink and has a sweet taste. It is readily metabolized if consumed within 30 minutes after being mixed in fluid. The side effects are minimal, but patients have reported mild diarrhea, slight nausea, and stomach discomfort that was reduced by consuming the drink with food (45–47). There had been some concerns about the safety of ribose therapy related to the inhibitory effects of ribose on cell proliferation in vitro. However, Pliml et al. investigated possible side effects of ribose on human lymphocytes. They found no significant inhibition of human lymphocyte proliferation in vitro in mitogen-stimulated cells and no evidence that ribose therapy was harmful to human cells (48).
CONCLUSION
Mitochondria regulate a multitude of metabolic and signaling pathways, but their primary function is the production of ATP. When mitochondrial function is compromised, there can be a reduced efficiency of cellular respiration and thus a loss of ATP production. D-ribose is an ATP substrate naturally occurring within cells. When nucleotides are reduced, supplemental D-ribose has been shown to be useful in enhancing the recovery of these energy molecules. Thus, D-ribose supplementation may help to return adenine nucleotides to the cell and thereby serve as a potential therapeutic option for various pathophysiologic conditions.
Acknowledgments
Sponsoring Information: Supported by the Department of Health and Human Services, National Institutes of Health, National Institute on Aging (Grant Number: 1R01AG054486-01A1).
Funding: None
Footnotes
Conflicts of interest: None
References
- 1.Martin-Fernandez B, Gredilla R. Mitochondria and oxidative stress in heart aging. Age (Dordr) 2016;38:225–238. doi: 10.1007/s11357-016-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 4.Nicolson GL. Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integr Med (Encinitas) 2014;13:35–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial Metabolism in Aging Heart. Circ Res. 2016;118:1593–1611. doi: 10.1161/CIRCRESAHA.116.307505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane N. Energetics and genetics across the prokaryote-eukaryote divide. Biol Direct. 2011;6:35. doi: 10.1186/1745-6150-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull. 2013;106:135–159. doi: 10.1093/bmb/ldt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauly DF, Pepine CJ. D-Ribose as a supplement for cardiac energy metabolism. J Cardiovasc Pharmacol Ther. 2000;5:249–258. doi: 10.1054/JCPT.2000.18011. [DOI] [PubMed] [Google Scholar]
- 10.Herrick J, St Cyr J. Ribose in the heart. J Diet Suppl. 2008;5:213–217. doi: 10.1080/19390210802332752. [DOI] [PubMed] [Google Scholar]
- 11.Leites EP, Morais VA. Mitochondrial quality control pathways: PINK1 acts as a gatekeeper. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 12.Ettema TJ. Evolution: Mitochondria in the second act. Nature. 2016;531:39–40. doi: 10.1038/nature16876. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am J Physiol Lung Cell Mol Physiol. 2016;310:L103–113. doi: 10.1152/ajplung.00320.2015. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Liu L, Chen Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Biophys Acta. 2015;1853:2784–2790. doi: 10.1016/j.bbamcr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol. 2017;595:6613–6621. doi: 10.1113/JP274472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida A, Ribeiro TP, de Medeiros IA. Aging: Molecular Pathways and Implications on the Cardiovascular System. Oxid Med Cell Longev. 2017;2017:7941563. doi: 10.1155/2017/7941563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng D, Liu L, Zhu Y, Chen Q. Molecular signaling toward mitophagy and its physiological significance. Exp Cell Res. 2013;319:1697–1705. doi: 10.1016/j.yexcr.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Polster BM, Carri MT, Beart PM. Mitochondria in the nervous system: From health to disease, Part I. Neurochem Int. 2017;109:1–4. doi: 10.1016/j.neuint.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes LC, Scorrano L. Mitochondrial morphology in mitophagy and macroautophagy. Biochim Biophys Acta. 2013;1833:205–212. doi: 10.1016/j.bbamcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Tamura Y, Sesaki H, Endo T. Phospholipid transport via mitochondria. Traffic. 2014;15:933–945. doi: 10.1111/tra.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Tamura Y, Roy M, Adachi Y, Iijima M, Sesaki H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell Mol Life Sci. 2014;71:3767–3778. doi: 10.1007/s00018-014-1648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer JN, Leuthner TC, Luz AL. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology. 2017;391:42–53. doi: 10.1016/j.tox.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasilewski M, Chojnacka K, Chacinska A. Protein trafficking at the crossroads to mitochondria. Biochim Biophys Acta. 2017;1864:125–137. doi: 10.1016/j.bbamcr.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Neupert W. A perspective on transport of proteins into mitochondria: a myriad of open questions. J Mol Biol. 2015;427:1135–1158. doi: 10.1016/j.jmb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68:513–519. doi: 10.1016/j.molimm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 31.Rieger B, Junge W, Busch KB. Lateral pH gradient between OXPHOS complex IV and F(0)F(1) ATP-synthase in folded mitochondrial membranes. Nat Commun. 2014;5:3103. doi: 10.1038/ncomms4103. [DOI] [PubMed] [Google Scholar]
- 32.Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matta CF, Massa L. Energy Equivalence of Information in the Mitochondrion and the Thermodynamic Efficiency of ATP Synthase. Biochemistry. 2015;54:5376–5378. doi: 10.1021/acs.biochem.5b00834. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh K, Debasis K, Purnendu R. Benzimidazolium-based simple host for fluorometric sensing of H2PO4, F–, PO43- and AMP under different conditions. Tetrahedron Letters. 2011;52:5098–5103. doi: 10.1016/j.tetlet.2011.07.110. [DOI] [Google Scholar]
- 35.Vyas NK, Vyas MN, Quiocho FA. Comparison of the periplasmic receptors for L-arabinose, D-glucose/D-galactose, and D-ribose. Structural and Functional Similarity. J Biol Chem. 1991;266:5226–5237. [PubMed] [Google Scholar]
- 36.Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentosephosphate pathway: a review. J Inherit Metab Dis. 2008;31:703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 37.Tanuma S, Sato A, Oyama T, Yoshimori A, Abe H, Uchiumi F. New Insights into the Roles of NAD+ -Poly(ADP-ribose) Metabolism and Poly(ADP-ribose) Glycohydrolase. Curr Protein Pept Sci. 2016;17:668–682. doi: 10.2174/1389203717666160419150014. [DOI] [PubMed] [Google Scholar]
- 38.Link H, Fuhrer T, Gerosa L, Zamboni N, Sauer U. Real-time metabolome profiling of the metabolic switch between starvation and growth. Nat Methods. 2015;12:1091–1097. doi: 10.1038/nmeth.3584. [DOI] [PubMed] [Google Scholar]
- 39.Frenguelli BG. The Purine Salvage Pathway and the Restoration of Cerebral ATP: Implications for Brain Slice Physiology and Brain Injury. Neurochem Res. 2017 doi: 10.1007/s11064-017-2386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayram M, St Cyr JA, Abraham WT. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther Adv Cardiovasc Dis. 2015;9:56–65. doi: 10.1177/1753944715572752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones K, Probst Y. Role of dietary modification in alleviating chronic fatigue syndrome symptoms: a systematic review. Aust N Z J Public Health. 2017;41:338–344. doi: 10.1111/1753-6405.12670. [DOI] [PubMed] [Google Scholar]
- 42.Thompson J, Neutel J, Homer K, Tempero K, Shah A, Khankari R. Evaluation of D-ribose pharmacokinetics, dose proportionality, food effect, and pharmacodynamics after oral solution administration in healthy male and female subjects. J Clin Pharmacol. 2014;54:546–554. doi: 10.1002/jcph.241. [DOI] [PubMed] [Google Scholar]
- 43.St Cyr JA, Bianco RW, Schneider JR, Mahoney JR, Jr, Tveter K, Einzig S, Foker JE. Enhanced high energy phosphate recovery with ribose infusion after global myocardial ischemia in a canine model. J Surg Res. 1989;46:157–162. doi: 10.1016/0022-4804(89)90220-5. [DOI] [PubMed] [Google Scholar]
- 44.Pliml W, von Arnim T, Stablein A, Hofmann H, Zimmer HG, Erdmann E. Effects of ribose on exercise-induced ischaemia in stable coronary artery disease. Lancet. 1992;340:507–510. doi: 10.1016/0140-6736(92)91709-h. [DOI] [PubMed] [Google Scholar]
- 45.Wagner DR, Gresser U, Kamilli I, Gross M, Zollner N. Effects of oral ribose on muscle metabolism during bicycle ergometer in patients with AMP-deaminase-deficiency. Adv Exp Med Biol. 1991;309B:383–385. doi: 10.1007/978-1-4615-7703-4_87. [DOI] [PubMed] [Google Scholar]
- 46.Teitelbaum JE, Johnson C, St Cyr J. The use of D-ribosein chronic fatigue syndrome and fibromyalgia: a pilot study. J Altern Complement Med. 2006;12:857–862. doi: 10.1089/acm.2006.12.857. [DOI] [PubMed] [Google Scholar]
- 47.Seifert J, Frelich A, Shecterle L, St Cyr J. Assessment of Hematological and Biochemical parameters with extended D-Ribose ingestion. J Int Soc Sports Nutr. 2008;5:13. doi: 10.1186/1550-2783-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pliml W, von Arnim T, Hammer C. Effects of therapeutic ribose levels on human lymphocyte proliferation in vitro. Clin Investig. 1993;71:770–773. doi: 10.1007/BF00190316. [DOI] [PubMed] [Google Scholar]