Abstract
Impulsivity is a variable behavioral trait that depends on numerous factors. For example, increasing the absolute magnitude of available choice options promotes farsighted decisions. We argue that this magnitude effect arises in part from differential exertion of self-control as the perceived importance of the choice increases. First, we demonstrated that frontal executive-control areas were more engaged for more difficult decisions and that this effect was enhanced for high-magnitude rewards. Second, we showed that increased hunger, which is associated with lower self-control, reduced the magnitude effect. Third, we tested an intervention designed to increase self-control and showed that it reduced the magnitude effect. Taken together, our findings challenge existing theories about the magnitude effect and suggest that visceral and cognitive factors affecting choice may do so by influencing self-control.
Keywords: self-control, decision making, delay of gratification, fMRI, open data
Decisions between outcomes available at different points in the future, called intertemporal choices, often require delaying gratification to maximize rewards. Intertemporal preferences are sensitive to the sizes of the rewards being considered (Thaler, 1981). Specifically, people will more often choose to wait for large rewards as the value of all options increases—a phenomenon known as the magnitude effect. We argue that increased self-control, arising from the perception of greater stakes that accompanies larger rewards, underlies the magnitude effect. Given that self-control is effortful, decision makers should flexibly allocate self-control according to the perceived importance of the decision (McGuire & Botvinick, 2010; Shenhav, Botvinick, & Cohen, 2013). Hence, along with the amount or number of rewards and the subjective value of the rewards, self-control is a critical determinant of intertemporal choices, and factors that influence self-control drive intra- and interindividual choice variability.
Magnitude can influence intertemporal choice via different mechanisms. Normative accounts of the effect make the following claims:
The risk model hypothesizes that larger rewards are intrinsically less risky and therefore have higher subjective value.
The reward-rate model posits that subjects expect a longer delay between opportunities for choices from among high-magnitude options. Therefore, if subjective value depends on reward rate, then a long delay until reward receipt has a relatively reduced impact on subjective value (Myerson & Green, 1995).
The utility account holds that subjects could assess rewards with a utility function that has increasing proportional sensitivity (Loewenstein & Prelec, 1992). According to this account, the ratio between the utilities of $20 and $10 is smaller than the ratio between the utilities of $2,000 and $1,000, and so the larger reward in the high-magnitude condition is relatively more valuable than the larger reward in the low-magnitude condition.
The consumption model—a process model—contends that rewards are further discounted by the time it takes to consume them. Because large rewards are generally consumed at a faster rate in terms of dollars per day, relatively more reward is available sooner as the magnitude of the delayed option increases (Raineri & Rachlin, 1993).
The sampling account—another process model—argues that people evaluate delayed rewards by sampling memory. Because larger rewards are generally associated with longer delays (e.g., monthly paychecks, retirement savings), the same time delay will seem comparatively shorter or longer when considered in the context of a large or small reward, respectively (Stewart, Chater, & Brown, 2006).
We tested the self-control account, a different explanation of the magnitude effect, in two behavioral experiments and explored its neural basis in a functional MRI (fMRI) study. In Study 1, we tested the prediction that executive-control functions in the prefrontal cortex are enhanced for choices among high-magnitude options. In Study 2, we explored whether the magnitude effect depended on state differences in self-control, and in Study 3, we tested whether an experimental manipulation that affects self-control would influence the magnitude effect.
Study 1
The lateral prefrontal cortex is part of an executive control network responsible for supporting goal-directed behaviors (Miller & Cohen, 2001). Recent neuroimaging work has demonstrated that the dorsolateral prefrontal cortex is active when self-control is exerted (Crockett et al., 2013; Hare, Camerer, & Rangel, 2009) and is necessary for self-control to be deployed (Figner et al., 2010). Precise anatomical work has suggested that the inferior frontal sulcus (IFS) is the central node in a prefrontal cognitive-control network (Waskom, Kumaran, Gordon, Rissman, & Wagner, 2014; Yeo et al., 2015) and exerts control flexibly according to task demands (Waskom, Frank, & Wagner, 2017). We performed a model-based analysis to directly assess whether control mechanisms in the IFS are more strongly engaged when evaluating high-magnitude choices.
Method and results
Subjects
Twenty subjects participated in data collection at the Princeton Neuroscience Institute. One subject was excluded because of excessive head motion, and 6 subjects were excluded because they chose all shorter and sooner options or all larger and later options. These choice patterns precluded behavioral and neural analysis because (a) it is not possible to estimate discount rates for these subjects and (b) they were probably not en-gaged in deliberative choice for each option. Nine subjects participated in data collection at the Banner Alzheimer’s Institute (Phoenix, AZ). Two subjects were excluded because they reported that they fell asleep. Thus, we analyzed data from 20 subjects. The original sample size (i.e., 20) was chosen to match that typically used in imaging studies of intertemporal choice, and the second sample size was chosen so that our total would match sample sizes more commonly used in current fMRI studies (i.e., 30). Our sample size provided moderate power for group-average analyses, but insufficient power to detect between-subjects correlation effects. We analyzed our full cohort together using the analysis procedures described in the Analysis section.
Procedure and materials
Subjects were asked to make a series of choices between small rewards that came after a short delay and large rewards that came after a long delay; the small reward was always less than the large reward, and the short delay was always less than the long delay. The magnitude of the small reward was varied so that it had a mean of either $20 (low-magnitude-options condition) or $2,000 (high-magnitude-options condition). For choices among low-magnitude options, the difference between the large and small rewards ranged between 1% and 50%, whereas for choices among high-magnitude options, the difference between the large and small reward options ranged between 0.1% and 5%. This difference between magnitude conditions is essential because the magnitude effect is so pronounced that fixing proportional differences would create large subjective value differences between conditions (e.g., an average subject is roughly ambivalent between $20 now and $24 in 2 weeks, but overwhelmingly prefers $2,400 in 2 weeks to $2,000 now). The short delay was either today, 2 weeks, or 4 weeks and was randomly selected; the difference between the short delay and the long delay was fixed at 2 weeks. All decisions in this and subsequent studies were hypothetical, which is necessary when studying the magnitude effect. Given this, it is important to note that discount rates are highly correlated within subjects for real and hypothetical rewards (Johnson & Bickel, 2002), and blood-oxygen-level dependent (BOLD) responses are similar for real and hypothetical rewards (Bickel, Pitcock, Yi, & Angtuaco, 2009). For details about data acquisition and analysis, see the Supplemental Material available online.
Analysis
To quantitatively assess the magnitude effect in our subjects’ choices, we estimated discount rates (k) for each subject in each condition by assuming that rewards were discounted according to the hyperbolic discount function D(t) = (1 + k t)−1, where D is amount of discounting applied to reward delayed by time (t). Larger discount rates imply a greater relative preference for immediate rewards. We estimated k for each subject and condition by assuming a logistic decision function and identifying parameter values that maximized the likelihood of the observed choices. Discount rates were nonnormal; thus, we used log-transformed values for statistical analysis. A paired t test confirmed a strong magnitude effect: Log-transformed discount rates were larger for the low-magnitude-options condition (M = −4.45, SD = 0.92) than for the high-magnitude-options condition (M = −7.29, SD = 1.72), t(19) = 7.98, Hedges’s average g (gav) = 2.11, 95% confidence interval for the difference in means (CI) = [2.10, 3.59], p < .001. For the neuroimaging analysis, we excluded an extreme outlier data from a subject who showed a strong antimagnitude effect (i.e., 3.4 SD from the mean) because we wished to isolate neural effects related to the magnitude effect.
To identify the areas of prefrontal cortex involved in valuation and self-control in our task, we implemented an approach involving region-of-interest (ROI) analysis (Fig. 1). We used ROIs taken from a parcellation of the cortex into networks that showed correlated resting-state activation reliably in a cohort of 1,000 subjects (Yeo et al., 2011). Recent research has suggested that the cortex can be divided into functional parcels with distinct resting-state profiles and that these parcels constrain the topography of functional activations (Glasser, Coalson, Robinson, & Hacker, 2015). This approach allows for a description of cortical task effects that is more precise than broad anatomical labels (e.g., “dorsolateral prefrontal cortex”) and is directly interpretable across experiments and subject groups.
Fig. 1.
Target regions for analysis. The top two images are right and left lateral views of the brain, respectively, and the bottom two images are right and left medial views of the brain, respectively. We chose these regions of interest (ROIs) to isolate the main components of the prefrontal executive-control network (IFS, or inferior frontal sulcus; pMFG, or posterior middle frontal gyrus; and FPC, or frontopolar cortex), the dorsal attention network (FEF, or frontal eye fields), and the prefrontal subjective-value-associated area (vmPFC, or ventromedial prefrontal cortex), as well as adjacent ROIs belonging to other functional networks. aINS = anterior insula; IFG = inferior frontal gyrus; aMFG = anterior middle frontal gyrus. For additional details and visualizations, see Bartra, McGuire, and Kable (2013) and Yeo et al. (2011).
This approach offers several methodological advantages. First, regions of interest are defined in the cortical surface, which allows for increased precision relative to normalization to a volumetric atlas. Second, it avoids the need for cluster-based multiple-comparisons correction, which is vulnerable to the choice of cluster-defining threshold and is variable across software packages. We focused our analyses on prefrontal ROIs that belonged to networks associated with attention and cognitive control. In particular, we expected to find magnitude effects in the IFS, which is the prefrontal hub of the top-down control network (Waskom et al., 2014). We also included other large prefrontal control ROIs—the frontopolar cortex and the posterior middle frontal gyrus. We included the human analogue of the monkey frontal eye fields as a central node in the top-down attention network, given that control may be implemented by biasing attention toward certain aspects of the choice. Finally, we included three adjacent ROIs in which we did not necessarily expect to find an effect: the anterior insula and the anterior middle frontal gyrus, which are part of the ventral or bottom-up attention network, and the inferior frontal gyrus, which is part of the default mode network, a set of brain areas in which activity decreases as subjects engage with most tasks. Other large, lateral prefrontal ROIs in the Yeo atlas (Yeo et al., 2011) belong to the default network and were not included.
To identify valuation effects, we included a ventromedial prefrontal cortex (vmPFC) ROI taken from a meta-analysis of valuation and decision-making studies of regions in which activity has a monotonic and modality-independent relationship with subjective value (Bartra, McGuire, & Kable, 2013). We adopted this ROI because it has been precisely identified with our experimental question of interest, and correlation-based parcellation methods are only moderately effective in areas of magnetic-resonance signal in homo gene ities (Yeo et al., 2011).
Our ROIs were chosen to align with our specific hypotheses about prefrontal cortical function in value-based decision making. We made our data publicly available so that researchers can conduct additional analyses according to their interests.
Cognitive control in intertemporal choice supports long-term behavioral goals and depends on (a) determining how much one ought to discount, (b) maintaining this goal, and (c) using this goal to bias reward valuation and choice. Encoding and maintaining a goal should be associated with a main effect of increased decision-period activation (Curtis & D’Esposito, 2003). To identify brain areas associated with cognitive control in this experiment, we first tested for brain activation during the decision period in all trials across both magnitude conditions. We fit a general linear model of the task design to each voxel (see the Analysis section for Study 1 in the Supplemental Material), extracted beta weights for each task event of interest, and performed a group mixed-effects analysis on these beta weights. Decision-related activity was observed in the IFS, t(18) = 5.40, 95% CI for the beta weights = [6.58, 14.10], dz = 1.24, p < .001 (false-discovery-rate, or FDR, corrected), and anterior insula, t(18) = 5.93, 95% CI for the beta weights = [6.43, 12.79], dz = 1.36, p < .001 (FDR corrected; Fig. 2a). In addition, we observed expected deactivation in the vmPFC, t(18) = −13.0, 95% CI for the beta weights = [−39.1, −28.9], dz = −2.97, p < .001 (FDR corrected), and inferior frontal gyrus, t(18) = −4.98, 95% CI for the beta weights = [−11.2, −4.9], dz = −1.14, p < .001 (FDR corrected), which are part of the default mode network.
Fig. 2.

Results from Study 1: beta weights associated with (a) the decision period, (b) difficulty, and (c) subjective value in the eight regions of interest. Error bars represent 95% confidence intervals. Asterisks indicate significant differences from zero (*p < .05, false-discovery-rate corrected). AU = arbitrary units; IFS = inferior frontal sulcus; FPC = frontopolar cortex; pMFG = posterior middle frontal gyrus; FEF = frontal eye fields; aINS = anterior insula; IFG = inferior frontal gyrus; aMFG = anterior middle frontal gyrus; vmPFC = ventromedial prefrontal cortex.
Cognitive control benefits decision making by enhancing representation of behavioral goals and by increasing the fidelity of neural representations of task variables (Waskom et al., 2017). Further, cognitive control is deployed adaptively in situations in which it is most useful (Shenhav et al., 2013; Waskom et al., 2017). Because cognitive control is most useful for difficult choices, we tested whether any brain regions showed a decreased response as absolute subjective-value difference increased (i.e., decision difficulty). As expected, all three of our cognitive-control ROIs showed this effect—IFS: t(18) = 5.09, 95% CI for the beta weights = [1.51, 3.93], dz = 1.17, p < .001 (FDR corrected); frontopolar cortex: t(18) = 2.69, 95% CI for the beta weights = [0.52, 3.31], dz = 0.62, p = .013 (FDR corrected); posterior middle frontal gyrus: t(18) = 3.29, 95% CI for the beta weights = [0.84, 3.32], dz = 0.75, p = .003 (FDR corrected)—indicating that these regions scale their activity with the difficulty of the choice (Fig. 2b). In summary, the IFS is activated during all decisions and increases its activation for more difficult decisions.
We next turned to subjective value, which is strongly associated with activity in the ventromedial prefrontal cortex. We examined two potential correlates of subjective value that have been shown to be represented in this area: the subjective value difference and the total subjective value of the options. The first is a decision-related signal that scales inversely with the relative difficulty of the choice, whereas the second is what would be expected if interlocked pools of neurons represent the value of each option. These regressors were included in the same model so that any shared variability did not systematically bias results. We found that the vmPFC tracked both average subjective value, t(18) = 3.13, 95% CI for the beta weights = [0.65, 2.81], dz = 0.72, p = .014 (FDR corrected; Fig. 2c), and the subjective-value difference, t(18) = 2.65, 95% CI for the beta weights = [0.61, 3.34], dz = 0.61, p = .013 (FDR corrected; Fig. 2b). We concluded that the vmPFC BOLD signal tracked multiple quantities related to the subjective value of the options.
We propose that decisions about high-magnitude options should engage control processes. We began by testing for decision-period differences between the high-magnitude-options condition and the low-magnitude-option condition, although this analysis was compromised because it required comparing conditions assessed against implicit baselines from different runs. None of our ROIs showed increased activation to high-magnitude options (see Fig. S1 in the Supplemental Material).
We next turned to our parametric measure of decision difficulty. Because control most strongly benefits difficult choices, and because high-magnitude options should engage control processes, we predicted a stronger relationship between IFS activation and choice difficulty for high-magnitude-option decisions than for low-magnitude-option conditions. We found increased activation as a function of decision difficulty for choices involving high-magnitude options relative to low-magnitude options in the IFS, t(18) = 2.58, 95% CI for the beta weights = [0.27, 1.99], dz = 0.59, p = .04 (FDR corrected), and, unexpectedly, the inferior frontal gyrus, t(18) = 2.61, 95% CI for the beta weights = [0.35, 2.47], dz = 0.60, p = .04 (FDR corrected; Fig. 3a). The IFS, which is the central prefrontal hub of the top-down control network, is more engaged during more difficult decisions and even further engaged during these decisions when the choices involve high-magnitude options. Finally, we conducted an exploratory analysis to test whether any regions showed increased activation as a function of total subjective value in the high-magnitude-options condition relative to the low-magnitude-options condition. We found that the frontal eye fields showed this effect, t(18) = 2.82, 95% CI for the beta weights = [0.28, 1.53], dz = 0.65, p = .038 (FDR corrected; Fig. 3b). This region is involved in directing top-down attention, and this result is consistent with a model in which choices among more valuable options elicit more top-down attention. Future studies manipulating attention should test this model directly.
Fig. 3.
Results from Study 1: beta weights associated with (a) decision difficulty and (b) subjective value. Error bars reflect 95% confidence intervals. Asterisks indicate a significant difference from zero (*p < .05, false-discovery-rate corrected). AU = arbitrary units; IFS = inferior frontal sulcus; FPC = frontopolar cortex; pMFG = posterior middle frontal gyrus; FEF = frontal eye fields; aINS = anterior insula; IFG = inferior frontal gyrus; aMFG = anterior middle frontal gyrus; vmPFC = ventromedial prefrontal cortex.
We found no differences in activity in the vmPFC between magnitude conditions for any of our analyses. This result may seem surprising given that this brain region is sensitive to reward magnitude. However, the dependence on magnitude is also thought to be relative to the best and worst outcomes within a behavioral context (Cox & Kable, 2014). Because our experiment was divided into blocks according to magnitude condition, a relative-value coding scheme would predict no difference in reward responses between magnitude conditions.
Discussion
We replicated previous work showing that prefrontal components of attention and control networks are involved in decisions regardless of delay, whereas vmPFC codes for subjective value. We found that the IFS, the central node of the top-down prefrontal control network, was activated for both high- and low-magnitude options and also is more strongly activated by more difficult decisions. Further, this effect is stronger in the high-magnitude-option condition than in the low-magnitude-option condition. Finally, we found that the human frontal eye fields, which constitute the central node of the prefrontal attention network, more strongly track the subjective value of the option in high-magnitude conditions. These findings show that prefrontal control areas that are adaptively recruited for difficult decisions are more strongly engaged in choices involving high-magnitude options. Future studies should assess whether control areas exert their effect on behavior by directing attention to the option with the higher subjective value.
Study 2
If the magnitude effect depends on self-control, then variability in self-control should correlate with differences in the size of the magnitude effect. Control is not exerted uniformly and is deployed adaptively (Shenhav et al., 2013). The factors that influence endogenous variability are an area of active study. Alcoholics Anonymous uses the acronym HALT (hungry, angry, lonely, tired) to describe circumstances in which self-control is vulnerable. It has been shown empirically that people tend to exert less self-control when they are hungry (Danziger, Levav, & Avnaim-Pesso, 2011; Gailliot, 2013; Read & van Leeuwen, 1998), and this tendency is evident in intertemporal choice (Wang & Dvorak, 2010). How hunger influences self-control remains controversial: Reduced control may derive from visceral factors (Loewenstein & Prelec, 1992) or psychological factors (Job, Walton, Bernecker, & Dweck, 2013). Regardless, if the magnitude effect depends on self-control, then hunger should reduce the magnitude effect by decreasing the tendency to employ self-control in high-stakes decisions.
Demonstrating that hunger reduces the magnitude effect would provide unique empirical support for the self-control theory. Hunger should not selectively influence estimated riskiness of large versus small rewards (the risk model). Hunger should not make large rewards seem to appear more frequently (the reward-rate model). Large, delayed rewards should not be expected to be consumed more slowly when hungry (the consumption model). Finally, memories of large delayed rewards should be no easier to recall when hungry than memories of small delayed rewards (the sampling model). Hunger could conceivably affect the shape of utility curves, but, to our knowledge, no empirical evidence or theoretical work supports this idea.
Method
Subjects
We collected data from 200 online subjects, recruited through Amazon’s Mechanical Turk (MTurk). We choose our sample size hoping for at least 100 subjects. Because the magnitude effect is large, we focused on having enough subjects to detect a moderate between-subjects correlation. Of the 200 subjects, we analyzed data from 153 who correctly answered a trick question designed to confirm task engagement (mean age = 33.80 years, SD = 7.38; for more detail, see the Supplemental Material). The data were not analyzed until all subjects’ data had been collected.
Procedure and materials
Subjects answered 40 intertemporal-choice questions (20 for the low-magnitude-option condition and 20 for the high-magnitude-option condition). The distribution of reward magnitudes and delays was very similar to that in Study 1, except that all rewards with a short delay were available “today.” After the delay-discounting questions, subjects provided ratings of hunger levels on a visual analogue scale with anchors labeled not hungry at all and very hungry. In addition, they reported their height and weight so we could compute body mass index (BMI).
Analysis
We estimated each subject’s discount rates for the low-magnitude-options condition (klow) and the high-magnitude-options condition (khigh) as described in the Method section for Study 1. Both discount rates and hunger scores were nonnormal, and thus all analyses were conducted on log-transformed k values. The size of the magnitude effect was calculated as the relative difference in discount rate between the two conditions: log(klow ) – log(khigh).
Results
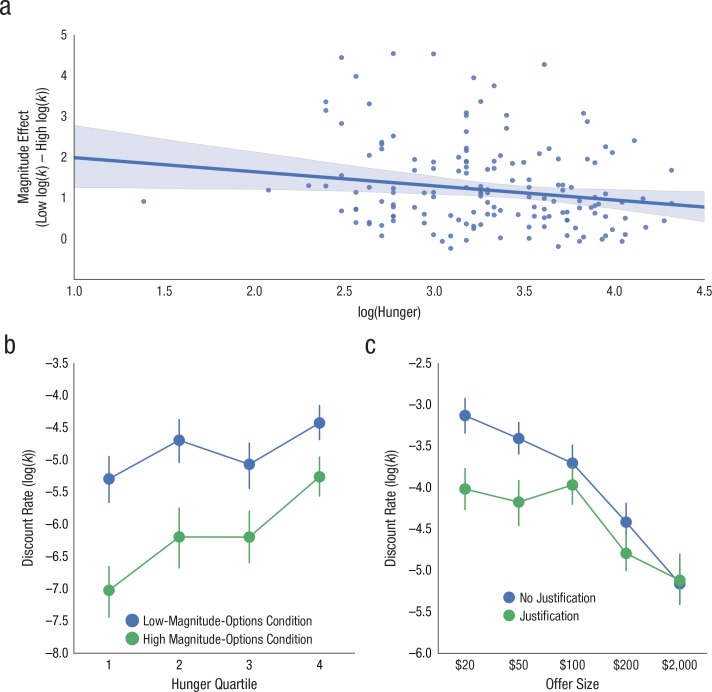
Overall, hunger significantly affected discount rates; hunger correlated with log discount rates in both the low-magnitude-options condition, r(151) = .25, p = .002, and the high-magnitude-options condition, r(151) = .41, p < .001. This effect was anticipated by previous work (Wang & Dvorak, 2010). We observed a strong magnitude effect: Log-transformed discount rates were larger for the low-magnitude-options condition (M = −4.87, SD = 1.07) than for the high-magnitude-options condition (M = −6.16, SD = 1.4), t(152) = 14.6, p < .001, Hedges’s gav = 1.04, 95% CI = [1.12, 1.47]. We next tested whether self-reported hunger was correlated with the size of the magnitude effect across subjects. We found a significant relationship between the two measures, r(151) = −.29, p < .001, even when we controlled for any effects of decision stochasticity, r(151) = −.21, p = .008 (Fig. 4a). To further examine this effect, we grouped subjects into quartiles (Q1, Q2, Q3, and Q4) according to their individual hunger ratings (the lowest hunger rating was assigned to Q1; see Table S2 in the Supplemental Material).
Fig. 4.
Results from Studies 2 and 3. The scatterplot (with best-fitting robust regression line) in (a; Study 2) shows the relationship between the magnitude effect and hunger (after we controlled for the effects of decision stochasticity). The shaded area indicates the 95% confidence interval (CI). The graph in (b; Study 2) shows the relationship between discount rate and hunger quartile, separately for the high- and low-magnitude-options conditions. Error bars indicate 95% CIs. In (c), discount rate is plotted against offer size, separately for the justification and no-justification conditions. Error bars indicate 95% CIs.
We conducted a Type III mixed analysis of variance (ANOVA) with Greenhouse-Geisser sphericity correction and with hunger as a between-subjects effect and magnitude as a within-subjects effect. There was a significant effect of hunger on log(k), F(3, 149) = 10.7, generalized η2 (ηg2) = .15, p < .001, as well as an effect of magnitude on log(k), F(1, 149) = 232, ηg2 = .24, p < .001 (Fig. 4b). As hypothesized, there was an interaction between magnitude and hunger; the size of the magnitude effect decreased as the level of hunger increased, F(3, 149) = 5.41, ηg2 = .02, p = .001 (Fig. 4b). Thus, although hunger increased discounting for all options, this change was most pronounced for choices involving high-magnitude options. Hunger accounts for roughly 8% of the variance in the magnitude effect in our sample, which suggests a moderately sized effect that could translate meaningfully to the realm of financial decision making.
In addition, we examined whether the temperature parameter from the model (a measure of choice variability) showed a relationship with the magnitude effect or with hunger. We predicted that hungry subjects would be more random in their responses, and we observed that the log inverse temperatures correlated with hunger in both the low-magnitude-options condition, r(151) = −.67, p < .001, and the high-magnitude-options condition, r(151) = −.67, p < .001. We repeated the quartile analysis and observed that subjects showed a main effect of hunger, F(3, 149) = 165.3, ηg2 = .61, p < .001 and, unexpectedly, that subjects were less stochastic for choices involving high-magnitude options relative to low-magnitude options, F(1, 149) = 86.6, ηg2 = .23, p < .001 (see Fig. S2 in the Supplemental Material). In addition, we observed an interaction between this magnitude effect and hunger: When they were hungry, subjects were relatively more stochastic for choices involving low magnitudes, F(3, 149) = 17.1, ηg2 = .15, p < .001 (see Fig. S3 in the Supplemental Material).
Finally, to determine whether the magnitude effects for discounting and stochasticity were independently related to hunger, we performed a multiple linear regression analysis. Hunger was related to the magnitude effect for discounting, b = −0.1, t(150) = −2.78, 95% CI = [−0.17, −0.03], p = .006, as well as the magnitude effect for stochasticity, b = −0.39, t(150) = −3.9, 95% CI = [−0.59, −0.19], p < .001. Together, the two magnitude effects accounted for a significant variance in hunger, r 2 = .17, F(2, 150) = 15.0, p < .001. Hunger may influence choice behavior via two processes: increasing impulsivity (especially for decisions involving high-magnitude options) and increasing stochasticity (especially for decisions involving low-magnitude options). Although impulsivity and stochasticity are related psychological phenomena, our modeling and analysis procedures dissociated the two processes. However, the inverse temperature parameter is more difficult to faithfully estimate than the discounting parameter k (see the Method section for Study 4 in the Supplemental Material), and strong conclusions about decision stochasticity should await future study.
Hunger ratings were low, on average (M = 30.4, SD = 15.0; scale from 0 to 100). This is reasonable because our subjects were not required to be food restricted and presumably had free access to food. In addition, we found no relationship between self-reported BMI and either discount rates or the magnitude effect (p > .25). Although negative findings must be interpreted with caution, this lack of relationship suggests that our findings are due to the state effect of hunger rather than to trait effects that may influence both hunger and discounting. Our observation that normal day-to-day differences in hunger influence the magnitude effect is striking and suggests that relatively modest changes in self-control can have pronounced effects on decision making.
Discussion
We aimed to determine how self-control affects variability in intertemporal choices by leveraging the fact that hunger affects self-control. We showed that hunger is inversely correlated with the size of the magnitude effect, a relationship not anticipated by existing accounts of the magnitude effect.
Study 3
We next tested whether instructions could serve as an experimental manipulation of self-control. Asking subjects to justify their preferences causes them to use a choice strategy consistent with increased cognitive control (Wilson & Schooler, 1991). Because we contend that the magnitude effect arises from the triggering of self-control by high magnitudes, and because exertion of self-control has a ceiling (van Veen, Cohen, Botvinick, Stenger, & Carter, 2001), a manipulation that enhances self-control should reduce the magnitude effect. For choices involving low-magnitude options, such instruction could produce increases in self-control and thus reduce discount rates. However, for choices involving high-magnitude options, the higher stakes should independently prompt increased self-control, and instruction effects should be reduced. We hypothesized that task instructions designed to increase cognitive control should have a greater effect on delay discounting in choices involving low-magnitude options than in those involving high-magnitude options.
No account of the magnitude effect predicts that justification should have this effect. Justifying responses should not selectively increase the probability of smaller rewards (risk model) and should not make small rewards seem to appear less frequently (reward rate model). Likewise, justifying decisions should not change expectations about how quickly large rewards are consumed (consumption model) and should not influence memory of small delayed rewards (sampling model). Justification could affect the shape of utility curves; however, to our knowledge, no empirical evidence or theoretical work supports this idea.
Method
Subjects
We collected data from 1,500 online subjects recruited through MTurk. After exclusions, we analyzed data from 1,382 subjects (mean age = 30.2 years, SD = 10.8), split approximately equally across 10 experimental conditions using a random number generator (for more detail, see the Supplemental Material). No analyses were performed until all subjects’ data had been collected. Sample size was chosen to approximately match that of Experiment 2 for each choice condition because we expected the effect sizes for the two experiments to be approximately equal.
Procedure and materials
Subjects read a scenario in which they were asked to imagine winning money in a raffle. They read that they could receive a reward imme-diately or they could receive more money if they accepted a delay of 1 month. Subjects reported the amount of delayed reward that would make waiting and getting the payment immediately equally attractive. Each subject was assigned to one immediate reward, which was either $20, $100, $200, $1,000, or $2,000. To manipulate cognitive control, we asked subjects in the justify conditions, but not those in the control conditions, to justify their responses in two or three written sentences. We screened the written justifications and excluded subjects who did not provide justifications (see the Supplemental Material).
Analysis
We calculated discount rates (k) for each subject using the equation D(t) = (1 + k t)−1. Because the distribution of discount rates was nonnormal, we analyzed log-transformed scores. We conducted a two-way Type III ANOVA with reward magnitude ($20, $100, $200, $1,000, $2,000) and instructions (justify, control) as separate factors.
Results
The main effect of reward magnitude was significant, F(4, 1372) = 57.0, p < .001, ηg2 = .14, which shows the magnitude effect (see Table S3 in the Supplemental Material). Subjects were also significantly more patient (i.e., they demanded less for the delayed reward) if they had to justify their responses, F(1, 1372) = 33.9, p < .001, ηg2 = .02. This result suggests that our instructions successfully increased self-control. Finally, the interaction between reward magnitude and task instructions was also significant, F(4, 1372) = 4.7, p < .001, ηp2 = .01 (Fig. 4c). The effect of the justification demand was significantly greater at smaller reward magnitudes than at larger reward magnitudes. Thus, manipulating self-control reduced delay discounting to a greater degree in low-stakes decisions. This effect is small but consistent with other framing effects in intertemporal choice (Bickel, Wilson, Chen, Koffarnus, & Franck, 2016). Although the interaction of justification and magnitude is unlikely to produce a meaningful change in any given decision, its impact could increase in domains such as retirement planning, which occurs across repeated decisions and many individuals. For choices involving high-magnitude options, when self-control should be high, the justification manipulation confers no additional change in discounting. Conversely, in choices involving low-magnitude options, when self-control is relatively lower, the justification manipulation enhanced self-control and, in turn, reduced discounting. This finding provides additional support for the hypothesis that the magnitude effect results from differences in self-control.
General Discussion
According to our proposed mechanistic account of the magnitude effect, high-magnitude rewards prompt greater self-control. In the studies reported here, neural activity related to cognitive control increased for decisions involving high-magnitude options. An internal state (hunger) and our justification manipulation reduced the magnitude effect. Prior accounts of the magnitude effect cannot explain our findings.
The risk model, in which delayed rewards are inherently risky, is influential among behavioral economists (Azfar, 1999) and psychologists (Raineri & Rachlin, 1993). However, the observation that humans discount large probabilistic rewards more steeply makes this model seem less likely as an explanation of the reduced discounting evident in intertemporal choice (Christensen, Parker, Silberberg, & Hursh, 1998). To our knowledge, there is no empirical evidence supporting the risk model or other hypotheses about the magnitude effect; moreover, the consumption and sampling models are difficult to test with available techniques.
Only the utility model, in which larger rewards seem proportionally smaller, can explain our findings. Yet empirical evaluation fails to support the central supposition that people show increasing proportional sensitivity with reward magnitude (only 25% of subjects show this effect; Chapman, 1996). In addition, the magnitude effect persists even when individual utility curves are independently estimated and controlled for. Still, variability in self-control implies that large magnitude rewards are evaluated differently, and future work should investigate how to best model the influence of self-control on utility functions.
An interesting avenue for future research is determining how self-control influences choice. Self-control could modulate the subjective values of each option by biasing the discount parameter (Kirby, 1997). This idea, in which the lateral prefrontal cortex biases valuation, is consistent with both the two-system and the one-system valuation accounts. In the two-system account, self-control biases the weighting of patient and myopic brain systems during choice (van den Bos & McClure, 2013). In the one-system account, self-control modulates value representations in vmPFC (Hare et al., 2009). Self-control may also bias choice toward delayed options without affecting valuation (Figner et al., 2010). Our studies did not discriminate between valuation schemes but rather demonstrated the importance of self-control in the magnitude effect. Future work delineating how subjective values are constructed in the vmPFC and used to determine choice will be necessary to isolate the mechanism by which self-control influences choice.
Although temporal discounting exists across species, direct tests for a magnitude effect have been conducted without success in pigeons, rats, and new world monkeys (Calvert, Green, & Myerson, 2010; Green, Myerson, Holt, Slevin, & Estle, 2004; Stevens, Rosati, Ross, & Hauser, 2005; but see Grace, Sargisson, & White, 2012). The fact that the magnitude effect is evident uniquely in humans is consistent with our species’ distinct capacity for cognitive control, and self-control in particular.
We observed large effects even though our studies used fictive rewards. The magnitude effect is commonly studied with fictive rewards for practical reasons, but it has been demonstrated in the laboratory for real rewards (Johnson & Bickel, 2002) and in real-world financial decisions (Schoenfelder & Hantula, 2003). Neural responses to fictive rewards are commonly observed. In fact, the brain appears to create fictive rewards to speed up learning (Iglesias et al., 2013). The finding that self-control can be triggered by large magnitudes, even for choices among fictive rewards, supports a model in which a critical determinant of self-control is perceived stakes (for additional discussion, see Study 4 in the Supplemental Material). Numerically higher magnitudes can act as a signal of increased stakes, indicating the need for greater self-control. This self-control account is not specific to intertemporal choices (Shenhav et al., 2013), and it should be an important priority for future research to determine which decision attributes increase self-control.
A mechanistic understanding of psychological and neurobiological underpinnings of intertemporal choice would lead to novel strategies for improving self-control. Our findings suggest interventions that trigger self-control, such as encouraging justifications for choices, could effectively improve decisions in financial and health domains.
Supplementary Material
Acknowledgments
We thank Matt Samberg for help with imaging data collection and George Loewenstein and Wouter van den Bos for useful comments.
Footnotes
Action Editor: Marc J. Buehner served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This work was supported by National Institute on Aging Grant R01-AG031310 (to J. D. Cohen) and National Science Foundation Grant 1358507 (to S. M. McClure).
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617711455
Open Practices:

All data have been made publicly available; the functional MRI data are available via OpenFRMI (at https://openfmri.org/dataset/ds000223/), and the code for the data analysis is available via Github (at https://github.com/iancballard/magnitude). The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617711455. This article has received the badge for Open Data. More information about the Open Practices badges can be found at https://www.psychologicalscience.org/publications/badges.
References
- Azfar O. (1999). Rationalizing hyperbolic discounting. Journal of Economic Behavior & Organization, 38, 245–252. [Google Scholar]
- Bartra O., McGuire J. T., Kable J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W. K., Pitcock J. A., Yi R., Angtuaco E. J. C. (2009). Congruence of BOLD response across intertemporal choice conditions: Fictive and real money gains and losses. The Journal of Neuroscience, 29, 8839–8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W. K., Wilson A. G., Chen C., Koffarnus M. N., Franck C. T. (2016). Stuck in time: Negative income shock constricts the temporal window of valuation spanning the future and the past. PLoS ONE, 11(9), Article e0163051. doi: 10.1371/journal.pone.0163051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert A. L., Green L., Myerson J. (2010). Delay discounting of qualitatively different reinforcers in rats. Journal of the Experimental Analysis of Behavior, 93, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G. B. (1996). Temporal discounting and utility for health and money. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22, 1–21. [DOI] [PubMed] [Google Scholar]
- Christensen J., Parker S., Silberberg A., Hursh S. (1998). Trade-offs in choice between risk and delay depend on monetary amounts. The Journal of Neuroscience, 8, 8839–8846. doi: 10.1901/jeab.1998.69-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. M., Kable J. W. (2014). BOLD subjective value signals exhibit robust range adaptation. The Journal of Neuroscience, 34, 16533–16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett M. J., Braams B. R., Clark L., Tobler P. N., Robbins T. W., Kalenscher T. (2013). Restricting temptations: Neural mechanisms of precommitment. Neuron, 79, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. E., D’Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7, 415–423. [DOI] [PubMed] [Google Scholar]
- Danziger S., Levav J., Avnaim-Pesso L. (2011). Extraneous factors in judicial decisions. Proceedings of the National Academy of Sciences, USA, 108, 6889–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B., Knoch D., Johnson E. J., Krosch A. R., Lisanby S. H., Fehr E., Weber E. U. (2010). Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience, 13, 538–539. [DOI] [PubMed] [Google Scholar]
- Gailliot M. T. (2013). Hunger and reduced self-control in the laboratory and across the world: Reducing hunger as a self-control panacea. Psychology, 4, 59–66. doi: 10.4236/psych.2013.41008 [DOI] [Google Scholar]
- Glasser M. F., Coalson T., Robinson E., Hacker C. (2015). A multi-modal parcellation of human cerebral cortex. Nature, 536, 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace R. C., Sargisson R. J., White K. G. (2012). Evidence for a magnitude effect in temporal discounting with pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 38, 102–108. [DOI] [PubMed] [Google Scholar]
- Green L., Myerson J., Holt D. D., Slevin J. R., Estle S. J. (2004). Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? Journal of the Experimental Analysis of Behavior, 81, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T. A., Camerer C. F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–648. [DOI] [PubMed] [Google Scholar]
- Iglesias S., Mathys C., Brodersen K. H., Kasper L., Piccirelli M., den Ouden H. E. M., Stephan K. E. (2013). Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron, 80, 519–530. [DOI] [PubMed] [Google Scholar]
- Job V., Walton G. M., Bernecker K., Dweck C. S. (2013). Beliefs about willpower determine the impact of glucose on self-control. Proceedings of the National Academy of Sciences, USA, 110, 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. W., Bickel W. K. (2002). Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior, 77, 129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K. N. (1997). Bidding on the future: Evidence against normative discounting of delayed rewards. Journal of Experimental Psychology: General, 126, 54–70. [Google Scholar]
- Loewenstein G., Prelec D. (1992). Anomalies in intertemporal choice: Evidence and an interpretation. The Quarterly Journal of Economics, 107, 573–597. [Google Scholar]
- McGuire J. T., Botvinick M. M. (2010). Prefrontal cortex, cognitive control, and the registration of decision costs. Proceedings of the National Academy of Sciences, USA, 107, 7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Myerson J., Green L. (1995). Discounting of delayed rewards: Models of individual choice. Journal of the Experimental Analysis of Behavior, 64, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri A., Rachlin H. (1993). The effect of temporal constraints on the value of money and other commodities. Journal of Behavioral Decision Making, 6, 77–94. [Google Scholar]
- Read D., van Leeuwen B. (1998). Predicting hunger: The effects of appetite and delay on choice. Organizational Behavior and Human Decision Processes, 76, 189–205. [DOI] [PubMed] [Google Scholar]
- Schoenfelder T. E., Hantula D. A. (2003). A job with a future? Delay discounting, magnitude effects, and domain independence of utility for career decisions. Journal of Vocational Behavior, 62, 43–55. [Google Scholar]
- Shenhav A., Botvinick M. M., Cohen J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79, 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. R., Rosati A. G., Ross K. R., Hauser M. D. (2005). Will travel for food: Spatial discounting in two new world monkeys. Current Biology, 15, 1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart N., Chater N., Brown G. D. A. (2006). Decision by sampling. Cognitive Psychology, 53, 1–26. [DOI] [PubMed] [Google Scholar]
- Thaler R. (1981). Some empirical evidence on dynamic inconsistency. Economics Letters, 8, 201–207. [Google Scholar]
- van den Bos W., McClure S. M. (2013). Towards a general model of temporal discounting. Journal of the Experimental Analysis of Behavior, 99, 58–73. doi: 10.1002/jeab.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Cohen J. D., Botvinick M. M., Stenger V. A., Carter C. S. (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage, 14, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Wang X. T., Dvorak R. D. (2010). Sweet future: Fluctuating blood glucose levels affect future discounting. Psy chological Science, 21, 183–188. [DOI] [PubMed] [Google Scholar]
- Waskom M. L., Frank M. C., Wagner A. D. (2017). Adaptive engagement of cognitive control in context-dependent decision making. Cerebral Cortex, 27, 1270–1284. [DOI] [PubMed] [Google Scholar]
- Waskom M. L., Kumaran D., Gordon A. M., Rissman J., Wagner A. D. (2014). Frontoparietal representations of task context support the flexible control of goal-directed cognition. The Journal of Neuroscience, 34, 10743–10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. D., Schooler J. W. (1991). Thinking too much: Introspection can reduce the quality of preferences and decisions. Journal of Personality and Social Psychology, 60, 181–192. [DOI] [PubMed] [Google Scholar]
- Yeo B. T. T., Krienen F. M., Eickhoff S. B., Yaakub S. N., Fox P. T., Buckner R. L., . . . Chee M. W. (2015). Func tional specialization and flexibility in human association cortex. Cerebral Cortex, 25, 3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B. T. T., Krienen F. M., Sepulcre J., Sabuncu M. R., Lashkari D., Hollinshead M., . . . Buckner R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neuro physiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.