Abstract
In vivo imaging of cancer cell growth and invasion is instrumental in studying cancer cell behavior and in developing effective anticancer agents. In this ROS Protocols article, we report the experimental protocol and steps involving the implantation of luciferase-expressing Lewis lung carcinoma (LLC) cells in normal syngeneic C57BL/6 mice. Using the Berthold NightOwl LB981 in vivo imaging system, we observe the time-dependent growth and invasion of the lung cancer cells following subcutaneous injection of luciferase-expressing LLC cells. The three-dimensional image and counts of photon emission of the tumor mass are obtained to estimate the relative size of the tumor. Ex vivo imaging of the isolated lungs supplemented with D-luciferin and adenosine triphosphate (ATP) is obtained to determine lung metastasis of the LLC cells. The LLC cell load in entire mouse lungs is further determined by quantitative bioluminometry with a concurrently run standard curve of the number of LLC cells versus bioluminescence intensity. This in vivo imaging system in live mice, in combination with ex vivo imaging of isolated lungs as well as quantitative bioluminometry of target tissues, may provide important information on the in vivo cancer cell dynamics in immunocompetent syngeneic C57BL/6 mice and offer a valuable tool for studying experimental anticancer agents, including redox-modulating compounds, which are promising anticancer modalities.
Keywords: Bioluminescence, In vivo imaging, Lewis lung carcinoma, Lung cancer, Metastasis, Quantitative bioluminometry
1. OVERVIEW
In vivo bioluminescence imaging of cancer cell growth and metastasis has been emerged as a major experimental approach in cancer research. In line with this notion, a number of luciferase-expressing cancer cell lines of both human and animal origins have been developed for in vivo imaging in experimental animals, especially immune-deficient mice [1–3]. Indeed, cancer cells of human origin are typically studied in mice of immune deficiency, such as nude mice, due to the inability of human cancer cells to grow in animals of competent immunosurveillance. On the other hand, cancer cells of animal origin may be implanted to normal syngeneic mice. In this context, the luciferase-expressing B16-F10 melanoma cells and Lewis lung carcinoma (LLC) cells are widely used in normal syngeneic immunocompetent C57BL/6 mice [4–6]. This is important because immunosuppression, as seen in nude mice, may promote spontaneous cancer development and might thereby confound the studies of cancer cell behavior in otherwise immunocompetent animals. In this ROS Protocols article, we report a simple in vivo imaging method involving the use of luciferase-expressing LLC cells in syngeneic C57BL/6 mice and ex vivo imaging and quantitative bioluminometry of lung metastasis of the LLC cells. We describe the detailed protocol and steps as well as discuss the advantages and limitations of using this method in studying cancer cell dynamics and anticancer therapeutics.
2. METHOD PRINCIPLES
Light emission has been used to detect experimental changes in biological assays for over a century. The term luminescence may be defined as light emission as a result of a chemical reaction without the concomitant production of heat or any thermal changes. As luminescence is caused by chemical reactions, the term chemiluminescence (CL) is frequently used. If the luminescence occurs as a result of biochemical reactions in a biological system, it is conventionally called bioluminescence (BL). Likewise, if the luminescence is from a non-biological source (e.g., a chemical reaction in a test tube), it is typically referred to as CL. Nevertheless, the distinction between the two terms is not strict as chemical reaction is the common denominator for both CL and BL.
The luciferase-expressing Lewis lung cancer (LL/2-Luc-M38) cells are inoculated subcutaneously to C57BL/6 mice. At the different time points following the cancer cell injection, D-luciferase is injected peritoneally and then the animals are subjected to whole-body imaging. Reaction of D-luciferin with luciferase in the cancer cells generates photon emission, which can be captured by an ultra-sensitive camera, thereby producing in vivo imaging of the tumor mass in the live animals. The intensity of the imaging correlates to the intensity of the light emission from the tissues where the cancer cells reside, which in turn correlates to the number of the cancer cells in the tissues. For internal organs that do not emit enough photons (due to small number of cancer cells metastasized) to penetrate the tissues so as so be captured by the camera, such organs can be collected and ex vivo imaging be obtained. In addition, the exact number of cancer cells metastasized to an internal organ can be determined by the quantitative bioluminometry as described before [7].
3. MATERIALS AND INSTRUMENTS
3.1. Animals and Major Materials
Animals: Male C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice at the age of 7–8 weeks were used in the experiments. These mice were housed in an institutional animal research facility with a light period from 6 am to 6 pm. Purified AIN-93G chow (BioServ, NJ, USA) and water were available ad libitum. All mice were allowed to acclimate for at least one week prior to the experiments. The animal procedures were approved by the Institutional Animal Care and Use Committee in compliance with the pertinent U.S. Federal policy.
Luciferase-expressing cancer cells: The luciferase-expressing Lewis lung cancer (LL/2-Luc-M38; LLC in short) cells were from PerkinElmer (Cat. No. BW119267, Waltham, MA, USA). The LLC cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 μg/ml of fungizone at 37°C in a humidified atmosphere of 5% CO2. For puromycin selection, the LLC cells were cultured in the above medium in the presence of puromycin (1 μg/ml) for 1 week.
D-Luciferin: D-Luciferin was obtained from PerkinElmer (Cat. No. 122799). D-Luciferin was dissolved in phosphate-buffered saline (see below) and stored at −90°C.
Adenosine triphosphate (ATP): ATP (Cat. No. A-2383) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Phosphate-buffered saline (PBS): PBS was prepared by the investigators, and the components include 8.1 mM Na2HPO4, 1.47 mM KH2PO4, 138 mM NaCl, and 2.67 mM KCl, pH 7.4 in deionized water.
Pentobarbital: Pentobarbital (50 mg/ml) was manufactured by Abbott Laboratories (Chicago, IL, USA)
3.2. Major Instruments
Berthold NightOwl LB981 bioluminescence imaging system (Wildbad, Germany).
Berthold multi-channel (6-channel) LB9505C luminometer (Wildbad, Germany)
4. PROTOCOLS AND STEPS
4.1. In Vivo Imaging
4.1.1. Experimental Layout
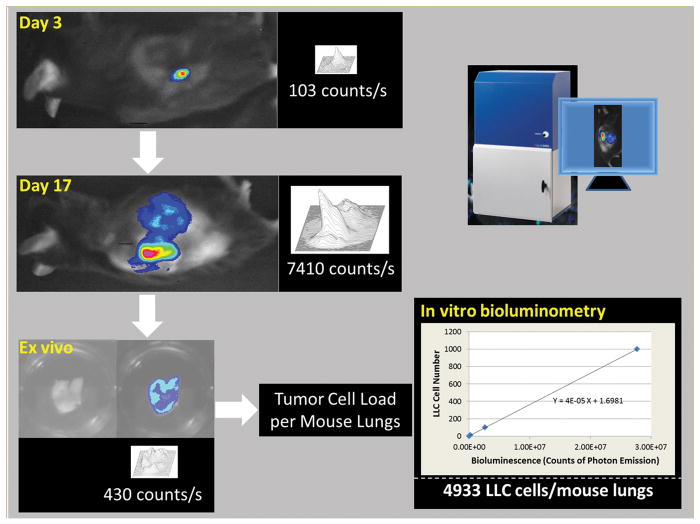
The overall experimental layout for the in vivo imaging is illustrated in Figure 1.
FIGURE 1. Lewis lung carcinoma (LLC) cell-based bioluminescence imaging modality for studying lung cancer cell growth and metastasis.
Shown are images indicative of local growth of LLC cells at days 3 and 17 after subcutaneous injection of LLC cells, and an ex vivo image of the mouse lungs at day 17. Also shown is the LLC cell load in the entire mouse lungs determined by in vitro quantitative bioluminometry in combination with a standard curve of LLC cell number versus bioluminescence intensity.
4.1.2. Assay Description
LLC cells (1 × 106 cells in 0.1 ml PBS) are injected subcutaneously to each mouse. In vivo imaging experiment is performed at day 3 and day 17 after LLC cell inoculation. Five min after intraperitoneal injection of D-luciferin (0.6 mg/30 g body weight) in a volume of 30 μl/30 g body weight (dissolved in PBS) to the anesthetized mouse, the image is acquired with a Berthold NightOwl LB981 bioluminescence imaging system for 10 min and then analyzed using the WinLight32 software. The final image is created by overlaying the bioluminescence image on top of the photo of the animal. The 3D image is constructed using the WinLight32 software, and the rate of total light emission of the tumor-bearing area is calculated and expressed as counts (numbers of photons emitted) per second (Figure 1).
4.1.3. Preparation of LLC Cells and Assay Reagents
LLC cell suspension: The LLC cells in culture (80–90% confluence) are harvested freshly and suspended in ice-cold PBS to yield 10 × 106/ml (or 1 × 106/0.1 ml). The cell suspension is used for subcutaneous injection within one hour following isolation.
D-Luciferin solution (20 mg/ml in PBS): 20 mg D-luciferin dissolved in 1 ml cold PBS (aliquot into microfuge tubes and stored at −90°C). Intraperitoneal injection of 30 μl of the above D-luciferin solution per 30 g body weight gives a final dosage of 0.6 mg/30 g body weight.
4.1.4. Steps
Inoculate 1 × 106 LLC cells suspended in 0.1 ml PBS to each mouse via subcutaneous injection. The in vivo imaging (see next) is performed on day 3 and day 17 after LLC cell inoculation.
On day 3 and day 17, anesthetize the mouse via intraperitoneal injection of pentobarbital (50 mg/ml) at a dose of 50 mg/kg body weight. It typically takes 3–5 min for the animal to become completely anesthetized.
Once the mouse becomes completely anesthetized, inject D-luciferin intraperitoneally at a dosage of 0.6 mg/30 g body weight, and 5 min later, transfer the mouse to the Berthold LB981 imager and start imaging acquisition (at high resolution for 10 min). A photograph of the mouse is taken immediately following imaging acquisition.
Overlay the image on top of the photograph to locate the body area (i.e., tumor bearing area) of light emission using the WinLight32 software and use the same software to calculate the rate of photon emission.
4.1.5. Calculation
The rate of photon emission is calculated using the WinLight32 software and expressed as counts per second.
4.2. Ex Vivo Imaging
4.2.1. Experimental Layout
The overall experimental layout of ex vivo imaging is depicted in Figure 1.
4.2.2. Assay Description
The entire lungs are harvested from the anesthetized mouse and immediately transferred into a well of a 24-well plate. The well is then filled with 1 ml of 37°C-prewarmed imaging solution (see below) with 100 μg/ml D-luciferin and 0.5 mM ATP in 25 mM phosphate buffer (pH 7.8) containing 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM MgSO4, and 0.1% Triton X-100. The plate is immediately transferred to the Berthold NightOwl LB981 bioluminescence imaging system for imaging acquisition (at high resolution for 10 min). A photograph is taken immediately after the above imaging acquisition. The image is analyzed using the WinLight32 software. The 3D image is constructed using the above software, and the rate of total light emission of the lungs is calculated and expressed as counts (numbers of photons emitted) per second (Figure 1).
4.2.3. Preparation of Assay Reagents
ATP stock solution (50 mM): Dissolve 27.6 mg ATP (Sigma-Aldrich, molecular mass = 555.1) in 1 ml of ice-cold deionized water and store at −90°C.
D-luciferin stock solution (10 mg/ml): Dissolve 10 mg D-luciferin in 1 ml of ice-cold PBS and store at −90°C.
Imaging solution: Add 10 μl of the above ATP stock solution and 10 μl of the above D-luciferin stock solution to 1 ml of 25 mM phosphate buffer (pH 7.8) containing 2 mM EDTA, 2 mM MgSO4, and 0.1% Triton X-100. The final concentrations for ATP and D-luciferin in the imaging solution are 50 mM and 100 μg/ml, respectively. Warm the imaging solution at 37°C before imaging experiment (see next).
4.2.4. Steps
Harvest the entire lungs from the phenobarbital-anesthetized mouse and place the lungs into a well of a 24-well plate.
Add 1 ml of 37°C-prewarmed imaging solution to the above well.
Quickly transfer the plate to the imager for imaging acquisition (at high resolution for 10 min). Take a photograph of the lungs after completion of the imaging acquisition.
Use the WinLight32 software to calculate the rate of photon emission.
4.2.5. Calculation
The rate of photon emission from the ex vivo lungs is calculated using the WinLight32 software and expressed as counts per second per entire mouse lungs (see Figure 1).
4.3. In Vitro Bioluminometry
4.3.1. Experimental Layout
The overall experimental layout of the in vitro quantitative bioluminometry is depicted in Figure 1.
4.3.2. Assay Description
In vitro bioluminescence is measured with a Berthold multi-channel (6-channel) LB9505C luminometer (Wildbad, Germany) at 37°C for 5 min in a clear CL vial filled with 1 ml of 25 mM phosphate buffer (pH 7.8) containing 2 mM EDTA, 2 mM MgSO4, and 0.1% Triton X-100 in the presence of luciferin (100 μg/ml) and ATP (0.5 mM). The reaction is started by adding cell or tissue samples to the above reaction mix. The luciferase/D-luciferin-derived bioluminescence intensity is expressed as integrated responses (total counts of photon emission) over the above 5 min. The LLC cell load per entire mouse lungs is determined by using a concurrently run standard curve of known numbers of LLC cells.
4.3.3. Preparation of Tissue Homogenates, Cell Lysates, and Assay Reagents
Lung tissue homogenates: Wash the entire mouse lungs with ice-cold PBS and then use a pair of small scissors to mince the lungs into small fragments in 1 ml of ice-cold imaging solution (see Section 4.2.3) in a 4 ml plastic tube. Then, subject the sample to homogenization using a homogenizer (Model 985370-395, Tissue-Tearor, BioSpec, Bartlesville, OK, USA) at the full speed for 1 min on ice. The resulting tissue homogenates are either kept on ice for measurement within 2 h or stored at −90°C for measurement within 4 weeks.
LLC cell lysates: Harvest LLC cells in culture at a confluence of 80–90% and suspend a known number of cells (1 × 106cells) in 1 ml of 25 mM phosphate buffer (pH 7.8) containing 2 mM EDTA, 2 mM MgSO4, and 0.1% Triton X-100, followed by sonication on ice for 1 min. The resulting cell lysates are either kept on ice for measurement within 2 h or stored at −90°C for measurement within 4 weeks. The cell lysates of known number of LLC cells are used to generate the standard curve (cell number versus bioluminescence intensity) which is then used to determine the LLC cell load in mouse lung tissue homogenates.
Assay reagents: see Section 4.2.3 above.
4.3.4. Steps
Standard curve: To each of the 6 CL vials, add 1 ml of 37°C-prewarmed imaging solution containing 100 μg/ml of D-luciferin and 0.5 mM ATP. Then add cell lysates derived from various known cell numbers in a final volume of 10 μl to the above vials and immediately transfer the vials to the multi-channel luminometer for measuring light emission at 37°C for 5 min. The final numbers of cells in the vials range from 0 to 1000 cells.
Tissue homogenates: To a CL vial, add 1 ml of 37°C-prewarmed imaging solution containing 100 μg/ml of D-luciferin and 0.5 mM ATP. Immediately after adding 10 μl of the lung tissue homogenate, transfer the vial to the multi-channel luminometer for measuring light emission at 37°C for 5 min. As the multi-channel luminometer can simultaneously measure light emission from 6 vials, as many as 6 samples can be measured at a time.
4.3.5. Calculation
Based on the standard curve, extrapolate the equivalent LLC cell number from the 10 μl of the lung tissue homogenate used in the assay. As the total volume of each mouse lung tissue homogenate is known, the total LLC cell number in each mouse lungs can then be calculated. The LLC cell load is expressed as the total number of the LLC cells per entire mouse lungs (Figure 1).
5. DISCUSSION OF ADVANTAGES AND LIMITATIONS
One of the major advantages of in vivo bioluminescence imaging is the allowance of the studies of the temporal progression of tumor development. This is due to the relatively non-toxic nature of D-luciferin, which allows repeated injections at different time points without causing significant effects on the animals as well as tumor growth [8]. Another advantage of using LLC cells for studying lung cancer is that the cell line is originally derived from the lung carcinoma developed in C57BL/6 mice. As such, the LLC cell-based assay would allow the investigation of the biology and experimental therapeutics of the primary carcinoma in the lungs. This is in contrast to the melanoma B16-F10 cells, another widely used cell line for studies of cancer (melanoma) cell metastasis to the lungs, rather than primary lung carcinoma.
Like other imaging techniques, in vivo bioluminescence imaging is not without limitations. One intrinsic drawback is the quenching of photons by internal tissues and skin. This is particularly evident with the C57BL/6 mouse, whose black fur and skin color significantly block photon emission from the target organs (e.g., the lungs). Such a phenotype may dramatically reduce the sensitivity of the imaging modality in visualizing lung tumor mass in live animals. Fortunately, an albino C57BL/6 mouse line is commercially available at a cost comparable to that of the regular C57BL/6 line, and this mouse line has been used for in vivo bioluminescence imaging [9].
In the present study, subcutaneous injection of LLC cells is used to investigate both the subcutaneous growth and lung metastasis of the cancer cells. Alternatively, the LLCs can be inoculated via intravenous injection. This would allow the rapid metastasis of the LLC cells to the lungs and the rapid development of lung carcinoma [10]. Studies are currently underway to develop a more sensitive bioluminescence imaging assay for visualizing in situ growth of LLC cells in the lungs of live albino C57BL/6 mice.
Following the in vivo imaging, the mouse lungs can be harvested for subsequent ex vivo measurement of organ bioluminescence. Such an ex vivo study is particularly relevant when the in vivo imaging in live animals fails to pick up light emission from the internal organs. Ex vivo imaging of various internal organs may also yield important insight into the organ-specific metastasis of LLC cells.
Although in vivo and ex vivo imaging may reveal the relative size of the tumor mass in target organs, both assays are semi-quantitative. To obtain quantitative information, in vitro biolumineometry may then be used. As demonstrated in the current study, in vitro bioluminescence measurement of lung tissue homogenates in combination with a standard curve derived from known numbers of LLC cells can yield quantitative information on the cancer cell load in the mouse lungs. The high sensitivity of quantitative bioluminometry (able to detect as few as 10 cancer cells per sample) makes it possible to map the cancer cell load in virtually all internal organs. Such quantitative studies may be instrumental in understanding cancer cell dynamics across various organs and devising organ-selective anticancer therapies.
Acknowledgments
This work was supported in part by an investigator-initiated grant (IIG) (09A084) from the American Institute for Cancer Research (AICR), and a grant (CA192936) from the U.S. National Institutes of Health/National Cancer Institute. The authors declare no conflicts of interest.
ABBREVIATIONS
- ATP
adenosine triphosphate
- BL
bioluminescence
- CL
chemiluminescence
- EDTA
ethylenediaminetetraacetic acid
- LLC
Lewis lung carcinoma
- PBS
phosphate-buffered saline
References
- 1.Bhang HE, Gabrielson KL, Laterra J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nat Med. 2011;17(1):123–9. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–5. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Evans MS, Chaurette JP, Adams ST, Jr, Reddy GR, Paley MA, Aronin N, et al. A synthetic luciferin improves bioluminescence imaging in live mice. Nat Methods. 2014;11(4):393–5. doi: 10.1038/nmeth.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74(24):7250–9. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata A, Baoum A, Ohta N, Jacquez S, Seo GM, Berkland C, et al. Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer Res. 2012;72(8):2057–67. doi: 10.1158/0008-5472.CAN-11-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H, Jia Z, Trush MA, Li YR. Nrf2 deficiency promotes melanoma growth and lung metastasis. Reactive Oxygen Species. 2016;2(4):308–14. doi: 10.20455/ros.2016.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Kauffman ME, Li JZ, Sarkar S, Trush MA, Jia Z, et al. Innovative bioluminometric quantification of cancer cell load in target organs: implications for studying anticancer drugs, including ros enhancers. Reactive Oxygen Species. 2016;1(2):157–64. doi: 10.20455/ros.2016.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiffen JC, Bailey CG, Ng C, Rasko JE, Holst J. Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol Cancer. 2010;9:299. doi: 10.1186/1476-4598-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci U S A. 2008;105(38):14342–6. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Furukawa K, Chen HH, Sakakibara T, Urano T, Furukawa K. Metastatic potential of mouse Lewis lung cancer cells is regulated via ganglioside GM1 by modulating the matrix metalloprotease-9 localization in lipid rafts. J Biol Chem. 2006;281(26):18145–55. doi: 10.1074/jbc.M512566200. [DOI] [PubMed] [Google Scholar]