Abstract
This study evaluated the longitudinal relationships among visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and peripheral bone strength during adolescence. Fat and lean mass, VAT and SAT area, and android/gynoid (A/G) ratio were estimated with DXA. Our main outcome was strength-strain index (SSI), an indicator of peripheral bone strength estimated by pQCT at the radius and tibia. Sex-specific analyses evaluated the longitudinal bone-fat relationship from ages 11 to 19 years with linear mixed models using biological age as the time variable and adjusted for limb length and lean mass in 182 girls and 167 boys. Variables were standardized (mean =0, SD =1) prior to model fitting and results shown are parameter estimates ± SE. Fat mass and SAT were positively associated with SSI (radius: 0.07 ± 0.02, p =0.003 and 0.05 ± 0.02, 0.041, respectively; tibia: 0.09 ± 0.02, p <0.001 and 0.08 ± 0.02, p <0.001, respectively) prior to, but not following adjustment for lean mass in girls. In contrast, fat mass and SAT were negatively associated with radial SSI, both before and after adjustment for lean mass in boys (fat mass: −0.05 ± 0.01, p =0.001; SAT: −0.04 ± 0.01, p =0.004). In full models, negative associations were limited to VAT in girls and included radial (−0.06 ± 0.02, p =0.001) and tibial SSI (−0.04 ± 0.02, p =0.033). For boys, there were no significant associations present between VAT and SSI at the radius or tibia. In analyses limited to obese participants, an A/G ratio was not significantly associated with SSI in girls, but was negatively associated with radial SSI regardless of adjustment for lean mass in boys (−0.06 ± 0.02, p =0.018). These results that show a negative relationship between peripheral bone strength and VAT in girls, but greater total and central adiposity in boys, suggest these factors play a role in adequate acquisition of bone strength during adolescence.
Keywords: BONE-FAT INTERACTIONS, BONE QCT, OSTEOPOROSIS, GENERAL POPULATION STUDY, DXA
Introduction
There is growing evidence that the fat-bone relationship varies according to region of fat deposition. In adult men, trunk fat, but not appendicular fat, has been associated negatively with cortical bone size.(1) Similarly, visceral and subcutaneous abdominal fat have, respectively, been associated negatively and positively with femur structure and strength in young women.(2) Limited data in children and adolescents also support a negative relationship between centrally-located fat and bone mineral density (BMD) and/or bone mineral content (BMC).(3–6) However, a longitudinal investigation that accounts for characteristic changes in body composition during growth would be beneficial in order to confirm these findings. Indeed, in a recent review of adiposity in children and adolescents, Katzmarzyk and colleagues(7) identified a need for additional research in several areas, including the measurement of subregions of adipose tissue as well as the clinical consequences of excess visceral adipose tissue (VAT) during childhood.
The possibility that visceral fat negatively influences bone metabolism during childhood and adolescence is supported by a small number of studies. Russell and colleagues(3) found VAT associated negatively, but subcutaneous adipose tissue (SAT) associated positively, with bone density measures in 30 girls 12 to 18 years old. Moreover, obese girls with a higher compared with a lower ratio of VAT to SAT had lower bone density measures, even though body fat and percent body fat did not differ significantly between groups. Although that study only included girls in early or late adolescence, there is evidence to indicate that the negative relationship between visceral fat and bone is also present in boys and prepubertal girls. Pollock and colleagues(4) evaluated 140 prepubertal, overweight boys and girls and found centrally-located fat was negatively associated with whole-body BMC in analyses adjusted for race, sex, fat-free soft tissue mass, fat mass, and SAT. Furthermore, they found that children with prediabetes also had lower whole-body BMC compared to those without prediabetes, indicating that insulin resistance might also influence bone metabolism in children. However, they also found that subcutaneous fat was negatively associated with whole-body BMC. Similarly, Afghani and Goran(8) found SAT negatively associated with BMC in girls and boys approximately age 11 years (n =256), although in that study the negative relationship between visceral fat and BMC only reached statistical significance in girls.
Although these findings, taken together, suggest a negative relationship between centrally-located adipose tissue and bone during growth, there are rapid and sex-specific changes in body composition during adolescence that are not fully accounted for in these cross-sectional investigations. In addition, because the majority of these studies involved overweight and obese individuals, additional investigations are necessary to determine whether there might be any relevance to bone strength from visceral fat in otherwise healthy-weight individuals. Finally, these studies assessed bone using DXA and a three-dimensional (3D) technique such as pQCT that measures volumetric BMD, bone geometry, and strength would be more informative. Because up to 90% of peak bone mass is accrued by late adolescence(9) and the growing prevalence of severe childhood obesity,(10) it is important to clarify the relationships between childhood obesity and bone development, particularly as they could relate to depot-specific adiposity. Therefore, the purpose of this study was to evaluate the relationships among visceral and subcutaneous fat and peripheral bone strength during adolescence using data from children participating in the Iowa Bone Development Study (IBDS), a prospective cohort study of the effects of fluoride and other factors on bone development.(11)
Subjects and Methods
For this longitudinal analysis, data from the age 11 years through age 19 years biennial visits of the IBDS were utilized. These data were collected from 2003 to 2013. Participants with medical conditions or medication use that could affect bone were excluded from analyses. Also excluded were participants without age 11 years baseline data or without follow-up data. This study was approved by the institutional review board. Parents and their children provided informed consent/assent.
Body composition
Estimates of fat and lean mass were obtained through analysis of dual-energy X-ray absorptiometry (DXA) whole-body scans (QDR 4500W, software version 12.3; Hologic, Inc., Bedford, MA, USA). The Hologic spine phantom was scanned daily for quality assurance, and the whole-body step phantom was scanned weekly per manufacturer’s guidelines to calibrate the machine for whole-body composition. Participants were scanned wearing street clothes but without shoes or metal items. VAT area and SAT area were estimated from whole-body scans according to manufacturer’s guidelines using Hologic Apex 4.0 software. This method for measuring visceral fat has been shown to correlate well with visceral fat measured by computed tomography in adults (r =0.92, p <0.01)(12) and overweight children (r =0.86, 95% CI, 0.80 to 0.90).(13) Because DXA does not directly separate subcutaneous from visceral fat, the amount of subcutaneous fat overlying visceral fat is estimated using an algorithm that incorporates information from the amount of fat tissue located between the abdominal muscle wall and outer edge of skin at the level of the fourth lumbar vertebra. VAT is estimated as the difference between total abdominal fat of the region of interest minus subcutaneous fat. The android/gynoid fat ratio (A/G ratio) was determined following standard manufacturer’s guidelines and utilized as a surrogate indicator of the relative amounts of visceral to subcutaneous fat.
Bone
pQCT scans of the radius (nondominant side) and tibia were acquired (XCT 2000/3000, software version 6.2; Stratec Medizintechnik GmbH, Pforzheim, Germany) by trained technicians. As part of scan acquisition, the length of the radius and tibia in millimeters (mm) was measured using a sliding ruler. To determine the starting position, a coronal computed radiograph was performed and the reference line was placed to bisect the medial border of the most proximal growth plate of the distal radius. When growth plates were no longer visible, the reference line was placed to bisect the medial border of the distal endplate.
Our primary bone outcome was diaphyseal bone strength. This was estimated from measurements taken at sites 20% of the limb length of the radius and 38% of the limb length of the tibia. These measurements provided estimates of polar strength-strain index (pSSI, mm3), an indicator of strength to resist torsion at radial and tibial shaft sites. Additional measurements utilized at the 20% and 38% sites included cortical bone mineral density (cBMD, mg/cm3), cortical bone area (CA, cm3), cortical bone mineral content (cBMC, mg), periosteal circumference (PC, mm), and cortical thickness (CTh, mm). For these measurements, contour mode 1 and contour mode 2 with thresholds of 710 mg/ cm3 and 480 mg/cm3, respectively, were applied. A circular ring model was used to obtain the cortical thickness measurement. Scans were acquired using a voxel size of 0.4 mm, scan speed of 20 mm/s, and slice thickness of 2.4 mm. Scans with poor quality, as assessed by two trained technicians, were excluded from analyses. This included review of reference line placement, progression of reference line placement between visits, and excessive motion. Scans were excluded for motion if there was a visible break in the cortical rim with displacement of the rim. Due to size constraints of the XCT 2000 for tibial scans, participants with a calf measurement greater than 15.5 inches were scanned at the tibia using the XCT 3000. Results of a previous calibration study, along with previous findings in this cohort,(14) did not suggest a significant effect on bone outcomes.
Biological age
Biological age (maturity offset), the number of years from peak height velocity, was estimated from the age 11 years, 13 years, and 15 years assessment visits using a predictive equation validated in white Canadian children and adolescents.(15)
Height, weight, and body mass index
Height was measured to the nearest 0.1 cm by stadiometer (Harpenden, Holtain, UK) and weight to the nearest kilogram (kg) by balance beam scale by trained research nurses at assessment visits. Body mass index (BMI, weight in kg/height in m2) was calculated for each visit. Participants were defined as healthy weight (HW) or overweight/obese (OW) according to CDC percentiles for age and sex: healthy weight (>5th to <85th percentile), overweight (≥85th to <95th percentile), and obese (≥95th percentile).(16)
Physical activity level
The Physical Activity Questionnaire for Children (PAQ-C) was administered at the age 11 years visit and the Physical Activity Questionnaire for Adolescents (PAQ-A) was administered starting at the age 13 years visit. These are 7-day recall questionnaires that differentiate between high-activity and low-activity children(17) and assess self-reported moderate to vigorous physical activity during the school year. The PAQ-A and PAQ-C scores range from 1 to 5, with higher activity levels indicated by higher mean scores.
Statistical methods
Sex-specific analyses were conducted. Participant characteristics were described using mean ± SD for continuous variables and percentages for categorical variables by measurement visit. Data were evaluated for normality using the Shapiro-Wilk test and by evaluating histograms. Mean differences between girls and boys for continuous variables were evaluated using t tests, while chi-square tests were used to evaluate differences in the frequency distributions of categorical variables. SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA) was utilized for analyses and statistical significance was defined as a p value <0.05.
Linear mixed models were used to evaluate the longitudinal relationships between VAT and our primary outcome, SSI, and secondary bone outcomes that included cortical BMD, BMC, area, CTh, and PC. Random intercepts and slopes were used to describe the variation in bone parameters at baseline (intercept) and the rate of growth (slope). Fixed effects included the time-varying predictor variable, VAT, as a continuous variable, biological age as the variable for time and to control for differences in maturation level, limb length to control for differences between participants in body size and limb lean mass (radius) or whole body lean mass (tibia) to control for differences in muscle size. Physical activity was also evaluated for inclusion in models. Polynomial functions of age (biological age2 and biological age3) were included in the models to allow for nonlinearity of growth. Interactions between VAT and biological age were evaluated for inclusion in models to determine whether potential associations between VAT and bone outcomes varied depending on age. SAT was evaluated as a predictor, but not included in models with VAT due to high correlations at each visit (r =0.89–0.99 in girls and r =0.70–0.87 in boys) in order to prevent multicollinearity. Instead, the relationships between SAT and bone measures were modeled separately from visceral fat area, but using similar methods. Models also were fit using fat mass as the main predictor variable for the purpose of assessing greater overall fat mass in the context of greater VAT or SAT. Because a fat-bone relationship could differ in healthy and overweight/obese, analyses were repeated to evaluate the relationship between greater relative amounts of visceral to subcutaneous fat (A/G ratio) and peripheral bone strength measured as SSI in participants with a BMI considered obese at baseline and who remained obese or overweight at each follow-up visit. Model variables, with the exception of biological age, were standardized (mean =0 and standard deviation [SD] =1) to improve interpretation of results. Each SD change in predictor variable corresponded with a SD change in bone parameter.
The linear mixed models were evaluated using Akaike’s Information Criterion (AIC) goodness-of-fit statistic. The AIC was used to select the structure of the variance-covariance matrices of the between-subject and within-subject variances. The unstructured covariance matrix was selected for the random effects covariance matrix based on the AIC statistic for each model. The random error (within person or residual covariance matrix) covariance matrix was also evaluated, but the majority of models utilizing both the random effects and random error covariance matrices indicated that the random error (residual covariance matrix) structure either would not converge or the model fit was not improved based on the AIC statistic.
Results
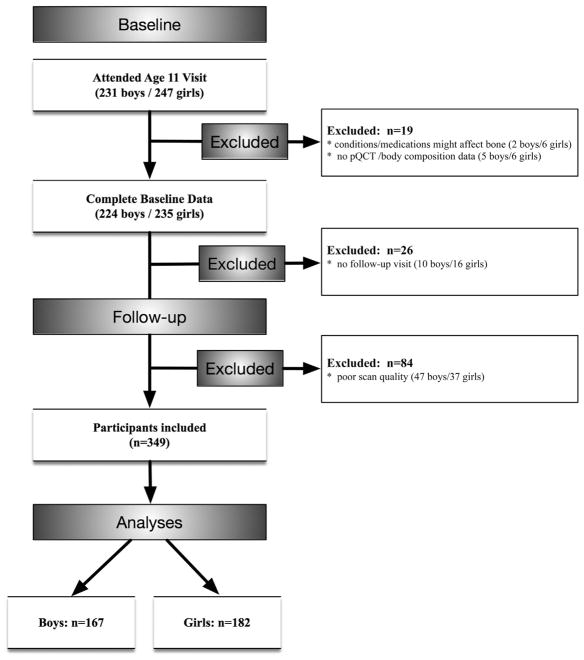
There were 478 children participating in the study at the time of the age 11 years baseline visit. Out of this number, eight were excluded due to medical conditions that could affect bone development, including cerebral palsy with hemiparesis (n =1), type 1 diabetes (n =3), ulcerative colitis (n =2), lupus erythematosus (n =1), and premature adrenarche (n =1). As shown in Fig. 1, 26 children (10 boys, 16 girls, 5.7%) were excluded for lack of follow-up. In addition, there were 211 observations (12.6% observations, 47 boys and 37 girls) excluded from analyses due to scan quality. Baseline characteristics were compared between those included in analyses and those excluded for lack of follow-up or poor scan quality. Boys excluded for lack of follow-up or scan quality did not differ from those included in baseline radial SSI (138.5 ± 40.6 versus 135.2 ± 33.9 mm3, p =0.552), tibial SSI (1045.9 ± 295.6 versus 1016.8 ± 254.4 mm3, p =0.502), percent body fat (25.1 ± 8.6 versus 24.6 ± 8.0%, p =0.662), lean mass (29.7 ± 7.3 versus 29.0 ± 5.6 kg, p =0.517), VAT (48.5 ± 21.6 versus 46.3 ± 20.2 mm2, p =0.478), or SAT (148.1 ± 133.4 versus 131.8 ± 110.4 mm2, p =0.358). Girls excluded for lack of follow-up or scan quality did not differ from those included in baseline radial SSI (129.1 ± 37.7 versus 122.0 ± 31.1 mm3, p =0.168), tibial SSI (950.2 ± 257.3 versus 951.7 ± 213.2 mm3, p =0.967), percent body fat (26.6 ± 7.2 versus 27.0 ± 7.4%, p =0.721), lean mass (28.5 ± 6.4 versus 27.3 ± 5.6 kg, p =0.149), VAT (34.3 ± 28.7 versus 31.3 ± 27.2 mm2, p =0.467), or SAT (210.1 ± 107.1 versus 202.7 ± 111.6 mm2, p =0.662).
Fig. 1.
Participant inclusion diagram.
Participant characteristics
Although the evaluation of differences between boys and girls was not a primary aim of this study, results shown in Table 1 indicate p values from sex comparisons for each measurement visit. We found boys had significantly greater VAT and girls had greater SAT compared with one another at each measurement visit. A greater percentage of girls compared with boys achieved peak height velocity (PHV) at the age 11 years and age 13 years measurement visits. There were no significant differences between boys and girls in the percentage of obese participants at each measurement visit and physical activity levels were generally similar. However, percentage body fat was greater at each measurement visit in girls versus boys. With the exception of cBMD, boys generally had greater pQCT-measured bone parameters compared with girls at each measurement visit (Table 2).
Table 1.
Participant Characteristics by Sex and Measurement Visit
| Variable | Girls | Boys | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Age 11 years (n =182) | Age 13 years (n =150) | Age 15 years (n =142) | Age 17 years (n =137) | Age 19 years (n =124) | Age 11 years (n =167) | Age 13 years (n = 138) | Age 15 years (n =124) | Age 17 years (n =107) | Age 19 years (n =94) | |
| Age (years) | 11.2 ± 0.3 | 13.3 ± 04 | 15.3 ± 0.3c | 17.5 ± 0.4 | 19.8 ± 0.7 | 11.2 ± 0.3 | 13.3 ± 0.4 | 15.4 ± 0.3 | 17.6 ± 0.4 | 19.7 ± 0.7 |
| VAT (cm2) | 31.4 ± 27.0a | 33.9 ± 28.5a | 36.9 ± 24.1a | 44.4 ± 28.7b | 50.2 ± 36.9c | 45.8 ± 19.4 | 48.3 ± 21.6 | 48.8 ± 21.3 | 56.0 ± 24.0 | 62.5 ± 29.2 |
| SAT (cm2) | 203.4 ± 111.3a | 221.1 ± 129.3a | 245.4 ± 127.9a | 282.1 ± 147.4a | 307.4 ± 162.4a | 129.4 ± 106.1 | 139.9 ± 125.6 | 131.4 ± 120.4 | 146.0 ± 126.3 | 170.9 ± 123.4 |
| Percent body fat | 27.1 ± 7.3c | 25.5 ± 7.6a | 26.7 ± 6.9a | 28.9 ± 7.2a | 32.5 ± 7.5a | 24.5 ± 7.9 | 21.4 ± 8.7 | 17.6 ± 7.4 | 17.2 ± 7.1 | 20.4 ± 6.4 |
| % Obese | 12.1% | 12.0% | 12.0% | 12.4% | 16.9% | 16.8% | 15.9% | 15.3% | 13.1% | 21.3% |
| Arm lean mass (kg) | 1.5 ± 0.3a | 1.9 ± 0.3a | 2.0 ± 0.3a | 2.0 ± 0.4a | 2.1 ± 0.4a | 1.6 ± 0.3 | 2.3 ± 0.5 | 3.1 ± 0.6 | 3.5 ± 0.7 | 3.7 ± 0.7 |
| Leg lean mass (kg) | 5.0 ± 1.0c | 6.4 ± 1.1a | 7.0 ± 1.2a | 7.3 ± 1.3a | 7.3 ± 1.6a | 5.3 ± 1.0 | 7.3 ± 1.4 | 9.4 ± 1.6 | 10.4 ± 1.7 | 10.4 ± 1.8 |
| Whole-body lean mass (kg) | 27.2 ± 5.6c | 35.0 ± 6.0a | 38.5 ± 5.8a | 40.6 ± 6.7a | 41.0 ± 8.0a | 28.9 ± 5.6 | 39.3 ± 7.5 | 50.9 ± 8.7 | 57.1 ± 9.0 | 58.8 ± 9.4 |
| Height (cm) | 148.8 ± 6.9 | 160.5 ± 5.9c | 164.5 ± 6.1a | 165.7 ± 6.1a | 166.2 ± 6.3a | 149.2 ± 7.5 | 163.3 ± 8.9 | 175.2 ± 7.7 | 179.1 ± 7.7 | 180.4 ± 7.8 |
| Achieved physical maturityd | 16.5%a | 98.7%a | 100.0% | 100.0% | 100.0% | 0.0% | 30.4% | 97.6% | 100.0% | 100.0% |
| Physical activity level score | 2.8 ± 0.7 | 2.6 ± 0.7c | 2.4 ± 0.7 | 2.3 ± 0.7 | 2.1 ± 0.7b | 2.9 ± 0.7 | 2.8 ± 0.8 | 2.6 ± 0.8 | 2.5 ± 0.9 | 2.4 ± 0.8 |
Values are mean ± SD or percentage.
Values of p for girls compared with boys at each measurement visit:
p <0.0001;
p <0.001;
p <0.05.
Physical maturity is defined as having achieved peak height velocity.
Table 2.
Bone Measurements by Sex and Measurement Visit
| Bone measures | Girls | Boys | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Age 11 years (n =182) | Age 13 years (n =150) | Age 15 years (n =142) | Age 17 years (n =137) | Age 19 years (n =124) | Age 11 years (n =167) | Age 13 years (n =138) | Age 15 years (n =124) | Age 17 years (n =107) | Age 19 years (n =94) | |
| Radius | ||||||||||
| pSSI (mm3) | 122.2 ± 31.6b | 166.7 ± 40.4b | 196.3 ± 42.0a | 210.5 ± 45.0a | 216.3 ± 45.8a | 135.0 ± 34.1 | 183.9 ± 43.4 | 264.6 ± 76.9 | 319.9 ± 74.0 | 342.4 ± 84.0 |
| cBMD (mg/cm3) | 1048.0 ± 26.4 | 1079.8 ± 32.4a | 1129.8 ± 24.3a | 1163.3 ± 18.0a | 1180.9 ± 14.8a | 1048.9 ± 26.5 | 1050.3 ± 27.8 | 1073.7 ± 30.4 | 1121.0 ± 23.2 | 1149.1 ± 17.3 |
| CA (cm3) | 49.4 ± 7.6a | 60.8 ± 8.6b | 67.0 ± 7.9a | 69.5 ± 8.5a | 70.1 ± 8.2a | 52.4 ± 7.8 | 65.3 ± 10.5 | 82.2 ± 13.6 | 92.9 ± 12.7 | 97.1 ± 13.1 |
| cBMC (mg) | 51.8 ± 8.2b | 65.8 ± 10.2c | 75.7 ± 9.1a | 80.8 ± 9.8a | 82.8 ± 9.5a | 55.0 ± 8.2 | 68.5 ± 11.0 | 88.4 ± 15.3 | 104.2 ± 14.2 | 111.5 ± 14.8 |
| PC (mm) | 29.5 ± 2.8a | 32.7 ± 2.8a | 34.3 ± 2.7a | 34.8 ± 2.8a | 34.9 ± 2.7a | 30.7 ± 2.8 | 34.2 ± 3.1 | 38.6 ± 4.0 | 40.7 ± 3.5 | 41.3 ± 3.7 |
| CTh (mm) | 2.2 ± 0.2 | 2.4 ± 0.2 | 2.6 ± 0.2a | 2.6 ± 0.2a | 2.6 ± 0.2a | 2.2 ± 0.2 | 2.5 ± 0.3 | 2.7 ± 0.3 | 3.0 ± 0.3 | 3.1 ± 0.3 |
| Tibia | ||||||||||
| pSSI (mm3) | 950.5 ± 217.2c | 1214.3 ± 262.4a | 1391.2 ± 291.0a | 1490.7 ± 322.2a | 1536.8 ± 329.7a | 1016.1 ± 255.5 | 1374.4 ± 302.9 | 1772.7 ± 413.7 | 2019.4 ± 395.6 | 2131.2 ± 403.3 |
| cBMD (mg/cm3) | 1046.8 ± 34.2a | 1094.5 ± 32.5a | 1129.4 ± 23.5a | 1147.3 ± 18.7a | 1159.4 ± 19.2a | 1031.1 ± 34.5 | 1035.5 ± 34.5 | 1076.2 ± 34.7 | 1116.6 ± 20.8 | 1134.9 ± 17.0 |
| CA (cm3) | 201.8 ± 32.2c | 237.4 ± 36.1a | 261.1 ± 39.8a | 274.5 ± 43.2a | 279.9 ± 43.2a | 212.8 ± 36.7 | 265.5 ± 40.6 | 315.7 ± 51.5 | 344.4 ± 52.0 | 360.6 ± 51.1 |
| cBMC (mg) | 211.2 ± 34.4 | 259.8 ± 39.6c | 294.7 ± 43.3a | 314.5 ± 47.5a | 324.2 ± 47.9a | 219.2 ± 37.2 | 274.9 ± 42.7 | 339.6 ± 55.1 | 384.3 ± 56.7 | 409.0 ± 56.4 |
| PC (mm) | 59.9 ± 4.8c | 64.0 ± 4.8a | 66.2 ± 4.8a | 67.4 ± 5.1a | 67.8 ± 5.1a | 61.5 ± 5.3 | 68.0 ± 5.4 | 73.1 ± 5.7 | 75.5 ± 5.1 | 76.4 ± 4.9 |
| CTh (mm) | 4.3 ± 0.5c | 4.9 ± 0.5b | 5.2 ± 0.6a | 5.4 ± 0.6a | 5.5 ± 0.6a | 4.5 ± 0.5 | 5.1 ± 0.6 | 5.7 ± 0.7 | 6.1 ± 0.8 | 6.4 ± 0.7 |
Primary outcome is pSSI. Values are mean ± SD.
pSSI =polar strength-strain index; cBMD =cortical bone mineral density; CA = cortical bone area; cBMC =cortical bone mineral content; PC =periosteal circumference; CTh =cortical thickness.
Values of p for girls compared with boys at each measurement visit:
p <0.0001;
p <0.001;
p <0.05.
Longitudinal relationships between VAT and bone strength (SSI)
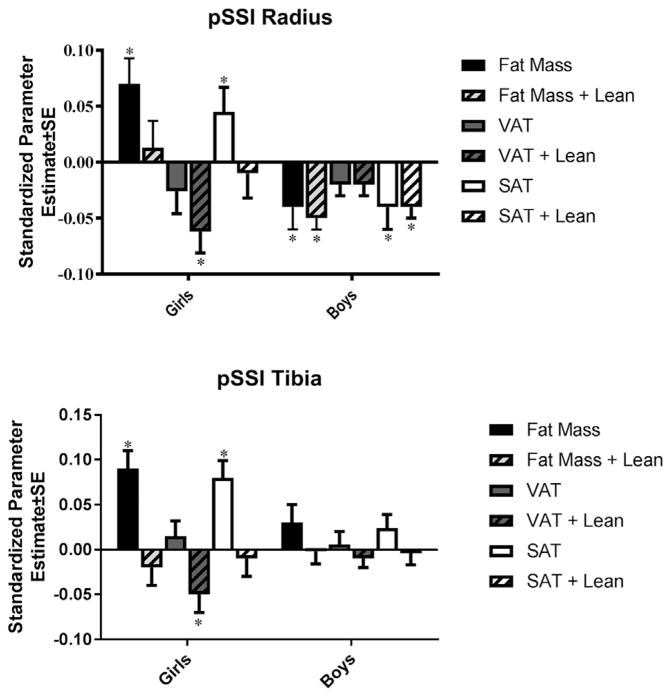
Linear mixed model analyses of the associations between VAT and SSI revealed the following (all results are presented as standardized beta ± SE unless otherwise specified). Results for the radius are shown in Figure 2A and for the tibia in Figure 2B. Physical activity was included in adjusted models where significant (only the analyses of PC and SSI at the radius in boys.) In girls, VAT was not significantly associated with SSI prior to adjustment for lean mass (Fig. 2); however, significant negative associations with SSI at the radius (−0.06 ± 0.02, p =0.001) and tibia (−0.05 ± 0.02, p =0.004) were present after adjustment. In addition, there was a significant interaction effect present between VAT and age for radial SSI (VAT main effect =−0.10 ± 0.02, p <0.001, interaction effect =0.012 ± 0.003, p <0.001). This indicated that the negative association was smaller in older than younger girls (−0.11 at 1 year pre-peak height velocity and −0.09 at 1 year post-peak height velocity). For boys, VAT was not significantly associated with SSI in unadjusted or adjusted models.
Fig. 2.
Linear mixed models for the relationships between adiposity measures and diaphyseal bone strength (SSI) from age 11 to 19 years in girls and boys at the radius (A) and tibia (B). *p <0.05. Models include biological age, lean mass, and limb length.
Longitudinal relationships between SAT and bone strength
In girls, SAT was positively associated with SSI (0.05 ± 0.02, 0.041;0.08 ± 0.02, p <0.001) at both the radius and tibia, prior to, but not following adjustment for lean mass (Fig. 2). In boys, significant negative associations were present between SAT and SSI at the radius both before (−0.04 ± 0.02, p =0.018) and after (−0.04 ± 0.01, p =0.004) adjustment for lean mass. In addition, there was a significant interaction effect between SAT and age for radial SSI (SAT main effect =−0.05 ± 0.01, p <0.001, interaction effect =−0.013 ± 0.003, p <0.001), indicating that this negative association was greater in older boys (−0.04 at 1 year pre-peak height velocity and −0.06 at 1 year post-peak height velocity).
Longitudinal relationships between whole-body fat mass and bone strength
Findings for fat mass were similar to those for SAT (Fig. 2). Like SAT, whole-body fat mass was positively associated with SSI at the radius (0.07 ± 0.02, p =0.003) and tibia (0.09 ± 0.02, p <0.001) prior to, but not following adjustment for lean mass in girls. In contrast, fat mass was negatively associated, both before and after adjustment for lean mass, with radial SSI (−0.04 ± 0.02, p =0.005; −0.05 ± 0.01, p =0.001) in boys. There was a significant interaction effect present with age in this relationship that indicated this negative association was larger in older boys (fat mass main effect =−0.05 ± 0.01, p =0.001, interaction effect =−0.012 ± 0.003, p <0.001).
Longitudinal relationships between VAT and secondary bone measures
In girls, VAT was negatively associated with cBMD (−0.06 ± 0.02, p =0.002) and cBMC (−0.04 ± 0.02, p =0.048) at the radius and cBMD (−0.08 ± 0.02, p <0.001) at the tibia, but positively associated with CA (0.06 ± 0.03, p =0.07) and PC (0.05 ± 0.02, p =0.007) at the tibia prior to adjustment for lean mass. Following adjustment for lean mass, a negative association with radial cBMC (−0.07 ± 0.02, p <0.001) and tibial cBMD remained significant (−0.08 ± 0.02, p <0.001), whereas newly significant negative associations were noted between VAT and radial CA (−0.06 ± 0.02, p =0.003) and CTh (−0.08 ± 0.03, p =0.013). There was a significant interaction effect present between VAT and age for CA (VAT main effect =−0.02 ± 0.02, p <0.001, interaction effect =0.012 ± 0.004, p =0.006), cBMC (VAT main effect =−0.12 ± 0.02, p <0.001, interaction effect =0.013 ± 0.003, p <0.001), and CTh (VAT main effect =−0.13 ± 0.04, p <0.001, interaction effect =0.01 ± 0.01, p =0.014) at the radius and cBMD at the tibia (VAT main effect =−0.16 ± 0.04, p <0.001, interaction effect =0.01 ± 0.01, p =0.005). These findings indicated that the negative relationships between VAT and these bone parameters were smaller in older girls. There were significant, negative associations noted in boys between VAT and radial CA (−0.05 ± 0.01, p <0.001), cBMC (−0.05 ± 0.01, p =0.001), and CTh (−0.07 ± 0.02, p =0.001) and tibial cBMD (−0.06 ± 0.02, p =0.007) that were present both before and after adjustment for lean mass. No significant interaction effects were noted for boys.
Longitudinal relationships between SAT and secondary bone measures
SAT was positively associated with CA (0.12 ± 0.02, p < 0.001), cBMC 0.10 ± 0.02, p <0.001), and CTh (0.09 ± 0.03, p =0.002) at the tibia, as well as with PC (0.05 ± 0.02, p =0.011;0.09 ± 0.02, p <0.001) at both the radius and tibia, prior to, but not following adjustment for lean mass in girls. There were also no significant interaction effects noted between SAT and secondary bone measures in girls. Positive associations were seen between SAT and tibial CA (0.04 ± 0.02, p =0.022) and PC (0.04 ± 0.02, p =0.020) prior to, but not following adjustment for lean mass in boys. In contrast, significant negative associations were present both before and after adjustment for lean mass in boys between SAT and CA (−0.07 ± 0.02, p <0.001; −0.09 ± 0.01, p <0.001), cBMC (−0.06 ± 0.02, p =0.002; −0.07 ± 0.01, p <0.001), and CTh (−0.11 ± 0.03, p <0.001; −0.12 ± 0.03, p <0.001) at the radius and cBMD at the tibia (−0.08 ± 0.02, p <0.001; −0.07 ± 0.03, p =0.004). In addition, radial PC was negatively associated with SAT (−0.04 ± 0.02, p =0.019) following adjustment for lean mass in boys. There were significant interaction effects between SAT and age for CA (SAT main effect =−0.08 ± 0.01, p <0.001, interaction effect =−0.013 ± 0.003, p <0.001) and cBMC (SAT main effect =−0.07 ± 0.01, p <0.001, interaction effect =−0.013 ± 0.003, p <0.001) at the radius. These findings indicated that the negative relationships between SAT and bone were larger in older boys
Longitudinal relationships between whole-body fat mass and secondary bone measures
Findings for fat mass were similar to those for SAT. Like SAT, whole-body fat mass was positively associated with PC (0.08 ± 0.02, p =0.003) at the radius and CA (0.13 ± 0.04, p <0.001), cBMC (0.11 ± 0.03, p <0.001), PC (0.08 ± 0.02, p <0.001), and CTh (0.10 ± 0.03, p =0.002) at the tibia prior to, but not following adjustment for lean mass in girls. Again, there were no significant interaction effects noted between fat mass and age for secondary bone measures in girls. In contrast, fat mass was negatively associated, both before and after adjustment for lean mass, with CA (−0.08 ± 0.02, p <0.001; −0.10 ± 0.02, p <0.001), cBMC (−0.07 ± 0.02, p <0.001; −0.09 ± 0.01, p <0.001), PC (−0.03 ± 0.02, p =0.088; −0.04 ± 0.02, p =0.006), and CTh (−0.12 ± 0.03, p <0.001; −0.13 ± 0.02, p <0.001) at the radius and cBMD (−0.08 ± 0.02, p =0.004; −0.08 ± 0.03, p =0.002) at the tibia. There were significant interaction effects noted between whole-body fat mass and age for CA (fat mass main effect =−0.08 ± 0.02, p <0.001, interaction effect =−0.011 ± 0.003, p <0.001) and cBMC (fat mass main effect =−0.07 ± 0.02, p <0.001, interaction effect =−0.011 ± 0.003, p <0.001) at the radius, indicating that these negative associations were larger in older boys.
Subanalyses in obese participants
There were 48 girls and 52 boys with a BMI defined as obese at baseline who were also overweight or obese at each measurement visit. We found no relationship between the A/G ratio and SSI in girls. However, we found a significant negative association between radial SSI and A/G ratio in boys, regardless of lean mass (−0.06 ± 0.02, p =0.018).
Discussion
Our study evaluated the longitudinal relationships among visceral and subcutaneous adipose tissue area and peripheral bone strength from ages 11 to 19 years in the Iowa Bone Development Study. We found whole-body fat mass and SAT were both negatively associated with radial bone strength in boys, but negative associations for SSI were limited to VAT in girls. These findings suggest that visceral fat in girls and central adiposity in boys influence acquisition of bone strength during adolescence.
It has been suggested that VAT and SAT exert opposing effects on measures of bone structure and strength.(2,3) Adipose tissue is metabolically active and secretes factors that influence bone metabolism. Inflammatory factors secreted by visceral fat(18,19) could increase bone resorption by stimulating osteoclast activity.(20,21) In contrast, leptin, a hormone produced and secreted in greater quantities by subcutaneous rather than visceral fat,(22,23) might increase bone mass by stimulating osteoblast activity.(24,25) Adiponectin, another hormone secreted by adipose tissue, is negatively related to visceral fat accumulation,(26) as well as insulin resistance,(27,28) and could improve insulin sensitivity.(29) This could have significance in terms of bone metabolism, as there is growing evidence that elevated insulin levels, as have been associated with greater visceral fat, could be harmful to bone.(4,30) Animal studies have shown that insulin signaling is an important component of osteoblast development,(31,32) but that insulin resistance could impair insulin signaling in osteoblasts and reduce their survival.(33) However, the mechanism for a potentially harmful influence from visceral fat on bone is still unknown and beyond the scope of this work.
Our longitudinal findings in girls that showed a negative association between VAT and cortical bone strength, but no significant associations for SAT, are partially consistent with previous studies that suggested opposing roles for VAT and SAT. Gilsanz and colleagues(2) found that SAT was associated positively, whereas VAT was associated negatively, with CT measures of femur mid-shaft structure and strength in women ages 15 to 25 years. Furthermore, the investigators found the strength of positive associations with bone measures were slightly greater for SAT than were the negative associations for VAT. Similarly, Russell and colleagues(3) found SAT associated positively and VAT associated negatively with bone density measures in 30 girls ages 12 to 18 years. We chose not to include VAT and SAT in the same analytic models in our main analyses because of very high correlations between them at each measurement visit (r >0.89). However, analyses completed with SAT and VAT both included in the same model predicting bone strength in our study yielded results more in line with those of previous studies. Specifically, we found VAT negatively and SAT positively associated with bone strength at both the radius (VAT =−0.14 ± 0.03, p <0.0001; SAT =0.11 ± 0.0.04, p =0.002) and tibia (VAT =−0.09 ± 0.03, p <0.001; SAT =0.07 ± 0.03, p =0.029) when included in the same model. Thus, differences in analytic models could contribute to some degree to discrepancies between these previous findings(2,3) and our findings in girls.
Our subgroup analyses in obese girls unexpectedly revealed no significant associations between A/G ratio and bone strength. Although a greater ratio of android to gynoid fat might be expected to be negatively associated with bone strength, this supposition only holds if the A/G ratio adequately reflects the relative amounts of overall VAT to SAT. However, an increase in body fat during adolescence in girls typically follows a gynoid fat distribution pattern, such that even were a greater amount of VAT present, there would likely be greater overall subcutaneous fat with obesity. In other words, a higher A/G ratio in obese girls might be in the setting of greater overall subcutaneous fat, which in our study was positively associated with both weight-bearing and non–weight-bearing bone strength, prior to adjustment for lean mass. In keeping with the idea of opposing roles for VAT and SAT, a greater amount of subcutaneous as opposed to visceral fat with obesity in girls, could negate or alter the direction of a fat–bone relationship from negative to positive and possibly explain the lack of the association between A/G ratio and SSI in subgroup analyses in girls in our study.
In contrast with our findings for girls, we found both greater overall fat mass and SAT were negatively associated with cortical bone strength at the radius in boys. Further, we found a negative association between A/G ratio and radial SSI in analyses restricted to obese boys that also lends support to a negative association between total central adiposity and bone strength. This might be explained partly by typical sex-specific differences in the allocation of body fat, in that boys tend to gain excess fat in central rather than peripheral regions, such that the greater overall fat mass in boys in our study was predominantly centrally located. Other studies have also found a negative association between SAT and bone measures. For example, SAT has been negatively associated with whole-body BMC in prepubertal, overweight children ages 7 to 11 years(4) and overweight children ages 8 to 14 years(8) in cross-sectional studies. Although involving an older population than our study, Taes and colleagues(1) also found trunk fat measured by DXA was negatively associated with cortical bone size by pQCT in 677 men 25 to 45 years old. Further, DXA estimates of abdominal adiposity have been significantly associated with insulin resistance,(34–36) diabetes,(37) and with metabolic risk factors such as circulating high-density lipoprotein (HDL) or low-density lipoprotein (LDL) levels.(36,38,39)
An additional explanation for differences in findings among studies might be due to the evaluation of abdominal SAT as a homogenous compartment. There is growing evidence that subcutaneous abdominal fat is not a homogenous tissue with a singular biochemical profile. Rather, it comprises two distinct compartments defined as superficial or deep,(40–42) with deep SAT more closely corresponding with the metabolic activity of VAT than does subcutaneous SAT.(41–43) Further, there could be greater amounts of deep rather than superficial abdominal SAT with adiposity in males,(44) though this has not been evaluated in adolescents to our knowledge. Thus, our findings in boys might be explained by a greater amount of deep SAT than present in girls.
There are several strengths to our study. Our study cohort is well-described and data were prospectively collected. Confirmation of previous, cross-sectional findings by our longitudinal study was informative, because we accounted for changes in body composition during adolescence. Our findings were also able to provide new insight into this relationship during growth with inclusion of weight-bearing and non–weight-bearing bone strength measurements. Furthermore, our study also included healthy weight individuals providing additional information on whether a potential relationship between visceral fat and bone varied across the spectrum of BMI. Finally, our differing results in girls and boys highlight the importance of sex-specific evaluations of factors that might influence bone during growth.
Despite these strengths, there are potential limitations to our findings. First, DXA measurements of VAT and SAT have not been validated in children at this time. However, a study evaluating the association between CTh-measured VAT and DXA-measured VAT showed a good correlation in children with a BMI in the overweight range (r =0.86; 95% confidence interval, 0.80 to 0.90).(13) Additionally, an investigator at our institution found measurement of visceral fat by MRI correlated highly with DXA VAT in a small sample of children (r >0.99, p <0.0001, n =4; Chadi Carlage, MD and colleagues, unpublished data). Second, it is possible that the negative associations of SAT, but not VAT, with bone strength could be due to inappropriate division of fat regions in male participants. Nevertheless, we were able to detect a negative association of VAT, but not SAT or fat mass, with radial SSI in our study in girls. We were also able to detect negative associations between VAT and our secondary bone outcomes, including cortical BMC, area, and thickness at the radius in boys. Importantly, it should be noted that our measure of physical activity was not significantly associated with bone measures in most statistical models. Thus, we might not be accounting adequately for dynamic loading that could exert greater influence on bone than adipose tissue. In addition, although we did not find significant differences in baseline characteristics between those included in analyses and those excluded for lack of follow-up or poor scan quality, the potential for selection bias remains a possibility. Another potential limitation is our use of biological age rather than Tanner stage to account for differences between participants in developmental stages. Furthermore, our results might not be applicable to measures of cortical bone strength from regions other than the radius and tibia. Because our study population is predominantly white and the study is conducted in the Midwestern region of the United States, our findings are not generalizable to other populations.
In conclusion, we found negative associations of diaphyseal strength at the radius with VAT in girls and central adiposity in boys. Thus, greater visceral and/or central adiposity may have an important role in bone growth during adolescence. These results suggest a compartment-specific influence of greater adiposity on bone and could help explain previous, inconsistent findings regarding childhood obesity and bone, as well as potential sex differences in these relationships. Additional studies are needed to evaluate whether this relationship persists into adulthood, evaluate the implications of deep versus superficial SAT effects on bone strength and evaluate biochemical factors that might better describe fat compartment-specific relationships with bone strength.
Acknowledgments
This study was supported by NIH grants: National Institute of Dental and Craniofacial Research (NIDCR) (R01-DE09551 and R01-DE12101), GCRCP General Clinical Research Centers Program award (M01-RR00059), and NIH Clinical and Translational Science Awards (CTSA) (UL1TR000442). We thank the Iowa Bone Development Study participants and staff; this project would not have been possible without their time, hard work and dedication.
Authors’ roles: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: all authors. Participated in drafting the manuscript or revising it critically for important intellectual contact: all authors. Approved the final version of the submitted manuscript: all authors. Agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: NG.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Taes YE, Lapauw B, Vanbillemont G, et al. Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab. 2009;94(7):2325–31. doi: 10.1210/jc.2008-2501. [DOI] [PubMed] [Google Scholar]
- 2.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–55. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollock NK, Bernard PJ, Wenger K, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25(12):2760–9. doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos RM, Lazaretti-Castro M, Mello MT, et al. Influence of visceral and subcutaneous fat in bone mineral density of obese adolescents. Arq Bras Endocrinol Metabol. 2012;56(1):12–8. doi: 10.1590/s0004-27302012000100003. [DOI] [PubMed] [Google Scholar]
- 6.Junior IF, Cardoso JR, Christofaro DG, Codogno JS, de Moraes AC, Fernandes RA. The relationship between visceral fat thickness and bone mineral density in sedentary obese children and adolescents. BMC Pediatr. 2013;13:37. doi: 10.1186/1471-2431-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Shen W, Baxter-Jones A, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatr Obes. 2012;7(5):e42–61. doi: 10.1111/j.2047-6310.2012.00073.x. [DOI] [PubMed] [Google Scholar]
- 8.Afghani A, Goran MI. The interrelationships between abdominal adiposity, leptin and bone mineral content in overweight Latino children. Horm Res. 2009;72(2):82–7. doi: 10.1159/000232160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15(4):263–73. doi: 10.1007/s00198-003-1542-9. [DOI] [PubMed] [Google Scholar]
- 10.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–6. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 11.Levy SM, Kiritsy MC, Slager SL, Warren JJ. Patterns of dietary fluoride supplement use during infancy. J Public Health Dent. 1998;58(3):228–33. doi: 10.1111/j.1752-7325.1998.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 12.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20(5):1109–14. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2015;10(3):172–9. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass NA, Torner JC, Letuchy EM, et al. The relationship between greater prepubertal adiposity, subsequent age of maturation, and bone strength during adolescence. J Bone Miner Res. 2016;31(7):1455–65. doi: 10.1002/jbmr.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–94. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski KC, Crocker PRE, Faulkner RA. Validation of the physical activity questionnaire for older children. Pediatr Exerc Sci. 1997;9:174–86. [Google Scholar]
- 18.Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26(5):1362–8. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- 19.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 20.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. 2008;41(7):495–510. doi: 10.5483/bmbrep.2008.41.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47(6):913–7. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 23.van Harmelen V, Dicker A, Ryden M, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51(7):2029–36. doi: 10.2337/diabetes.51.7.2029. [DOI] [PubMed] [Google Scholar]
- 24.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85(4):825–36. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 25.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatagai T, Nagasaka S, Taniguchi A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52(10):1274–8. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 29.Ahima RS. Overcoming insulin resistance with CNTF. Nat Med. 2006;12(5):511–2. doi: 10.1038/nm0506-511. [DOI] [PubMed] [Google Scholar]
- 30.Srikanthan P, Crandall CJ, Miller-Martinez D, et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res. 2014;29(4):796–803. doi: 10.1002/jbmr.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–19. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pramojanee SN, Phimphilai M, Kumphune S, Chattipakorn N, Chattipakorn SC. Decreased jaw bone density and osteoblastic insulin signaling in a model of obesity. J Dent Res. 2013;92(6):560–5. doi: 10.1177/0022034513485600. [DOI] [PubMed] [Google Scholar]
- 34.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–8. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 35.Aucouturier J, Meyer M, Thivel D, Taillardat M, Duche P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch Pediatr Adolesc Med. 2009;163(9):826–31. doi: 10.1001/archpediatrics.2009.148. [DOI] [PubMed] [Google Scholar]
- 36.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358(9291):1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 37.Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27(12):2948–53. doi: 10.2337/diacare.27.12.2948. [DOI] [PubMed] [Google Scholar]
- 38.Kang SM, Yoon JW, Ahn HY, et al. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011;6(11):e27694. doi: 10.1371/journal.pone.0027694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99(4):541–5. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 40.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 41.Walker GE, Verti B, Marzullo P, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15(8):1933–43. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 42.Cancello R, Zulian A, Gentilini D, et al. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity (Silver Spring) 2013;21(12):2562–70. doi: 10.1002/oby.20417. [DOI] [PubMed] [Google Scholar]
- 43.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278(5):E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 44.Marinou K, Hodson L, Vasan SK, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37(3):821–9. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]