Abstract
Apolipoprotein E (ApoE) is an important lipid carrier in both the periphery and the brain. The ApoE ε4 allele (ApoE4) is the single most important genetic risk-factor for Alzheimer’s disease (AD) while the ε2 allele (ApoE2) is associated with a lower risk of AD-related neurodegeneration compared to the most common variant, ε3 (ApoE3). ApoE genotype affects a variety of neural circuits; however, the olfactory system appears to provide early biomarkers of ApoE genotype effects. Here, we directly compared olfactory behavior and olfactory system physiology across all three ApoE genotypes in 6-month- and 12-month-old mice with targeted replacement for the human ApoE2, ApoE3, or ApoE4 genes. Odor investigation and habituation were assessed, along with, olfactory bulb and piriform cortical local field potential activity. The results demonstrate that while initial odor investigation was unaffected by ApoE genotype, odor habituation was impaired in E4 relative to E2 mice, with E3 mice intermediate in function. There was also significant deterioration of odor habituation from 6 to 12 months of age regardless of the ApoE genotype. Olfactory system excitability and odor responsiveness were similarly determined by ApoE genotype, with an ApoE4 > ApoE3 > ApoE2 excitability ranking. Although motivated behavior is influenced by many processes, hyper-excitability of ApoE4 mice may contribute to impaired odor habituation, while hypo-excitability of ApoE2 mice may contribute to its protective effects. Given that these ApoE mice do not have AD pathology, our results demonstrate how ApoE affects the olfactory system at early stages, prior to the development of AD.
Keywords: Olfaction, apolipoprotein E, piriform cortex, olfactory bulb, Alzheimer’s disease, odor habituation
Introduction
Apolipoprotein E (ApoE) is the primary carrier of cholesterol within the brain, and ApoE genotype is an important determinant of an individual’s risk for developing Alzheimer’s disease (AD) (Corder et al., 1993; Farrer et al., 1997; Bu, 2009; Liu et al., 2013). Three alleles of ApoE occur in humans: ε2 (cys112, cys158; ~6% of the ApoE alleles in the population), ε3 (cys112, arg158; the most abundant allele at ~80%), and ε4 (arg112, arg158; ~14%) (Mahley, 1988; Mahley and Rall, 2000). ApoE4, in a dose-dependent manner, is the single most important genetic risk-factor for AD (Corder et al., 1993; Farrer et al., 1997; Bu, 2009; Liu et al., 2013). ApoE3 is viewed as the neutral allele in terms of neurodegenerative risk (Corder et al., 1993; Mahley and Rall, 2000; Liu et al., 2013) (and most closely resembles murine ApoE (Raffai et al., 2001)). ApoE2 is associated with a lower risk of AD-related neurodegeneration (Corder et al., 1994; Liu et al., 2013), delayed age of onset of AD, and a greater likelihood of survival to advanced age compared to the ApoE3 and ApoE4 alleles [reviewed in (Suri et al., 2013)]. In addition to AD, there is increasing evidence that ApoE is involved in several other disorders, with ApoE4 often exerting a deleterious and ApoE2 a protective effect; while not all studies have found a difference between ApoE2 and ApoE3 carriers [reviewed in (Suri et al., 2013), both ApoE4 and ApoE2 appear to be associated with hemorrhagic and ischemic cerebrovascular disease [reviewed in (Liu et al., 2013; Suri et al., 2013; Lopez et al., 2014)], with a high risk of argyrophilic grain disease and frontotemporal dementia [reviewed in (Suri et al., 2013)]. ApoE2 also appears to confer an increased incidence and severity of posttraumatic stress disorder (PTSD) (Freeman et al., 2005; Kim et al., 2013; Johnson et al., 2015). While the effects of ApoE4 have been extensively studied, the inconsistencies among a small number of studies regarding the effect of ApoE2 may in part be due to challenges related to its low frequency allele.
While ApoE genotype may affect a myriad of neural circuits and functions, the olfactory system and olfactory perception appear to be a unique and early biomarker of ApoE genotype. In humans, ApoE4 carriers show early emergence of olfactory dysfunction (Price et al., 1991; Bacon et al., 1998; Mesholam et al., 1998; Murphy et al., 1998; Graves et al., 1999; Gilbert and Murphy, 2004; Josefsson et al., 2017; Peng et al., 2017), show impaired odor identification (Murphy et al., 1998; Olofsson et al., 2010; Olofsson et al., 2016), and modified olfactory related evoked potentials (Kowalewski and Murphy, 2012; Morgan and Murphy, 2012) prior to other forms of cognitive impairment, including AD impairment associated with amyloid β and tau pathology. In mice, ApoE4 impairs short-term odor memory and induces olfactory system hyperexcitability including in the olfactory bulb (OB) and piriform cortex (PCX) (Peng et al., 2017) as well as the lateral entorhinal cortex (Nuriel et al., 2017) The olfactory system is suitable for assessing links between cell biology, circuit function and behavior given its relatively simple circuitry and the availability of reliable, robust behavioral assays in both humans and non-human animals. ApoE is important for neuroregeneration of the rodent olfactory system (Nathan et al., 2005), and ApoE knock-out mice show impaired olfactory detection (Nathan et al., 2004). This olfactory dysfunction is associated with neurophysiological (Mesholam et al., 1998; Corby et al., 2012; Kowalewski and Murphy, 2012; Peng et al., 2017), structural (Tanaka et al., 1998; Hashimoto et al., 2001) and cellular changes (Tsuboi et al., 2003; Nathan et al., 2005; Hussain et al., 2013) in olfactory regions of the brain. Understanding how ApoE isoforms may influence olfactory system function and perception would provide important insights into ApoE4 as a major risk factor for olfactory and cognitive decline.
Here, we directly compared olfactory behavior and olfactory system physiology across all three ApoE genotypes in mice that are homozygous for human ApoE2, ApoE3, or ApoE4. We hypothesized a genotype-dependent gradient in odor habituation and olfactory system excitability, such that, for example, ApoE4 mice would display hyper-excitability and impaired behavioral habituation, and ApoE2 mice hypo-excitability and increased behavioral habituation, relative to ApoE3 mice. The results were in line with our hypotheses and they extend previous work (Peng et al., 2017).
Experimental Procedures
Study approval
All animal procedures were performed in accordance with the Nathan S. Kline Institute (NKI) for Psychiatric Research Institutional Animal Care Committee’s approval.
Mice
Mice used in this experiment were homozygous for human ApoE2, ApoE3, and ApoE4 genes on a C57BL/6 background which are from long-standing colonies at NKI. These targeted-replacement mice express human ApoE under the control of the endogenous murine promoter (Sullivan et al., 1997), which allows for the expression of human ApoE at physiologically regulated levels in the same temporal and spatial pattern as endogenous murine ApoE. The native mouse ApoE protein has structural similarities to human ApoE4, but functional similarities to human ApoE3 (Raffai et al., 2001). Thus, a direct comparison between human and mouse ApoE genotypes would be, at best, difficult to interpret. A total of 40 mice were used for the habituation component of this study (6-month, ApoE2 = 9; 6-month, ApoE3 = 6; 6-month, ApoE4 = 6; 12-month, ApoE2 = 6, 12-month ApoE3 = 9; 12-month, ApoE4 = 4). A total of 46 mice were used for the electrophysiology component of this study (6-month ApoE2 = 6; 6-month ApoE3 = 13; 6-month ApoE4 = 10; 12-month ApoE2 = 6, 12-month ApoE3 = 6; 12-month, ApoE4 = 5).
Odor Habituation
To investigate for simple behavioral odor memory deficits, mice were screened using an odor habituation test. Prior to behavioral assessment (24-48 hours), mice were single-housed in new home cages with fresh corncob bedding. Test odors (2-heptanone, isoamyl acetate, (+) enantiomer of limonene, and ethyl valerate; Sigma Aldrich, St. Louis, MO, USA) were diluted in mineral oil to a concentration of 100 ppm. The dilution was then applied to a cotton-tipped applicator that was subsequently enclosed in a piece of odorless plastic tubing. The tubing allowed for volatile odor delivery while preventing the liquid from coming in direct contact with either the chamber or animal. Each trial consisted of a 20 second odor presentation (inserting the applicator into a port on the side of the animal’s home cage) followed by a 30 second intertrial interval with four presentations of a single odor in each block. Each odor was presented four times and odor sequence was the same for all mice. The duration of time spent investigating, defined as snout-oriented sniffing within 1cm of the odor presentation port, was recorded by a single observer blind to animal genotype. Data was averaged across odors to provide a single odor habituation curve for each mouse. All testing took place during the light portion of the animals’ 12-hour dark/light cycle over two daily sessions and food-bins and water bottles were removed from cages immediately prior to testing.
In vivo electrophysiology
Mice were given i.p. injections of urethane (1.25 g/kg) and then placed in a stereotaxic apparatus. Incisions were made down the midline of the scalp to expose the skull and ipsilateral holes were drilled above the olfactory bulb and anterior piriform cortex. Monopolar tungsten recording electrodes were placed in both the OB and anterior piriform cortex (aPCX). Electrical stimulation of the OB was used to confirm electrode placement in the aPCX using evoked responses. Local field potentials (LFP) were amplified (200×), band-pass filtered (0.3 Hz-3 kHz), and digitized (1 kHz) for analyses.
Once the electrodes were placed, a series of odors (isoamyl acetate, ethyl valerate, 1,7-octadiene, 2-heptenone (Sigma Aldrich, St. Louis, MO, USA), and peppermint extract; (McCormick, Sparks, MD, USA)) were presented through a flow-dilution olfactometer by a computer-controlled pinch valve at a rate of 0.1 liter per minute to a constant 1 liter per minute flow of compressed air. Odors were presented for 2 seconds with at least 60 seconds between odor presentations and odor-evoked LFPs were assessed throughout. Each odor was presented four times per mouse in pseudorandom order.
Data Analysis
For behavioral odor habituation, the amount of time spent investigating the odor was normalized to the investigation time on trial 1 per animal for each odor with trial 1 investigation time assigned a value of 1. Differences in age and genotype were analyzed using univariate ANOVAs. Post hoc comparisons were made using one-tailed independent samples t-tests.
For LFP data, Spike2 software (Cambridge Electronic Design Ltd., Cambridge, England) was used to record both spontaneous and odor-evoked activity. The two second period immediately preceding odor presentation was used to assess spontaneous LFP’s whereas the two second period immediately following odor onset was used to assess odor-evoked LFP’s. LFP’s were analyzed using an off-line Fast-Fourier transform (FFT) examining three different frequency bands: theta (5-15 Hz), beta (15-40 Hz), and gamma (40–80 Hz). Analyses were made by calculating the average odor-evoked response for each individual odor (4 trials per odor per mouse) and subsequently averaging each odor together into an overall set of odor-evoked responses resulting in a single odor-evoked response metric for each mouse. This analysis allows identification of stable trait responses to odors and avoids noise due to minor variations in electrode placement, variation in stimulus presentation, and variation in individual mouse responsiveness to specific odors. Odor-evoked and spontaneous LFP’s were analyzed by age and genotype using repeated-measures ANOVAs and one-tailed t-tests, using SPSS 20 software. Heterogeneity of variances was assessed by Mauchly’s test and alpha levels were adjusted when appropriate.
Results
Olfactory behavior
Independent of age or genotype, mice readily investigated novel odors presented to their homecage, suggesting no difference in behavioral odor responsiveness. As shown in Fig. 1, there was no age (F(1,34) = 0.001, p = 0.97) or genotype (F(2,34) = 1.3, p = 0.3) difference in the amount of time spent investigating the initial presentation of a novel odor.
Figure 1.
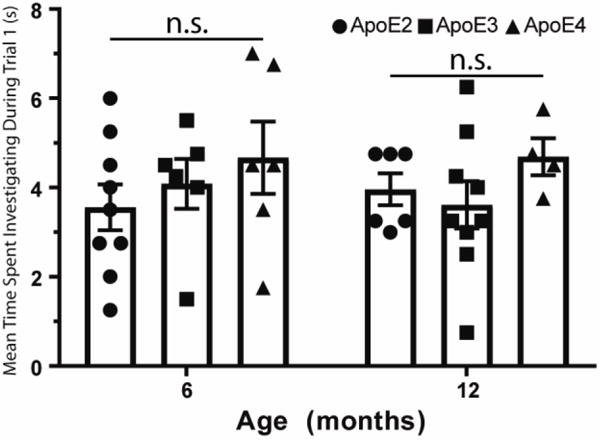
Initial odor investigation (trial 1 of odor habituation sessions) was unaffected by ApoE genotype or age (n.s. = not significant), suggesting normal odor guided behavior in the animals tested here. Histograms are means ± s.e.m. All individual data for each group are also plotted.
In contrast, odor habituation was significantly affected by genotype (Fig. 2). For habituation, the analysis strategy was informed by prior results from an identical experimental protocol (Peng et al., 2017), where results indicated that while habituation was notable following the initial exposure, inspection times were similar on trials 2-4. As habituation effects were not expected to interact further with ApoE genotype or age among trials 2-4, and as single-trial data from individual habituation trials are affected by noise, data from trials 2-4 were collapsed to obtain a robust habituation index for each animal (i.e. mean odor inspection time on trials 2-4 as a proportion of inspection time on trial 1; larger values indicate less habituation). Behavioral data were submitted to a univariate ANOVA where ApoE genotype (three levels: ApoE 2, 3 and 4) and Age (two levels: 6 months and 12 months) were included as fixed factors, and the habituation index constituted the dependent variable.
Figure 2.
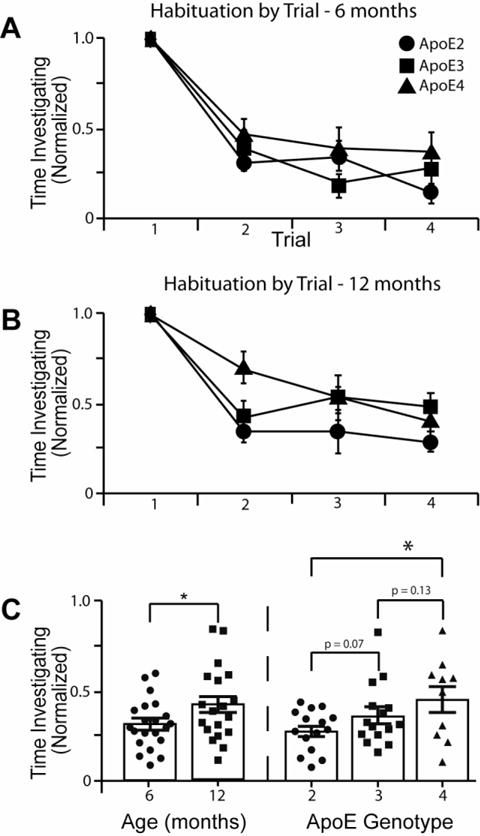
Odor habituation was impaired in ApoE4 mice compared to ApoE2 mice, with ApoE3 mice performing intermediately. (A) Habituation in 6-month-old mice, normalized to investigation on the first trial (ApoE2 n = 9, ApoE3 n = 6, ApoE4 n = 6). (B) Habituation in 12-month-old mice, normalized to investigation on the first trial (ApoE n = 6, ApoE3 n = 9, ApoE4 n = 4). (C) Direct comparison of age and genotype effects on odor habituation (average over trials 2-4). Histograms are means ± s.e.m. All individual data for each group are also plotted. Older mice showed less habituation than younger mice (left panel, p = 0.04). ApoE4 mice showed less habituation compared to ApoE2 (right panel, p = 0.02) while ApoE3 mice performed nominally, but not significantly, in-between ApoE2 and ApoE4 (right panel).
Results indicated main effects of ApoE genotype (F(2,40) = 3.6, p = 0.039) and Age (F(1,40) = 4.3, p = 0.046). Based on the directional a priori hypothesis that there would be a rank order difference between ApoE2, 3, 4 (e.g., Fig 2; (Huang et al., 2017)), additional one-tailed post-hoc analyses collapsed across age indicated that ApoE2 mice showed more habituation than ApoE4 mice (p = 0.02), with ApoE3 mice showing habituation values that were nominally in between the ApoE2 (p = 0.07) and ApoE4 (p = 0.13) groups, but neither trend reached statistical significance threshold. The effect of age was due to older mice showing less habituation than younger mice, again consistent with our prediction (Figure 1). There was no interaction effect between ApoE and Age (F(2,40) = 0.3, p = 0.7), indicating that age affected olfactory behavior independent of ApoE.
Olfactory system physiology
LFP data underwent FFT, which was subsequently divided into theta, beta, and gamma frequency bands for statistical analysis. LFP data were submitted to a repeated measures ANOVA with frequency band (three levels: theta, beta, and gamma) and brain region (two levels: OB and PCX) as within-subjects variables, age (two levels: 6 months and 12 months) and ApoE genotype (three levels: ApoE 2, 3 and 4) as between-subjects variables, and odor-evoked response magnitude as the dependent variable.
The ANOVA revealed a main effect of frequency band (F(2,80) = 7.1, p = 0.007), a main effect of genotype (F(2,40) = 4.2, p = 0.02), as well as a frequency band by genotype interaction (F(4,80) = 3.5, p = 0.031). However, there was no main effect of age (F(2,40) = 0.012, p = 0.913), indicating similar brain responses across age groups. As is clear from the FFT data (Fig. 3), the largest odor-evoked effect was an enhancement in beta band (15-40 Hz) activity. Thus, a subsequent omnibus repeated measures ANOVA was performed analyzing the odor-evoked response magnitudes specifically in the beta band revealing a significant main effect of genotype (F(2,40) = 4.2, p = 0.02). No effects of genotype were observed in the other frequency bands, indicating the ApoE by frequency band interaction effect was driven by the beta band.
Figure 3.
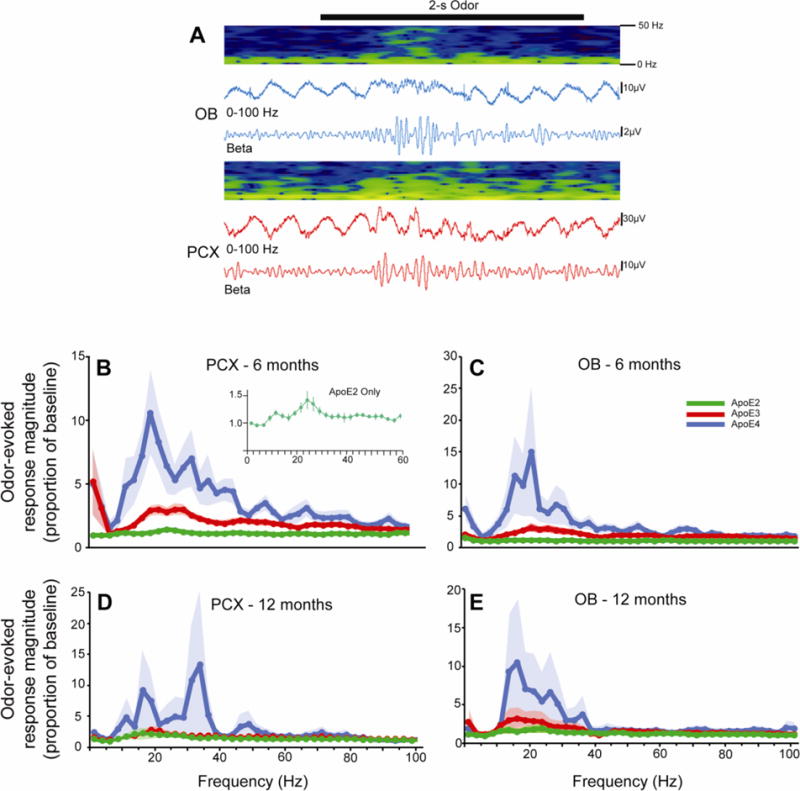
Representative recording of odor-evoked activity in the olfactory bulb and piriform cortex of ApoE mice (A). The 2s odor presentation is represented by the black bar with contemporaneous LFP recordings from the entire 0-100Hz spectrum as well as restricted to only the beta band (15-40 Hz). Pseudocolor spectrograms show the temporal structure of LFP power in which dark blue is low and yellow is high. FFT analyses of odor-evoked activity (difference from spontaneous activity) in the olfactory bulb (B, D) and piriform cortex (C, E) of 6 and 12 month old mice in each genotype. The inset in panel B is at a modified scale to highlight that while the odor evoked activity in ApoE2 mice is small relative to the other genotypes, there is a reliable evoked activity.
Based on the directional a priori hypothesis that there would be a rank order difference between ApoE 2, 3, 4 (e.g., Fig 2; (Huang et al., 2017)) subsequent one-tailed independent samples t-tests were performed to compare the effects of ApoE genotype on odor-evoked activity in both the OB and the PCX, collapsed across age (Fig. 4). As previously reported (Peng et al., 2017), ApoE4 mice showed exaggerated odor-evoked response magnitudes relative to ApoE2 in both the OB (t(25) = −2.1, p = 0.03) as well as the PCX (t(25) = −2.6, p = 0.01). Exaggerated responses in ApoE4 relative to ApoE3 were also shown in the PCX (t(32) = −2.10, p = 0.03) and a similar trend was observed in OB (t(32) = −1.6, p = 0.07). In contrast, ApoE2 mice were shown to have significantly lower odor-evoked response magnitudes than ApoE3 mice in both OB (t(29) = −2.6, p = 0.008) and PCX (t(29) = −3.1, p = 0.002).
Figure 4.
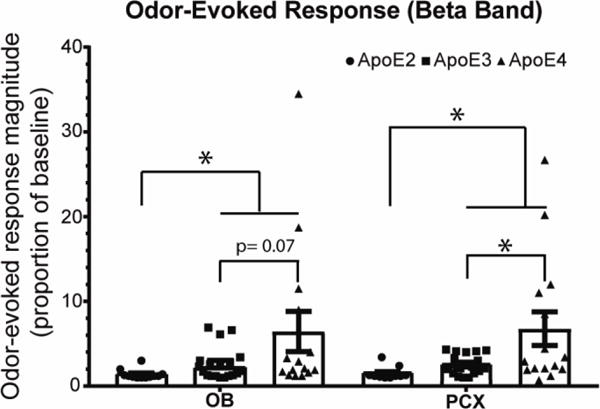
Odor-evoked activity in the beta frequency band was significantly ApoE genotype dependent, with an ApoE4 > ApoE3 > ApoE2 excitability relationship. In the OB, ApoE2 mice showed lower odor-evoked responses than both ApoE3 (p = 0.008) and ApoE4 (p = 0.03), but ApoE3 were only nominally different from ApoE4. In the PCX, ApoE2 mice showed lower odor-evoked responses than both ApoE3 (p = 0.002) and ApoE4 (p = 0.01), and ApoE3 mice showed lower responses than ApoE4 (p = 0.03). Histograms are means ± s.e.m. All individual data for each group are also plotted.
Discussion
Despite olfaction emerging as a system of importance to study the behavioral and physiological effects of ApoE, research remains limited. The present results demonstrate that while the initial tendency of mice to investigate novel odors is unaffected by ApoE genotype, odor habituation is impaired in E4 relative to E2 mice, with E3 mice presenting an intermediate functional level. Memory impairment in this simple task emerges in E4 mice by 6 months, as previously reported (Peng et al., 2017). The E2 allele appears to improve habituation memory relative to E4 and, although not significant in our sample, relative to E3 mice, consistent with a protective effect of the E2 allele. Odor habituation deteriorated as the animals aged from 6 months to 12 months, regardless of genotype. Similarly, while E4 mice showed olfactory system hyper-excitability relative to E3 mice, E2 mice displayed hypo-excitability relative to both E4 and E3 mice. As with the odor habituation behavioral effect, these effects were observed by 6 months. Together, these results demonstrate a pronounced, ApoE genotype-dependent regulation of olfactory system physiology and behavior that might suggest an explanation for the known ApoE effects on human olfaction.
Precisely how ApoE genotype leads to modified olfactory system function in humans and non-human animals is not known. The observed hyper-excitability induced by ApoE4 could be mediated by a variety of effects, including subtle shifts in excitation/inhibition balance, impaired synaptic homeostasis, synaptic strength, and synaptic connectivity, but also excitotoxicity and epileptogenesis in extreme cases. Using a well-characterized mouse model of human APOE isoform expression that does not develop amyloid plaques or neurofibrillary tangles (Sullivan et al., 1997; Sullivan et al., 2004), we were able to demonstrate that ApoE4 expression directly causes olfactory system abnormality. The genotype-dependent effect, that is independent of AD-associated pathology, is a potential major contributor to human cognitive decline during aging (Haan et al., 1999; Verghese et al., 2011; Mahley and Huang, 2012; Zlokovic, 2013). Further evidence for this link comes from studies in which the lack of brain β-amyloid deposits is confirmed by in vivo neuroimaging, yet individuals with an ApoE4 allele show greater cognitive impairment over time than age- and other risk-matched non-ApoE4 individuals (Sullivan et al., 1997; Haan et al., 1999; Sullivan et al., 2004; Verghese et al., 2011; Villemagne et al., 2011; Andrews et al., 2013; Liu et al., 2013; Reinvang et al., 2013; Zlokovic, 2013). Our findings thus fit well with recent behavioral results suggesting that APOE4-associated odor identification decline starts already in the 5th decade of life, likely before AD-associated pathology emerges (Josefsson et al., 2017).
Additionally, hyper-excitability of neural networks is also induced by amyloid β deposition in both the hippocampal formation (Palop and Mucke, 2016) and the olfactory system (Wesson et al., 2011; Xu et al., 2015). The hyper-excitability observed in human amyloid β precursor protein transgenic mice is associated with inhibitory interneuron dysfunction (Sun et al., 2009; Verret et al., 2012), as is the ApoE4 induced hyper-excitability (Nuriel et al., 2017). Preventing or reversing this amyloid β-induced hyper-excitability can restore cognitive (Sanchez et al., 2012; Verret et al., 2012) and olfactory (Wesson et al., 2011; Cramer et al., 2012) function. Epileptic activity has also been observed in ApoE4 mice (Hunter et al., 2012), and increased risk reported in ApoE4 humans (Palop and Mucke, 2016). Importantly, we show here that the ApoE2 allele, which can have protective effects against AD and other aging related pathologies (Corder et al., 1994; Liu et al., 2013; Suri et al., 2013), induces hypo-excitability in the olfactory system. Thus, if the hyper-excitability induced by ApoE4 and amyloid β is causative for their associated cognitive impairments, the hypo-excitability induced by ApoE2 may contribute to its protective effects. Further work is ongoing to explore mechanism of protective effects of the ApoE2. As noted in the methods, the WT mouse ApoE allele is not identical to any human allele (Raffai et al., 2001). Ergo, comparison of WT mouse ApoE alleles with human ApoE alleles is worthy of a future, separate study.
It is not clear why the olfactory system appears particularly sensitive to ApoE genotype effects. For example, human ApoE4 carriers can demonstrate odor identification impairment in the absence of other detectable cognitive impairments (Olofsson et al., 2010). The olfactory epithelium and olfactory bulb both display robust, life-long neurogenesis, which may make them susceptible to ApoE effects given ApoE’s role in olfactory nerve regeneration (Nathan et al., 2005). However, ApoE4-related olfactory deficits are most often observed in odor identification tasks. Pathology of the epithelium or olfactory bulb might be more likely to result in problems of odor detection or discrimination, which are often intact in ApoE4 humans and mice. Rather, ApoE-related olfactory impairments may reflect more central circuit dysfunction, either in olfactory cortical processing of odor perceptual objects (Gottfried, 2010; Wilson and Sullivan, 2011), or in the transmission of that information to other circuits critical for translating odor information into cognitive representations that are critical for identification (Olofsson et al., 2014). The observed sensitivity of olfactory perception to ApoE genotype is similar to the early appearance of olfactory deficits observed in early stages of mild cognitive impairment leading to AD (Devanand et al., 2000; Devanand et al., 2008; Fischer et al., 2016), though mechanisms are not known in either case.
In summary, we show that the excitability of the olfactory system is regulated by ApoE genotype, with an ApoE4 > ApoE3 > ApoE2 excitability ranking. The hyper-excitability of the olfactory system in ApoE4 mice is associated with impairment of odor habituation memory ability, while the corresponding hypo-excitability observed in ApoE2 mice may be linked to its protective effects. Our results in mice show that behavioral and physiological olfactory effects of ApoE genotype manifest without AD amyloid β or tau pathology, consistent with the sensitivity of the olfactory pathway in humans to show ApoE genotype-dependent effects to manifest early vulnerability in AD.
HIGHLIGHTS.
ApoE genotype does not affect initial odor investigation.
ApoE genotype modulates behavioral odor habituation.
ApoE genotype produces an ApoE4 > ApoE3 > ApoE2 excitability ranking.
Deleterious effects of ApoE4 may be due to induced olfactory hyperexcitability.
Protective effects of ApoE2 may be due to induced olfactory hypoexcitability.
Acknowledgments
We thank Dr. Monika Pawlik for her expert assistance with our mouse colonies. This work was supported by the NIH (P01 AG017617 to EL and PMM, and R01 AG057517 to EL, PMM, and DAW; KP was additionally supported by an NIH postdoctoral research training grant T32-AG052909). JKO was supported by a Pro Futura Scientia VII fellowship and research grants from the Marianne and Marcus Wallenberg Foundation (MMW 2014:0178) and the Swedish Foundation for the Humanities and Social Sciences (M14–0375:1).
Abbreviations
- ApoE
Apolipoprotein E
- AD
Alzheimer’s Disease
- OB
Olfactory Bulb
- PCX
Piriform Cortex
- aPCX
Anterior Piriform Cortex
- LFP
Local Field Potential
- FFT
Fast-Fourier Transform
- NKI
The Nathan S. Kline Institute for Psychiatric Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews KA, Modat M, Macdonald KE, Yeatman T, Cardoso MJ, Leung KK, Barnes J, Villemagne VL, Rowe CC, Fox NC, Ourselin S, Schott JM, Australian Imaging Biomarkers LFSoA Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PloS one. 2013;8:e58816. doi: 10.1371/journal.pone.0058816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of Apolipoprotein E in olfaction. Ann Ny Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby K, Morgan CD, Murphy C. Abnormal event-related potentials in young and middle-aged adults with the ApoE epsilon4 allele. Int J Psychophysiol. 2012;83:276–281. doi: 10.1016/j.ijpsycho.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer’s Disease. Nature Genetics. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BE, Klein R, Tweed TS. Age-Related Sensory Impairments and Risk of Cognitive Impairment. J Am Geriatr Soc. 2016;64:1981–1987. doi: 10.1111/jgs.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WS. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2005;17:541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yasuda M, Tanimukai S, Matsui M, Hirono N, Kazui H, Mori E. Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology. 2001;57:1461–1466. doi: 10.1212/wnl.57.8.1461. [DOI] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, Sudhof TC. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell. 2017;168:427–441 e421. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JM, Cirrito JR, Restivo JL, Kinley RD, Sullivan PM, Holtzman DM, Koger D, Delong C, Lin S, Zhao L, Liu F, Bales K, Paul SM. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 2012;1467:120–132. doi: 10.1016/j.brainres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Hussain A, Luong M, Pooley A, Nathan BP. Isoform-specific effects of apoE on neurite outgrowth in olfactory epithelium culture. J Biomed Sci. 2013;20:49. doi: 10.1186/1423-0127-20-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, Raber J. ApoE2 Exaggerates PTSD-Related Behavioral, Cognitive, and Neuroendocrine Alterations. Neuropsychopharmacology. 2015;40:2443–2453. doi: 10.1038/npp.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson M, Larsson M, Nordin S, Adolfsson R, Olofsson J. APOE-varepsilon4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. 2017;7:1286. doi: 10.1038/s41598-017-01508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Chung HG, Shin HS, Kim SJ, Choi JH, Chung MY, An SK, Choi TK, So HS, Cho HS. Apolipoprotein E gene polymorphism, alcohol use, and their interactions in combat-related posttraumatic stress disorder. Depress Anxiety. 2013;30:1194–1201. doi: 10.1002/da.22138. [DOI] [PubMed] [Google Scholar]
- Kowalewski J, Murphy C. Olfactory ERPs in an odor/visual congruency task differentiate ApoE epsilon4 carriers from non-carriers. Brain Res. 2012;1442:55–65. doi: 10.1016/j.brainres.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Krastins B, Ning M. The role of apolipoprotein E in neurodegeneration and cardiovascular disease. Expert Rev Proteomics. 2014;11:371–381. doi: 10.1586/14789450.2014.901892. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease - A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol-Chicago. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Murphy C. Individuals at risk for Alzheimer’s disease show differential patterns of ERP brain activation during odor identification. Behav Brain Funct. 2012;8:37. doi: 10.1186/1744-9081-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Nisar R, Short J, Randall S, Grissom E, Griffin G, Switzer PV, Struble RG. Delayed olfactory nerve regeneration in ApoE-deficient mice. Brain Res. 2005;1041:87–94. doi: 10.1016/j.brainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Nuriel T, Angulo SL, Khan U, Ashok A, Chen Q, Figueroa HY, Emrani S, Liu L, Herman M, Barrett G, Savage V, Buitrago L, Cepeda-Prado E, Fung C, Goldberg E, Gross SS, Hussaini SA, Moreno H, Small SA, Duff KE. Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nature communications. 2017;8:1464. doi: 10.1038/s41467-017-01444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-4 is independent of clinical dementia. Neurobiol Aging. 2010;31:567–577. doi: 10.1016/j.neurobiolaging.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Hurley RS, Bowman NE, Bao X, Mesulam MM, Gottfried JA. A designated odor-language integration system in the human brain. J Neurosci. 2014;34:14864–14873. doi: 10.1523/JNEUROSCI.2247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Josefsson M, Ekstrom I, Wilson D, Nyberg L, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson LG, Larsson M. Long-term episodic memory decline is associated with olfactory deficits only in carriers of ApoE-4. Neuropsychologia. 2016;85:1–9. doi: 10.1016/j.neuropsychologia.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng KY, Mathews PM, Levy E, Wilson DA. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience. 2017;343:364–371. doi: 10.1016/j.neuroscience.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The Distribution of Tangles, Plaques and Related Immunohistochemical Markers in Healthy Aging and Alzheimers-Disease. Neurobiology of Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Raffai RL, Dong LM, Farese RV, Jr, Weisgraber KH. Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc Natl Acad Sci U S A. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT. APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neurosci Biobehav Rev. 2013;37:1322–1335. doi: 10.1016/j.neubiorev.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. 2012;109:E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sun B, Halabisky B, Zhou Y, Palop JJ, Yu G, Mucke L, Gan L. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer’s Disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013;37:2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kawamata J, Shimohama S, Akaki H, Akiguchi I, Kimura J, Ueda K. Inferior temporal lobe atrophy and APOE genotypes in Alzheimer’s disease. X-ray computed tomography, magnetic resonance imaging and Xe-133 SPECT studies. Dement Geriatr Cogn Disord. 1998;9:90–98. doi: 10.1159/000017029. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of Neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J Neurosci. 2011;31:15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Fitzgerald S, Nixon RA, Levy E, Wilson DA. Early hyperactivity in lateral entorhinal cortex is associated with elevated levels of AbetaPP metabolites in the Tg2576 mouse model of Alzheimer’s disease. Exp Neurol. 2015;264:82–91. doi: 10.1016/j.expneurol.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein e: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]


