Supplemental digital content is available in the text.
Abstract
Background
Prolonged warm ischemia time (WIT) is associated with graft failure and mortality, however less is known about factors associated with prolonged WIT.
Methods
In a cohort of United States deceased donor kidney transplant recipients identified using the Scientific Registry of Transplant Recipients (Jan 2005-Dec 2013), we identified factors associated with prolonged WIT (defined as ≥ 30 minutes versus 10-30 minutes) using hierarchical multilevel models adjusting for center effect, and WIT as a continuous variable using multiple linear regression of log-transformed data.
Results
Among 55 829 patients, potentially modifiable risk factors associated with prolonged WIT included increased recipient body mass index (BMI) (odds ratio [OR], 1.57; 95% confidence interval [CI], 1.44-1.72 for BMI > 35), right donor kidney (OR, 1.14; 95% CI, 1.08-1.19), and a prolonged cold ischemic time (OR, 1.23; 95% CI, 1.13-1.33 for cold ischemia time > 24 hours). Transplanting a right kidney into an obese recipient further prolonged WIT (OR, 1.75; 95% CI, 1.55-1.98; for BMI > 35), increasing overall WIT by 11.0%. There was no correlation between median WIT for a given center and annual center transplant rate (pairwise correlation coefficient, 0.0898).
Conclusions
In conclusion, several modifiable factors are associated with prolonged WIT and may represent strategies to improve WIT and subsequent posttransplant outcomes.
Prolonged renal graft ischemia has been associated with early and delayed adverse outcomes after deceased donor kidney transplantation. There are 3 potential periods for ischemic injury during kidney transplant. The first includes the period before organ retrieval in the donor, referred to as “donor warm-ischemia time.”1 Second, there is a period of cold ischemia time (CIT), defined as the period wherein the kidney is transported from donor to recipient on ice or pump perfused under hypothermic conditions.2 This is followed lastly by a period of warm ischemia time (WIT) during reanastomosis in the recipient (“recipient WIT”) between the period that the kidney is taken out of cooling and the time that it is reperfused by the recipient's blood.1
A prolonged recipient WIT has been associated with poor short- and long-term graft outcomes. Irish et al3 demonstrated a linear relationship between WIT and delayed graft function in a risk prediction model using United Network for Organ Sharing data, though the effect was found to be small. A positive association between prolonged WIT and days to hospital discharge posttransplant has been demonstrated4 and more recently, 2 large-scale cohort studies have shown an association between WIT and reduced long-term graft survival (hazard ratio, 1.23; 95% confidence interval [CI], 1.15-1.33, for death or graft loss with 60 minutes or longer WIT relative to a reference category with 10 to 20 minutes WIT.5,6
Despite the association between WIT and transplant outcomes, little is known about the modifiable and nonmodifiable donor, recipient and transplant center factors associated with prolonged WIT. This information may lead to novel strategies to reduce WIT and patient risk. Therefore, the purpose of this study is to identify risk factors that are associated with prolonged WIT in a cohort of deceased donor kidney transplant recipients in the United States.
MATERIALS AND METHODS
Data Source and Study Population
Data from the Scientific Registry of Transplant Recipients (SRTR) were used for this analysis. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the Organ Procurement and Transplantation Network (OPTN) and SRTR contractors.
The study population included all patients receiving a solitary deceased donor kidney transplant in the United States, between January 1, 2005, and December 31, 2013. These dates were chosen due to the large amount of missing WIT data before 2005. We excluded recipients of living donor kidneys, patients younger than 18 years of age, those receiving multiple organs, en bloc or sequential transplants and patients with missing values for WIT. Warm ischemia time less than 10 minutes and longer than 200 minutes were excluded because these were assumed to represent miscoded values.
Outcome
The outcome of interest was recipient WIT defined as the anastomosis time between the kidney being removed from cooling to the time it was reperfused by the recipient's blood.5 For the primary analysis, WIT was dichotomized as 10 to less than 30 minutes (referent category) and 30 to 200 minutes (prolonged WIT).5 In a secondary analysis, WIT was treated as a log-transformed continuous variable.
Covariates
Donor, recipient, and transplant center factors were collected from SRTR, including donor and recipient age, race, sex, body mass index, donor type (standard versus expanded criteria; donation by cardiac versus neurologic death), CIT, recipient end-stage renal disease cause, dialysis vintage, prior kidney transplant, medical comorbidities, year of transplant, and donor kidney side.
Average annual center transplant rates were determined by calculating the total number of patients transplanted at a given center (both living and deceased) divided by the number of years over which that center performed transplants within the study period. The median center WIT was graphed in a scatter plot against the annual center transplant rate, and a Pearson correlation coefficient was determined.
Primary and Secondary Analyses
Descriptive statistics were used to report baseline characteristics stratified by WIT (10 to < 30 minutes and 30 to 200 minutes). Counts and percentages were used to describe categorical variables, and means and standard deviations or medians and quartiles were used for continuous normal and continuous nonnormally distributed variables, respectively.
The adjusted odds of prolonged WIT (30 to 200 minutes compared with 10 to < 30 minutes) was determined using a multivariable logistic regression analysis. In a secondary analysis, multivariable linear regression was used to determine factors associated with prolonged WIT. Warm ischemia time was log-transformed because it was nonparametric with a right skew. To account for center level variation in WIT, transplant center was included in all models as a random effect. In each analysis, variables with missing data were assigned a category of “missing” to allow inclusion of all patients in the models.
Sensitivity and Subgroup Analyses
Baseline characteristics were compared between those with recipient WIT reported, those with extreme WIT values excluded, and those with missing WIT.
In a sensitivity analysis, we repeated our primary analysis using a cut point of 45 minutes or longer to define prolonged WIT.
In a subgroup analysis, we analyzed the primary outcome for centers with 75% or greater reporting of recipient WIT, because it was felt that this may lead to reporting bias.
We examined the interaction of body mass index (BMI) and kidney side and their association with prolonged WIT because earlier studies have suggested transplantation of a right donor kidney may be more technically challenging than left, and even more so in obese recipients.2,7
To characterize the transplant center level variation in WIT, we determined percentiles of the median recipient WIT across transplant centers.
All statistical analyses were performed using Stata version 13.1 (Stata Corp., College Station, TX). Institutional ethics approval to conduct this study was provided by the Nova Scotia Health Authority research ethics board.
RESULTS
After relevant exclusions, 55 829 patients were included in the primary analysis, Figure S1, http://links.lww.com/TXD/A78.
Of these, 43 564 patients (78.0%) had a WIT between 30 and 200 minutes. A comparison of the characteristics of our study population versus eligible patients with missing WIT data or extreme WIT measures is shown in Table S1, http://links.lww.com/TXD/A78. There were no clinically meaningful differences between the 3 groups.
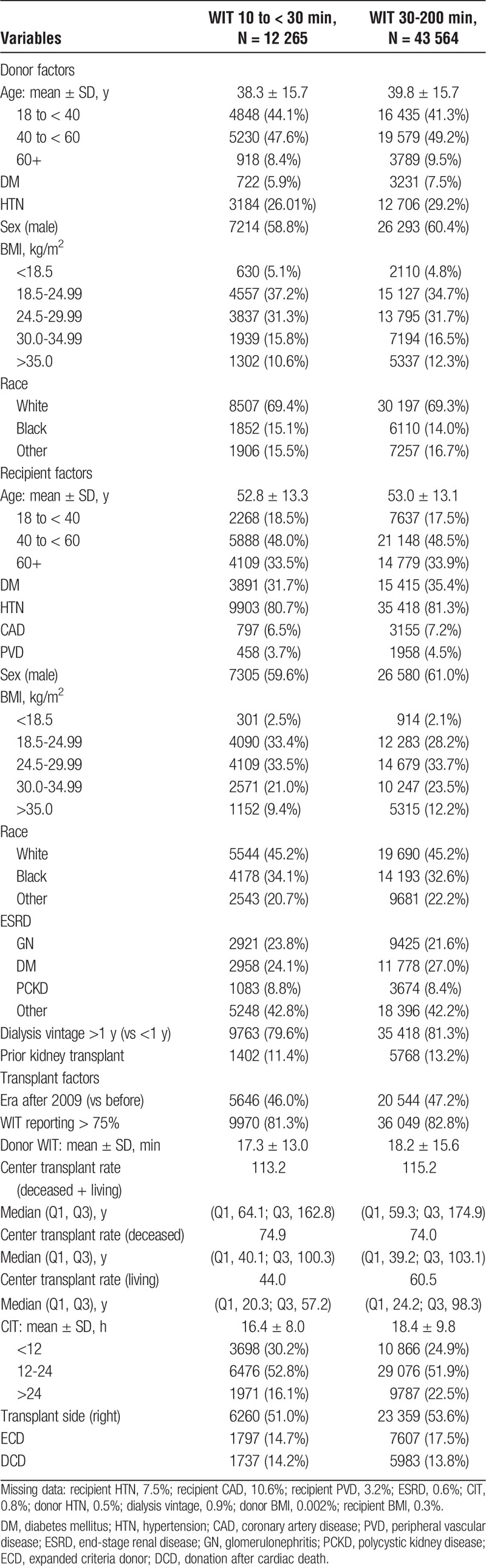
Warm ischemia time followed a slight right-skewed distribution with a mean WIT of 40.5 ± 17.5 minutes and median WIT of 37 minutes (Q1, 30; Q3, 46). Baseline characteristics stratified by WIT (10 to < 30 vs 30 to 200 minutes) are noted in Table 1.
TABLE 1.
Baseline characteristics
Primary Analysis
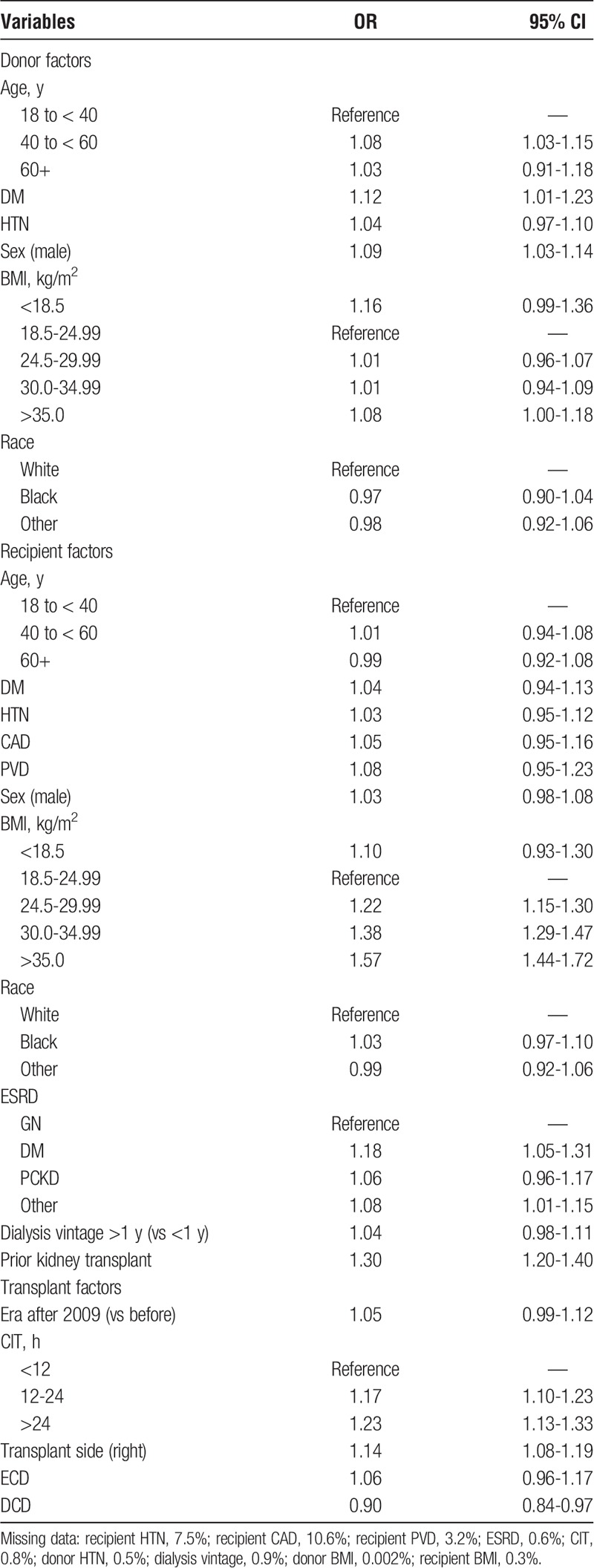
Factors associated with prolonged WIT are shown in Table 2. Male donor sex, donor age 40 to younger than 60 years vs 18 to younger than 40 years, donor history of diabetes, recipient BMI greater than 25 kg/m2, end-stage renal disease due to diabetes or “other,” history of a prior kidney transplant, receiving a right donor kidney versus left, and a prolonged CIT (>24 hours vs < 12 hours) were all associated with a prolonged WIT.
TABLE 2.
Multivariable logistic regression model for donor, recipient, and transplant variables associated with a prolonged recipient WIT (≥30 minutes) treating transplant center as a random effect
Secondary Analysis
Multivariable linear regression confirmed the association between recipient BMI greater than 25 kg/m2 and prolonged recipient WIT, with 8.8% longer WIT in class II and class III obese recipients (BMI, > 35 kg/m2). Additionally, transplantation of a right versus left donor kidney prolonged minutes of WIT by 2.1%. For every additional hour of CIT, WIT was prolonged by 0.5%. A CIT longer than 24 hours prolonged WIT by 8.6% relative to less than 12 hours of CIT (Table S2). This was determined using the exponential function of e to the beta-coefficient for log-transformed WIT for more than 24 hours of CIT (β = 0.0827) versus the reference category of less than 12 hours. For example, e0.0827 = 1.086, or an increase of 8.6% compared with the reference category.
Sensitivity Analyses
In a sensitivity analysis, we analyzed factors that were associated with a very long WIT (≥45 minutes, n = 17,688), based on quartiles of WIT. These followed a similar trend as in the primary analysis, with exaggerated odds ratios (ORs) (for example, for recipient BMI > 35 kg/m2: OR, 1.98; 95% CI, 1.76-2.23; Table S3, http://links.lww.com/TXD/A78). Restricting the cohort to only those centers with ≥ 75% reporting on WIT did not considerably change the study results (Table S4, http://links.lww.com/TXD/A78).
Center Level Variation in WIT
Variability in WIT between centers was explored by comparing percentiles of WITs between centers. WIT varied markedly across centers. The median WITs in the centers at the 10th and 90th percentiles were 30 and 47 minutes, respectively. There was no association between annual center transplant rate and median center WIT (Pearson correlation coefficient = 0.0898; Figure S2, http://links.lww.com/TXD/A78). The shortest median center WIT (13 minutes) was from a center performing 28 transplants annually, whereas the longest median center WIT (87 minutes) was from a center performing 72 transplants annually.
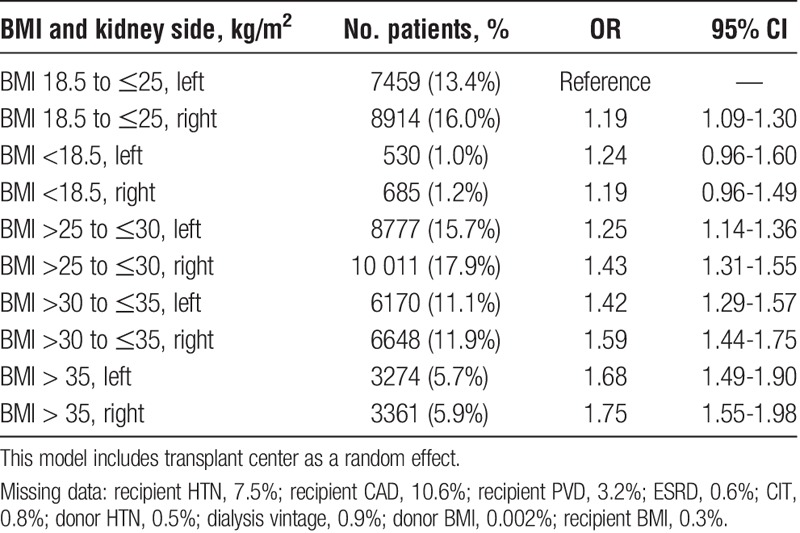
In a second sensitivity analysis, donor kidney side in obese recipients was investigated for its association with prolonged WIT (Table 3). Transplantation of a right donor kidney into an obese recipient was the highest risk for prolonged WIT overall (OR, 1.75; 95% CI, 1.55-1.98 for BMI > 35 kg/m2). Using multiple linear regression, transplanting a right kidney into an obese recipient prolonged WIT by 11.0% compared with transplanting a left kidney into a nonobese recipient. The interaction between donor kidney side and recipient BMI was small but significant (mean recipient BMI 28.3 ± 5.7 for left-sided kidney transplant, and 28.0 ± 5.6 for right-sided kidneys, P < 0.001 by Wilcoxon rank-sum).
TABLE 3.
Multivariable logistic regression model examining the interaction of BMI and kidney side and their association with prolonged WIT (30-200 minutes)
DISCUSSION
In this study, we identified an association between modifiable and nonmodifiable donor, recipient, and transplant factors and prolonged WIT. Male donor sex, older donor age, a right donor kidney, higher recipient BMI, end-stage renal disease due to diabetes, prior kidney transplant, and a prolonged CIT were all associated with a prolonged WIT.
Multiple previous studies (including those conducted out of our institution) have shown that a prolonged recipient WIT is associated with adverse outcomes in the early posttransplant period including delayed graft function, prolonged hospital admission,4 and graft loss.5,6 Given the association between WIT and outcome, identifying potentially modifiable factors contributing to prolongation of WIT is of importance.
Our study identified obesity in the recipient as a risk factor for prolonged WIT. We presume that this relates to the increased technical challenge when operating on obese individuals, with recent studies showing obesity to be independently associated with prolonged operation times in nontransplant surgeries.8 Obesity in the donor would be less expected to influence recipient anastomosis time as was shown in this study. Similar to Heylen et al,6 we also demonstrated that transplanting a right donor kidney versus left was associated with prolonged WIT. It has been proposed that the shorter right renal vein may prolong venous anastomosis time compared with the left,2,7,9 and that right kidneys are more inclined to have multiple vessels and longer arteries which may be susceptible to kinking, all of which may potentially prolong WIT.7 The surgical challenges associated with right donor kidneys are likely exaggerated in obese recipients with deep iliac vessels.7 Although we did not have information about the number of vessels and arterial length, our study confirmed this hypothesis with the highest relative odds observed for prolonged WIT occurring in obese recipients when transplanted with a right sided donor kidney.
Interestingly, our study showed that a longer CIT is associated with prolonged WIT: The cause for this is unclear. It may relate to a tendency for smaller centers to transfer more complex anatomical kidneys to tertiary centers, increasing both CIT transfer time and WIT due to increasingly complex surgical anatomy. It would be interesting to know if this relationship persists when adjusted for the use of pump perfusion; however, unfortunately, because of the very large amounts of missing data, we were unable to adjust for perfusion versus ice transport. To date, there is no literature to suggest that a prolonged CIT in itself leads to prolongation of WIT; however, this requires further exploration.
We showed that there was variability in WIT between centers, but unexpectedly, annual center transplant rate did not associate with WIT. We anticipated that centers with higher annual transplant rates may have more experienced surgeons (greater annual operative rates for a given procedure) who frequently perform the same procedure, thereby becoming more proficient with shorter operative times.10 A potential confounder however may be that centers performing more annual transplants are more likely to be academic centers with trainees involved in surgical practice.11 This may reflect a slower operation due to surgical fellows learning the technical skills for anastomosis; however, we did not have information on academic versus nonacademic practice to confirm this hypothesis.
Importantly, aside from recipient weight loss and reduction of CIT which are already advocated for and often difficult factors to modify, the use of a left donor kidney rather than right is a modifiable intervention which appears to be associated with shorter WIT. Most deceased donors contribute 2 kidneys with the side of transplant being chosen for a number of reasons including the surgeon’s discretion. If anatomically feasible, recipients with other predictors of prolonged WIT (for example, obesity) may benefit from transplantation with a left kidney to minimize this risk. In fact, some centers are already allocating right kidneys in a nonrandom manner to recipients with lower BMI’s and shorter CITs.2
This study was conducted using the SRTR which is a robust record of United States kidney transplant recipients used in many earlier studies. There are limitations to our study, however. As with any retrospective analysis of registry data, there is the potential for miscoding or misclassification, but any coding error would be expected to be distributed evenly between the reference and prolonged WIT categories and thus would be unlikely to significantly bias results. Additionally, we excluded extremes of WIT (WIT, < 10 and > 200 minutes) which were felt to likely represent coding errors. Although there is a potential for reporting bias (acknowledging that there were many missing WITs), the study findings were similar after restricting the cohort to those centers wit h ≥ 75% reporting. Although we had data on a number of potential factors associated with WIT, we did not have data regarding the number of transplant blood vessels. This has been identified as an important factor associated with prolongation of WIT.6 Furthermore, we did not have data about academic versus nonacademic centers. Our annual transplant rate was determined by dividing the total number of transplants done at a given center by the number of years that that center was performing transplants. This implies a stable annual transplant rate which may be a misrepresentation. Centers with low kidney transplant rates initially who then expanded to achieve very high transplant rates over the study period would have their annual transplant rates averaged out to a single rate over the years. Lastly, any changes in modifiable risk factors for prolonged WIT would need further investigation to determine if they also affect posttransplant outcomes above and beyond simply reducing WIT.
CONCLUSIONS
This study identifies donor, recipient, and transplant factors associated with prolonged WIT and highlights potential therapeutic targets to reduce anastomosis time. Acknowledging the association between WIT and outcome, identifying and applying mechanisms to minimize WIT is an important consideration for future study and may have implications for short- and long-term graft outcomes.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Footnotes
Published online 17 April, 2018.
The authors declare no funding or conflicts of interest.
A.V. is the primary author who completed the initial analysis and wrote the first draft of the article. C.R. and A.O. provided assistance with the analysis and study design, as well as with writing the manuscript. B.K., J.K., and I.A. reviewed the proposed study plan and provided feedback regarding analyses and content. K.T. oversaw the entire project from original study design to analysis and article review. All authors reviewed and approved the final article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Moers C, Smits HM, Maathuis MHJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. . 2009;360:7–19. [DOI] [PubMed] [Google Scholar]
- 2.Vad der Vliet JA, Warle MC. The need to reduce cold ischemia time in kidney transplantation. . 2013;18:174–178. [DOI] [PubMed] [Google Scholar]
- 3.Irish WD, Ilsley JN, Schnitzler MA, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. . 2010;10:2279–2286. [DOI] [PubMed] [Google Scholar]
- 4.Marzouk K, Lawen J, Alwayn I, et al. The impact of vascular anastomosis time on early kidney transplant outcomes. . 2013;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tennankore KK, Kim SJ, Alwayn IP, et al. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. . 2016;89:648–658. [DOI] [PubMed] [Google Scholar]
- 6.Heylen L, Pirenne J, Samuel U, et al. The impact of anastomosis time during kidney transplantation on graft loss: a Eurotransplant Cohort Study. . 2017;17:724–732. [DOI] [PubMed] [Google Scholar]
- 7.Janschek EC, Rothe AU, Holzenbein TJ, et al. Anatomic basis of right renal vein extension for cadaveric kidney transplantation. . 2004;63:660–664. [DOI] [PubMed] [Google Scholar]
- 8.Tjeertes EEKM, Hoeks SSE, Beks SSBJC, et al. Obesity—a risk factor for postoperative complications in general surgery? . 2015;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vacher-Coponat H, McDonald S, Calyton P, et al. Inferior early posttransplant outcomes for recipients of right versus left deceased donor kidneys: an ANZDATA registry analysis. . 2013;13:399–405. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. . 2010;145:634–640. [DOI] [PubMed] [Google Scholar]
- 11.Ross SW, Oommen B, Kim M, et al. A little slower, but just as good: postgraduate year resident versus attending outcomes in laparoscopic ventral hernia repair. . 2014;28:3092–3100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


