Abstract
Background
Biliary strictures (BS) are common complication after liver transplantation. We aimed to determine the accuracy of magnetic resonance cholagiopancreatography (MRCP) in diagnosing BS in liver transplant recipients (LTRs) when compared to direct cholangiographic methods (endoscopic resonance cholagiopancreatography [ERCP] and/or percutaneous transhepatic cholangiography [PTC]).
Methods
Retrospective chart review of 910 LTRs (July 2008 to April 2015) was performed, and a total of 39 patients with duct-to-duct anastomosis (22 males; 56.4%; mean age, 52.8 ± 8.3 years) were included who had an MRCP followed by either ERCP and/or PTC within 4 weeks. A cholangiographic narrowing (on ERCP and/or PTC) that required balloon dilation and/or stent placement was considered a BS and was considered clinically significant if the intervention resulted in at least 30% improvement of bilirubin within 2 weeks. Sensitivity, specificity, accuracy, positive predictive values and negative predictive values of MRCP in diagnosing BS were calculated.
Results
Magnetic resonance cholagiopancreatography showed anastomotic BS in 17 of 39 patients, and subsequent ERCP and/or PTC revealed a total of 25 BS (positive predictive value of 0.94). Nine BS on cholangiography (ERCP, 8; PTC, 1) were not detected on earlier MRCP (sensitivity, 0.64; 95% CI, 0.45-0.82); 2 were clinically significant BS and 6 of the remaining 7 had no improvement in their liver function test with biliary intervention. Thirteen LTRs had no BS on either modality (specificity, 0.93; 95% CI, 0.66-0.99). The negative predictive value of MRCP was 0.59 for cholangiographic BS. The overall accuracy of MRCP is 0.74 (exact 95% CI, 0.58-0.87). Inclusion of age, race, and alanine aminotransferase level improved the predictive value of MRCP (area under the curve = 0.94, 95% CI: 0.86-1.00).
Conclusions
Magnetic resonance cholagiopancreatography has high specificity but low sensitivity in diagnosing cholangiographic BS in LTRs, although the predictive value further improved with inclusion of age, race, and alanine aminotransferase. Clinical significance of BS in LTRs not identified on MRCP is questionable because ERCP with intervention did not improve their liver function tests in the vast majority.
Biliary strictures (BS) are an important complication encountered in the posttransplant setting. In fact, these are the second leading cause of morbidity (after graft rejection) in the post-liver transplant (LT) setting with an estimated incidence of 5% to 30% after orthotopic LT and a mortality rate of up to 10%.1 The incidence of biliary complications does decrease after about a year of LT.2 Although endoscopic retrograde cholangiography is the gold standard test for diagnosis of BS and commonly performed in these patients but its invasive nature and adverse event profile (up to 10%3) have prompted increasing use of magnetic resonance cholangiopancreatography (MRCP) in this setting.
Magnetic resonance cholagiopancreatography has been shown to have a sensitivity and specificity of 97% and 98%, respectively, in identifying BS.4 However, this meta-analysis4 (67 studies) did not specifically evaluate post-LT strictures. This is important because upstream biliary dilation, if a distal stricture is present, is almost always present in a native liver (in the absence of parenchymal liver disease) but maybe absent in up to 50% of LT recipients (denervation and/or fibrosis of donor ducts).5
At our center, endoscopic resonance cholagiopancreatography (ERCP) and/or percutaneous transhepatic cholangiography (PTC) have been the mainstay of managing suspected post-LT BS. With the advent and availability of MRCP, the trend has shifted toward this noninvasive modality first, followed by ERCP or PTC, if ductal pathology is found. Lack of adverse events, noninvasive and accurate nature of MRCP is the basis of this trend change. What is not clearly known is if these accurate results of MRCP reported in literature hold true in the post-LT setting.
MATERIALS AND METHODS
This was a retrospective study, done to assess accuracy of MRCP when compared with ERCP in diagnosing BS in post-LT setting. Our center is a tertiary care liver transplant center (with over one hundred LTs a year). Institutional review board approval was obtained before the start of data collection. We performed a retrospective chart review of all LTs from July 2008 until April 2015.
The search strategy was to identify patients who, in the post-liver transplant period, had an MRCP followed by either ERCP or PTC to evaluate a suspected anastomotic BS. Furthermore, direct cholangiography had to be performed within 28 days of MRCP for those patients to be included. This inclusion criterion was used to minimize any bias that may occur as a result of progression or regression of the biliary pathology. We did not include patients with ERCP or PTC performed without prior MRCP or if it occurred more than 28 days after MRCP. Similarly, those patients in whom MRCP was not followed by direct cholangiographic evaluation were excluded. Because the main purpose of the study was to compare the diagnostic accuracy (for anastomotic BS) of MRCP and direct cholangiographic methods, we also excluded those patients who underwent surgery directly after an MRCP (even if a stricture was confirmed at surgery) or had nonanastomotic BS.
A total of 910 charts were reviewed of which we identified a total of 39 patients that fulfilled the inclusion criteria. We collected demographic and clinical information that included LT indication, type of immunosuppression and anastomosis, indirect and direct cholangiographic information, liver function test (LFT) values, among others (Table 1).
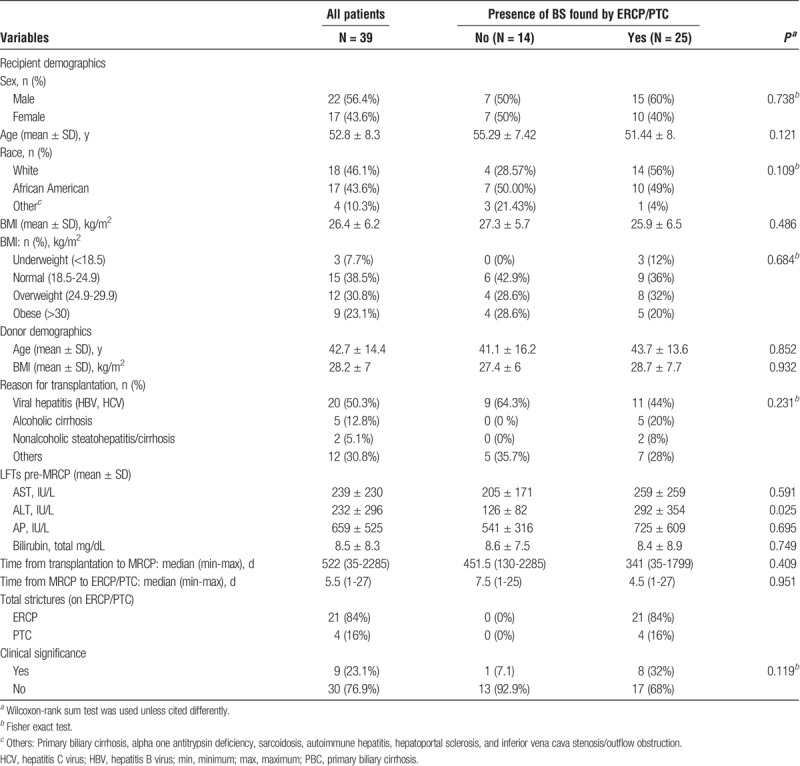
TABLE 1.
Characteristics of the 39 patients included in the study
MRCP Technique
Magnetic resonance examinations were performed on the 1.5 T General Electric magnet (GE, Boston, MA). Although a few early studies were performed on a 3T General Electric magnet. They were either MRCP studies dedicated to the biliary tree or part of a complete MRI abdomen examination. There were 4 sequences dedicated to the biliary tree.
On the 1.5 T magnet, this consisted of the following: axial T2 single shot fast spin echo with fat saturation, 8 mm thickness/1 mm gap; repetition time (TR), 550-635; echo time (TE), 88; axial single shot fast spin echo without fat saturation, 8 mm thickness/1 mm gap; TR, 550-635; TE, 88; coronal T2 single shot fast spin echo with fat saturation, 4 mm thickness/0 mm gap; TR, 850-113; TE, 201; and coronal 3D respiratory triggered fast spin echo, 1.4 mm thickness/0 mm gap reconstructed with 50% overlap; TR, 3750; TE, 505-515.
On the 3 T magnet, the series consisted of the following: axial single shot fast spin echo without fat saturation, 6 mm thickness/1 mm gap; TR, 1030-1125; TE, 139-141; coronal single shot fast spin echo without fat saturation, 7 mm thickness/2 mm gap; TR, 1472-1599; TE, 139; coronal thin single shot fast spin echo, 4 mm thickness/0 gap; TR, 1472-1614; TE, 139-141; 3D fast recovery respiratory triggered fast spin echo, 1.2 mm thickness/0 gap reconstructed with 50% overlap; TR, 4615-500; TE, 663-710.
All of the examinations were technically adequate except for 2 examinations on the 3 T magnet for which the images were somewhat compromised due to dielectric effect from large volume ascites and patient motion.
Definition of Stricture
Anastomotic stricture was defined as a significant narrowing of the biliary tree at the anastomotic site, with or without upstream biliary dilation on MRCP per the judgement of the radiologist interpreting the images. Narrowing at the anastomosis seen on the direct cholangiographic methods was considered a stricture only if it required either balloon dilation and/or stent placement (cholangiographic stricture). Any narrowing at the anastomosis that did not require any of these 2 interventions (irrespective of MRCP description) was not considered a stricture for the purpose of analysis. Once a stricture was defined cholangiographically, it was considered clinically significant only if the intervention (dilation or stent placement) resulted in at least 30% improvement in bilirubin within the following 2-week period.
Immunosuppression Protocol
Routinely our center has been using a steroid free immunosuppression protocol which consisted of induction immunosuppression with rabbit antithymocyte globulin given at 3 mg/kg in 2 divided doses of 1.5 mg/kg; the first dose given during the anhepatic phase, and the second dose given on posttransplant day 2. A single dose of 500 mg intravenous methylpredisolone is administered as premedication before the first dose of rabbit antithymocyte globulin to minimize cytokine release syndrome. Mycophenolate mofetil (MMF) is initiated on posttransplant day 1 at a dose of 1000 mg 2 times per day for a total of 3 months and then discontinued unless the patient's primary disease was autoimmune hepatitis, primary biliary cirrhosis, or primary sclerosing cholangitis. The MMF dose and administration frequency adjustments are made for gastrointestinal side effects or the development of cytopenias. The initiation of tacrolimus is delayed for a minimum of 3 days and a maximum of 7 days and started when the serum creatinine is less than 2.0 mg/dL. Primary sirolimus is used in lieu of tacrolimus if the recipient's creatinine level remained over 2.0 mg/dL beyond posttransplant day 7; patients receive an initial dose of 5 mg daily with daily trough levels after the first dose. Goal trough levels for tacrolimus and sirolimus during the first 3 months postoperatively are 6 to 8 ng/dL and 5 to 8 ng/dL, respectively. Patients with biopsy-proven rejection are initially treated with increasing doses of tacrolimus with a goal trough level of 10 to 12 ng/dL. Second-line therapy include the addition of sirolimus or restarting MMF. Steroid treatment is reserved for those patients with rejection resistant to this protocol or those who can't tolerate increased tacrolimus doses. In the current analysis, 35 patients (89.7%) were on tacrolimus, 3 (7.7%) on mycophenolate, 8 (20%) on rapamycin, 2 (5%) on everolimus, and 1 (2.5%) on cyclosporine at the time of MRCP.
Statistical Analysis
Descriptive statistics were performed for all of the key variables. Frequencies and percentages were measured for categorical variables; mean and standard deviation were calculated for continuous variables. Comparison of categorical variables were made using Fisher exact test or χ2 test; Student t test or Wilcoxon Rank-Sum tests were applied for comparison of continuous variables. Wilcoxon-signed rank tests were used to compare between matched data points, for example, preintervention and postintervention measures on liver function panel. Sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV) of MRCP in diagnosing BS were calculated. Relationship between diagnosing accuracy of MRCP and the predictor variables were investigated through multivariable logistic regression model. Statistical analyses were performed using statistical analysis software (SAS) version 9.3 (SAS Institute Inc., Cary, NC). The level of statistical significance for analyses was set at P less than 0.05 unless otherwise stated. Multiple hypothesis testing was adjusted using the false discovery rate method (proc multtest in SAS). Sensitivity, specificity, accuracy, PPV, and NPV are described in terms of true positives (TP), true negatives (TN), false negatives (FN) and false positives (FP) (Table 1), and Accuracy defined as (TN + TP)/(TN + TP + FN + FP).
RESULTS
Of the 910 liver transplant recipients (LTRs), 39 patients were included in the study who met the inclusion criteria. Among those, 25 patients had cholangiographic BS as found by ERCP/PTC. They were majority male (56.4%), white (46.1%), normal or overweight (53.9% combined), and about 53 years old (Table 1).Age, race, alanine aminotransferase (ALT) (pre-MRCP), and etiology of liver disease leading to LT showed a potential association with BS (P < 0.2, Table 1); however, sex (P = 0.738), BMI (P = 0.684), aspartate aminotransferase (AST) (P = 0.591), alkaline phosphatase (AP) (P = 0.695), and total bilirubin (P = 0.749) did not predict the presence of BS.
In the included cohort of patients, hepatitis B and C virus–related cirrhosis were the leading underlying etiology for LT (50.3%), followed by alcohol related disease (12.8%) and nonalcoholic steatohepatitis (5.1%) (Table 1). Other etiologies (30.8% combined) included primary biliary cirrhosis, alpha one antitrypsin deficiency, sarcoidosis, autoimmune hepatitis, hepatoportal sclerosis, and inferior vena cava stenosis/outflow obstruction. For those that had anastomotic BS, hepatitis B and C related cirrhosis were the underlying etiology for LT in 44%. Reason for transplantation did not predict the presence of BS (P = 0.231).
Endoscopic resonance cholagiopancreatography and PTC revealed a total of 25 BS (ERCP, 21; PTC, 4). Type of anastomosis was duct-to-duct in all patients, as we did not include any with bilio-digestive anastomosis. All patients had MRCP followed by either ERCP or PTC within 4 weeks. None of the included patients had any ERCP and PTC performed before the MRCP. The median time from LT to MRCP was not significantly different between the stricture and nonstricture groups (P = 0.409). Patients with BS had MRCP performed at a median of 341 days after LT with the minimum and maximum of 35 and 1799 days, respectively. Among the 25 BS that ERCP/PTC identified, 8 of them were clinically significant, that is, these patients had their bilirubin level decreased more than 30% compared to their pre-MRCP levels (Table 1).
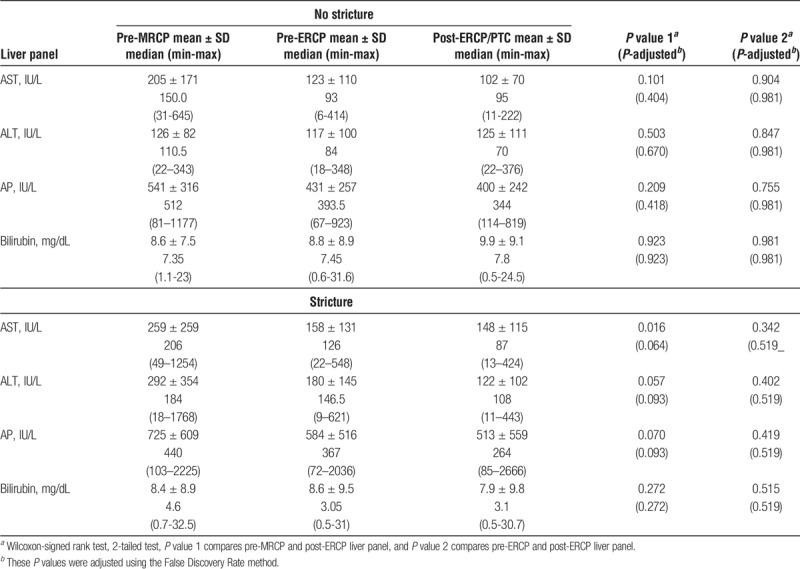
All measurements of LFTs such as AST, ALT, AP and bilirubin were all elevated before MRCP (Table 1). After ERCP/PTC intervention, there was a significant reduction in the levels of AST (P adjust = 0.012), and a trend for overall improvement in the ALT (P adjust = 0.084), and AP (P adjust = 0.058) after adjusting for multiple hypothesis testing using the false discovery rate method; but not bilirubin (P = 0.414). These improvements were indeed pertained to those that had strictures (Table 2). Among those that did not have strictures, post-ERCP/PTC intervention, a lower AST, ALT, and AP levels were noted, but these reductions did not reach statistically significant.
TABLE 2.
LFTs measures before and after ERCP/PTC intervention between stricture and nonstricture patients
MRCP Had Low Sensitivity, But High PPV
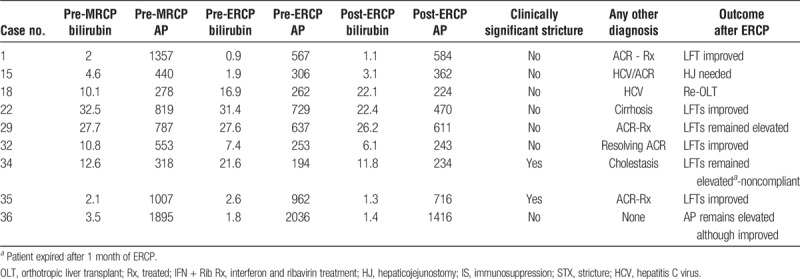
Magnetic resonance cholagiopancreatography showed anastomotic BS in 17 of 39 patients. Among these 17 cases, 16 were confirmed by ERCP/PTC. However, it failed to identify 9 other patients with anastomotic BS, which were subsequently found by ERCP, leading to a sensitivity of 0.64 for cholangiographic BS with 95% confidence interval (CI) (0.45, 0.82) (Table 3). However, only 2 of those 9 FN cases (MRCP negative but ERCP/PTC positive) were clinically significant (Table 4). Of those two, 1 had cirrhosis, 1 had resolving acute cellular rejection (ACR) (treated 3 weeks earlier) (Table 4). In the rest 7 patients, no clinical improvement in LFTs (Bilirubin reduction > 30% postintervention) was noted. Additionally, 6 out of the 7 patients had alternate explanations for elevated LFTs; recurrent hepatitis C (n = 1), acute cellular rejection (n = 3; 1 also concurrent hepatitis C virus), severe cholestasis of unclear etiology (n = 1), graft cirrhosis (n = 1). Of the 8 patients with anastomotic BS who had clinical response to ERCP/PTC (30% reduction in total bilirubin), only 2 (25%) patients had BS noted beyond 1 year (397, 419 days). In contrast, 8 (47%) of 17 patients with BS who did not respond to ERCP/PTC had BS beyond 1 year.
TABLE 3.
Sensitivity, specificity, accuracy, PPV, and NPV of MRCP using ERCP/PTC as the gold standard

TABLE 4.
Outcomes of LT recipients with BS diagnosed on ERCP/PTC but was negative on MRCP following biliary intervention
Magnetic resonance cholagiopancreatography also falsely identified a stricture, yielding the PPV of 94% (95% CI, 0.71-0.99) (patient had a possible bile leak and bile duct opacification could not be performed at ERCP; however, surgery performed on the very next day revealed common bile duct stricture requiring revision of the anastomosis). There were 13 LTRs that had no BS on either modality, yielding a specificity of 0.93 (95% CI, 0.66-0.99) (Table 3) and an NPV of 0.59 for cholangiographic BS. The overall accuracy of MRCP is 0.74 (95% CI, 0.58-0.87). The area under the receiver operating characteristic curve (ROC) curve was 0.78 (95% CI, 0.67-0.90) (Figure 1).
FIGURE 1.
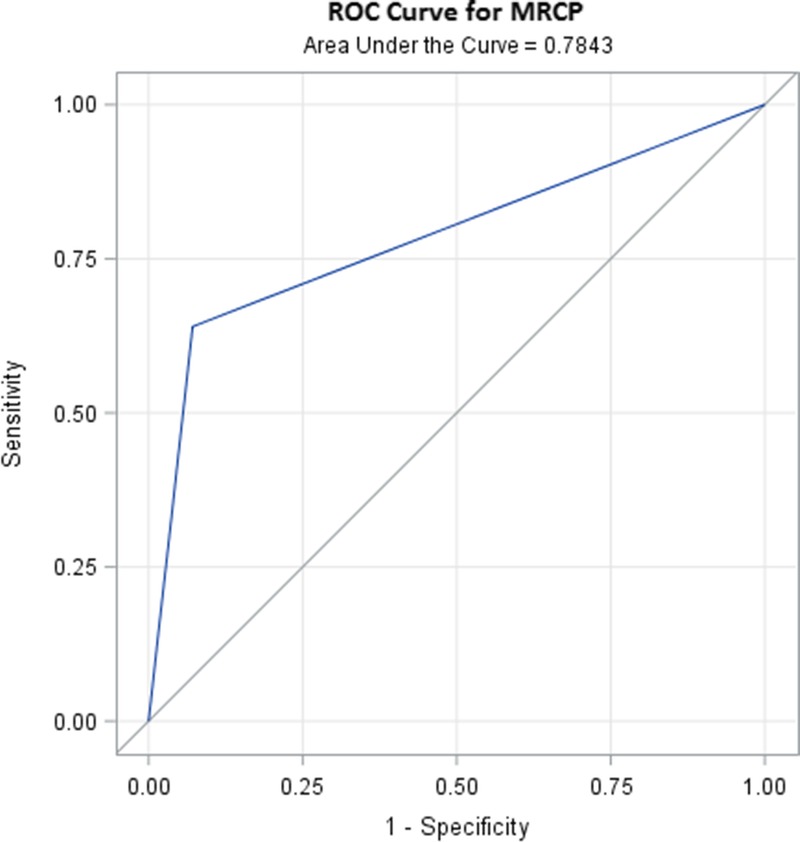
ROC curve for MRCP for the diagnosis of BS. ROC was performed for LTRs (n = 39; 25 strictures and 14 nonstrictures as found by ERCP/PTC). AUC for MRCP is 0.78 with 95% CI, 0.67-0.90.
Of the 16 patients with BS defined by both by MRCP and ERCP, clinically significant anastomotic biliary stricture was noted in 6 patients (30% reduction in total bilirubin within 2 weeks). In 5 patients, concomitant acute cellular rejection (4 mild, and 1 with moderate ACR) was noted that was treated concurrently post-ERCP/PTC intervention (Table 5). Five patients had BS without any other concurrent medical condition to explain their elevated LFTs, and all had improvement in LFTs, 4 had at least 30% improvement in both bilirubin and AP postintervention, and 1 had significant improvement in bilirubin by more than 70%, but AP reduction was less than 30%. The remaining 6 patients had concurrent underlying liver disease in addition to a BS as defined by MRCP and ERCP; these included chronic rejection with advanced fibrosis (n = 1), sickle cell hepatopathy (n = 1), marked hepatic steatosis (n = 1), recurrent hepatitis C on antiviral treatment with pegylated interferon and ribavirin (n = 1), ischemic intrahepatic stricture (n = 1), and advanced fibrosis (n = 1). Liver function tests either did not improve or worsen in these patients except for one who had concurrent diagnosis of sickle cell hepatopathy. This resulted in an overall sensitivity of 0.67 (95% CI, 0.36-0.97), specificity of 0.63 (95% CI, 0.46-0.81), PPV of 0.35 (95% CI, 0.13-0.58), and NPV of 0.86 (95% CI, 0.72-1.00) in diagnosing clinically significant cholangiographic anastomotic biliary stricture by MRCP (Table 6).
TABLE 5.
Outcomes of LT recipients with BS diagnosed on MRCP and ERCP/PTC post-biliary intervention
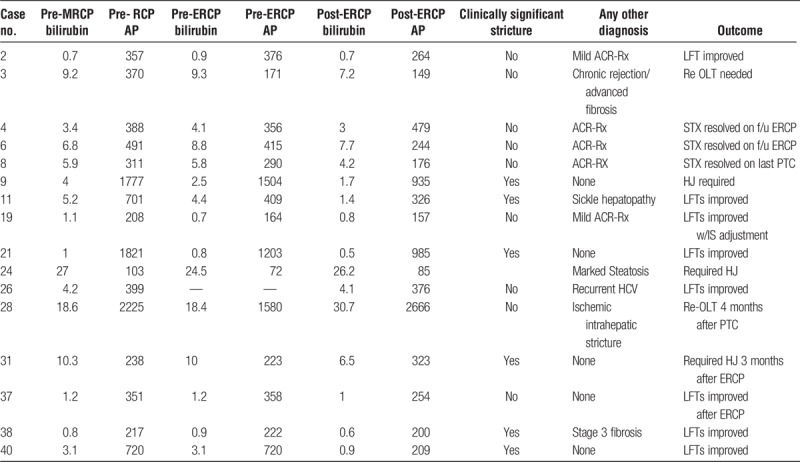
TABLE 6.
Sensitivity, specificity, accuracy, PPV, and NPV of MRCP in predicting ERCP/PTC defined but clinically significant stricture (>30% reduction in total bilirubin in 2 weeks postintervention)

Predictive Models for BS Using MRCP Together With Clinical Variables and Laboratory Tests
The performance of the 5 progressive models for predicting the presence of BS is shown in Table 7 and Figure 2. Results from MRCP performed modestly for predicting the presence of BS (area under the curve [AUC], 0.78; 95% CI, 0.67-0.90). Addition of clinical variables (age and race) and laboratory test (pre-ALT) improved the performance of the MRCP alone model (Table 7). These variables were selected because they were shown to be associated (P < 0.05) or had a potential to be associated with BS (P < 0.2, Table 1). Specifically, adding ALT levels before ERCP/PTC intervention increased the AUC to 0.90 (95% CI, 0.80-0.99; P-adjust = 0.017). When race or age was additionally included in the model, the AUC further increased to 0.92 (95% CI, 0.84-1.000; P-adjust = 0.001) or 0.91 (95% CI, 0.83-1.000; P-adjust = 0.017), respectively. The AUC of the full model was 0.94 (95% CI, 0.86-1.000), and this curve was significantly different from the AUC of the MRCP only model (P-adjusted = 0.012). Addition of etiology of LT (viral vs nonviral), did not increase the AUC significantly, hence this was not further considered in the model building (AUC, 83; 95% CI, 0.70-0.95; P = 0.285). Figure 2 shows the different ROC curves and the AUCs for each model shown in Table 7.
TABLE 7.
Areas under the ROC curves (AUC) for diagnostic BS of LTRs using MRCP along with clinical features and laboratory tests

FIGURE 2.
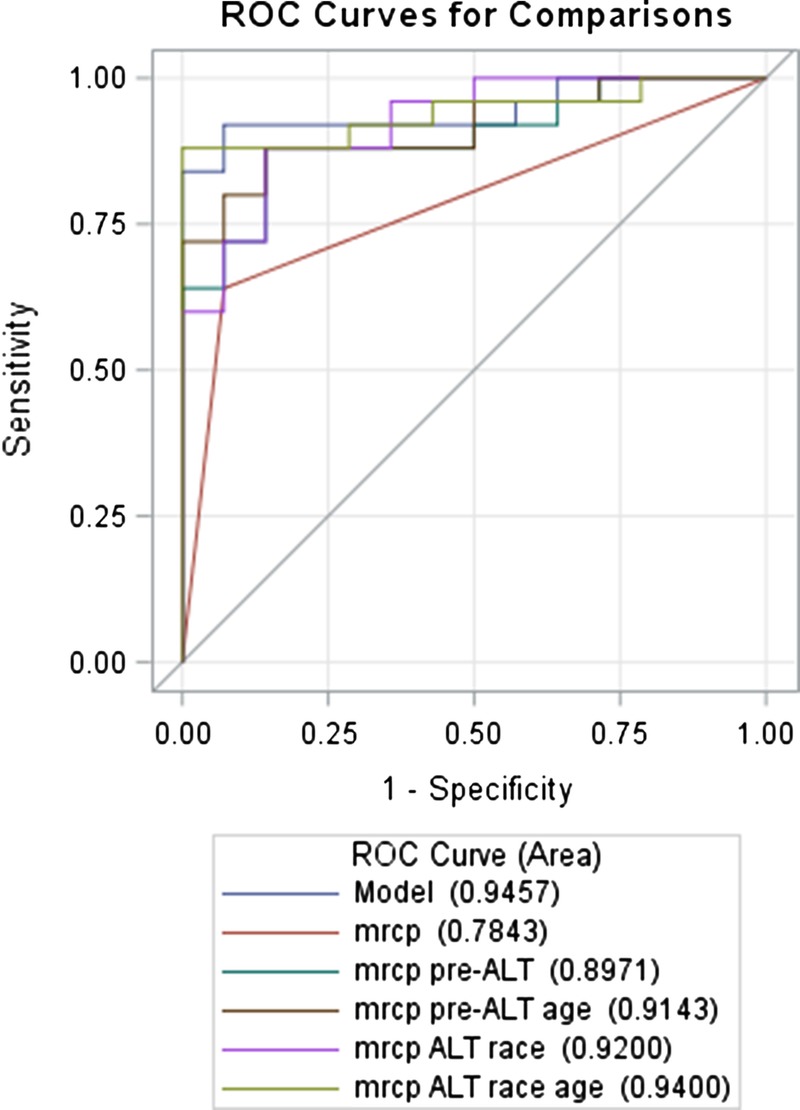
ROC curves for MRCP for the diagnosis of BS with or without adding clinical variables and laboratory test. ROC was performed for all LTRs.
DISCUSSION
Early diagnosis and subsequent timely management of a postliver transplant BS is of paramount importance for ensuring graft and patient survival. Need for an early diagnosis in this setting has prompted various studies looking at available diagnostic modalities for such evaluation. Direct cholangiographic methods (ERCP and PTC) are widely considered as gold standard for diagnosing BS. A meta-analysis of 67 studies published in 2003 including more than 4000 patients (all pretransplant) showed a high overall pooled sensitivity (95% [±1.96 SD, spread of SD, 75% to 99%]) and specificity (97% [spread of SD, 86% to 99%]) of MRCP in diagnosing BS.4 Also, because of the reported high accuracy of MRCP in diagnosing biliary pathology and its noninvasive nature, there has been an interest, over the years, to compare its accuracy with direct cholangiographic methods in post-LT settings.6-23 However, most of the earlier studies have included a heterogeneous group of patients, which for example have included bile leak, anastomotic as well as non-anastomotic BS including ischemic BS, and/or included small number of patients precluding any definite conclusions.9-11,14-23 Furthermore, the vast majority of the published studies did not use the ideal reference standard of ERCP consistently when assessing MRCP accuracy.9-11,14-23 An updated meta-analysis that was published in 2013 have noted most of the studies have used clinical follow-up, surgery and other imaging modalities when reporting results of MRCP accuracy.7 Some did not use the same reference standard for positive and negative MRCP results.7 Even when ERCP was used as the gold standard test for such a comparison, its performance protocols were not the same across those studies, for example, patients with ERCP performed before or even months after MRCP were included.7 The duration of clinical follow-up was also varied across the studies. Reported sensitivities and specificities hence could be subject to various types of bias. A study published in 2010 included 27 patients (post-LT) with MRCP (18 abnormal) however only 14 of the 27 had follow up ERCP and/or PTC.9 Differing follow up methods were used on the remainder of the patients.9 In a meta-analysis, 10 of the included studies did not report the time interval between MRCP and the reference standard, thus raising the possibility of disease progression (in this case strictures) or regression and its related bias.7 Another meta-analysis was done looking specifically at studies that have reported accuracy of MRCP when compared to ERCP in diagnosing BS in posttransplant setting.24 It included 9 studies from 1998 to 2008. Interestingly, 8 of the 9 included studies did not use ideal reference standard (ie, ERCP) after MRCP was performed and only one study used it (but had less than 10 patients).24
Based on paucity of prior good quality comparative studies we have undertaken this study in an attempt to address majority of these issues. First, we have included a uniform group of LTRs who were investigated with MRCP for a possible BS followed by ERCP within an interval of 4 weeks, thereby obviating possible disease progression or regression. ERCP and/or PTC were used in our series of patients as the reference standard, and patients undergoing surgery without confirmation of BS were excluded. Additionally, we defined stricture noted in ERCP or PTC as cholangiographic if any intervention was done (dilation or stent placement) and they were considered clinically significant only if a 30% reduction in total bilirubin was noted postintervention within 2 weeks, thereby obviating any false interpretation by the endoscopist or concomitant liver disease to account for the elevated LFTs.
On the basis of our data, MRCP predicted cholangiographic biliary stricture in 94%, and excluded cholangiographic biliary stricture in 59% of cases. The high PPV of MRCP in the current study (94%) in LTRs reaffirms a continued role of MRCP as diagnostic tool before therapeutic intervention with ERCP. In the current analysis, MRCP failed to identify 9 BS, 2 of them were clinically significant. Six of the rest 7 had concurrent explanation for elevated LFTs, and biliary intervention did not improve their LFTs, raising questions about the clinical significance of these cholangiographically defined BS in the setting of a negative MRCP diagnosis of anastomotic BS. This further validates the diagnostic role of MRCP in identifying clinically significant strictures. Pecchi et al16 have reported sensitivity, specificity, PPVs and NPVs, and accuracy of MRCP to detect BS as 96%, 96%, 95%, 97%, and 96%, respectively in 121 postliver transplant patients (53 confirmed with direct cholangiography). Its diagnostic yield may be lower in the presence of fluid collection of any etiology around biliary tract in a recent study based on single-center experience and raised questions about sensitivity of MRCP.25 Magnetic resonance cholagiopancreatography can accurately show the site of strictures, one of its advantages being the visualization of the bile ducts above and below the stricture or obstruction, which is also very important when planning possible interventional treatment. Despite overall relatively low accuracy of MRCP (74%) to predict cholangiograhic biliary stricture, MRCP overestimated a stricture only in a single patient in the current study. Although MRCP could potentially overestimate BS at the anastomotic site (only single FP case in the current study), this drawback can be reduced by ensuring a preliminary knowledge of the biliary anatomy of donor and recipient and carefully examining MRCP source images and subsequent MR examinations. In the current study, this particular transplant recipient in whom this “misdiagnosis” was made, had a biliary leak as noted on subsequent ERCP which most likely resulted in our inability to identify the stricture cholangiographically (subsequent surgery confirmed anastomotic stricture).
Of the 9 cases with BS which were missed by MRCP and were later identified by ERCP and/or PTC, only 2 were clinically significant, the rest despite being identified as stricture by the endoscopist, did not show clinical improvement with intervention, and likely has alternate etiology to explain their cholestatic liver dysfunction. Hence, MRCP was more accurate in ruling out clinically significant cholangiographic biliary stricture which is defined as greater than 30% improvement in bilirubin within 2-week period following endoscopic intervention. Specifically, the NPV of MRCP for identifying clinically significant cholangiographic biliary stricture was 0.86, while the PPV was only 0.35 (Table 6). The accuracy of MRCP for predicting cholangiographic biliary stricture can be substantially increased by adding clinical and laboratory variables, such as pre-ERCP ALT, age, and race (Table 7). Of note, accuracy of ERCP in identifying the BS as gold standard was reinforced by the fact that overall LFTs (AST, ALT, bilirubin, and AP) were improved post-ERCP intervention in patients diagnosed with BS. Off note, patients with cholangiographic BS in whom no significant improvement in LFTs were noted (Bil > 30% reduction postintervention), concurrent explanation for elevated LFTs were noted (Table 4 and 5) raising questions about the clinical significance of these BS. Patients in whom no stricture was noted by ERCP, no significant improvement in LFTs were noted suggesting alternate etiology for elevated LFTs. Additionally, interpretation of anastomotic stricture in the postliver transplant setting could be quite challenging due to edema at the anastomosis site early after surgery, donor duct recipient duct mismatch, and also probable reduced likely hood biliary dilatation in the LTRs.
The limitations of the current study are those inherent to any retrospective, single-center study. First, MRCP has been read by multiple radiologists potentially leading to interobserver variations in interpretation. Second, ERCP and PTC have been performed by several gastroenterologists and interventional radiologists, respectively, and their interpretation of BS could be different. We have tried to address these issues by using a strict clinical response definition (30% improvement of bilirubin within 2 weeks) to define the stricture. Third, the study included subjects with anastomotic BS only, and as such not generalizable to patients with nonanastomotic BS. Lastly, the number patients included overall are small, and as such prospective, larger, multicenter study focusing on these variables as identified in the current study might be able to further clarify the role of MRCP as a diagnostic investigation pre-ERCP in LT recipients.
In summary, this study demonstrates that MRCP has a low sensitivity, but high specificity and accuracy for diagnosis of cholangiographic BS. Additionally, clinically significant BS are missed less often in patients who have undergone orthotropic liver transplant with MRCP guided approach. The difficulty related to the differential diagnosis, and concurrent explanations for elevated LFTs in post-LT setting, and the potential risks with ERCP first approach, we recommend MRCP guided approach in all LTRs investigated for BS due to its high specificity along with high PPV. A negative MRCP does not rule out anastomotic BS, ERCP is warranted in such cases, if supported by appropriate clinical, biochemical, and/or histological evidence in a small subset of patients.
Footnotes
Published online 23 April, 2018.
A.A. and Q.T.T. contributed equally.
The authors declare no funding or conflicts of interest.
S.K.S., A.A., Q.T.T. conceptualized and designed the study, and wrote the initial draft. A.A. is responsible for data collection. Q.T.T. and S.K.S. performed the statistical analysis. S.K.S., A.A., Q.T.T. revised the article with intellectual input from S.N., S.P., B.M., M.I., J.M.V., J.E. All authors participated in additional discussions, and approved the final version of the article.
REFERENCES
- 1.Atwal T, Pastrana M, Sandhu B. Post-liver transplant biliary complications. . 2012;2:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moser MA, Wall WJ. Management of biliary problems after liver transplantation. . 2001;7(11 Suppl 1):S46–S52. [DOI] [PubMed] [Google Scholar]
- 3.Freeman ML. Adverse outcomes of ERCP. . 2002;56(Suppl 6):S273–S282. [DOI] [PubMed] [Google Scholar]
- 4.Romagnuolo J, Bardou M, Rahme E, et al. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. . 2003;139:547–557. [DOI] [PubMed] [Google Scholar]
- 5.St Peter S, Rodriquez-Davalos MI, Rodriguez-Luna HM, et al. Significance of proximal biliary dilatation in patients with anastomotic strictures after liver transplantation. . 2004;49:1207–1211. [DOI] [PubMed] [Google Scholar]
- 6.den Dulk AC, Wasser MN, Willemssen FE, et al. Value of magnetic resonance cholangiopancreatography in assessment of nonanastomotic biliary strictures after liver transplantation. . 2015;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu YB, Min ZG, Jiang HX, et al. Diagnostic value of magnetic resonance cholangiopancreatography for biliary complications in orthotopic liver transplantation: a meta-analysis. . 2013;45:2341–2346. [DOI] [PubMed] [Google Scholar]
- 8.Beswick DM, Miraglia R, Caruso S, et al. The role of ultrasound and magnetic resonance cholangiopancreatography for the diagnosis of biliary stricture after liver transplantation. . 2012;81:2089–2092. [DOI] [PubMed] [Google Scholar]
- 9.Katz LH, Benjaminov O, Belinki A, et al. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography—long-term follow-up. . 2010;24:E163–E169. [DOI] [PubMed] [Google Scholar]
- 10.Linhares MM, Coelho RD, Szejnfield J, et al. Evaluation of the efficacy and reproducibility of cholangiopancreatography by magnetic resonance for detecting biliary complications following orthotopic liver transplantation. . 2010;25:249–256. [DOI] [PubMed] [Google Scholar]
- 11.Kinner S, Dechêne A, Ladd SC, et al. Comparison of different MRCP techniques for the depiction of biliary complications after liver transplantation. . 2010;20:1749–1756. [DOI] [PubMed] [Google Scholar]
- 12.Aufort S, Molina E, Assenat E, et al. Value of MRCP for diagnosis of biliary complications after liver transplantation. . 2008;89:221–227. [DOI] [PubMed] [Google Scholar]
- 13.Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Diagnosis of biliary strictures after liver transplantation: which is the best tool? . 2005;11:2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cereser L, Girometti R, Como G, et al. Impact of magnetic resonance cholangiography in managing liver-transplanted patients: preliminary results of a clinical decision-making study. . 2011;116:1250–1266. [DOI] [PubMed] [Google Scholar]
- 15.Collettini F, Kroencke TJ, Heidenhain C, et al. Ischemic-type biliary lesions after ortothopic liver transplantation: diagnosis with magnetic resonance cholangiography. . 2011;43:2660–2663. [DOI] [PubMed] [Google Scholar]
- 16.Pecchi A, De Santis M, Gibertini MC, et al. , Role of magnetic resonance imaging in the detection of anastomotic biliary strictures after liver transplantation. . 2011;43:1132–1135. [DOI] [PubMed] [Google Scholar]
- 17.Boraschi P, Donati F, Gigoni R, et al. , MR cholangiography in orthotopic liver transplantation: sensitivity and specificity in detecting biliary complications. . 2010;24:E82–E87. [DOI] [PubMed] [Google Scholar]
- 18.Beltran MM, Marugán RB, Oton E, et al. Accuracy of magnetic resonance cholangiography in the evaluation of late biliary complications after orthotopic liver transplantation. . 2005;37:3924–3925. [DOI] [PubMed] [Google Scholar]
- 19.Kitazono MT, Qayyum A, Yeh BM, et al. Magnetic resonance cholangiography of biliary strictures after liver transplantation: a prospective double-blind study. . 2007;25:1168–1173. [DOI] [PubMed] [Google Scholar]
- 20.Boraschi P, Braccini G, Gigoni R, et al. Detection of biliary complications after orthotopic liver transplantation with MR cholangiography. . 2001;19:1097–1105. [DOI] [PubMed] [Google Scholar]
- 21.Laghi A, Pavone P, Catalano C, et al. MR cholangiography of late biliary complications after liver transplantation. . 1999;172:1541–1546. [DOI] [PubMed] [Google Scholar]
- 22.Valls C, Alba E, Cruz M, et al. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. . 2005;184:812–820. [DOI] [PubMed] [Google Scholar]
- 23.Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. . 1999;211:715–722. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen JE, waljee AK, Volk ML, et al. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. . 2011;73:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydelotte JD, Ali J, Huynh PT, et al. Use of magnetic resonance cholangiopancreatography in clinical practice: not as good as we once thought. . 2015;221:215–219. [DOI] [PubMed] [Google Scholar]


