Supplemental digital content is available in the text.
Abstract
Background
Steatosis is a major risk factor for primary nonfunction in liver transplantations. Steatotic livers recover poorly from ischemia reperfusion injury, in part due to alterations in the microcirculation, although the exact mechanism is unclear. In this study, we tested if there were any alterations in the shear stress sensing Kruppel-like factor 2 (KLF2) and its likely downstream consequences in the ex vivo perfused human liver endothelium, which would imply perturbations in microcirculatory flow in macrosteatotic livers disrupts laminar flow to evaluate if this is a potential therapeutic target for steatotic livers.
Methods
Using a subnormothermic machine perfusion system, 5 macrosteatotic and 4 nonsteatotic human livers were perfused for 3 hours. Flow, resistance, and biochemical profile were monitored. Gene expression levels of nitric oxide synthase 3 (eNOS), KLF2, and thrombomodulin were determined. Nitric oxide (NO) was measured in the perfusion fluid and activation of eNOS was measured with Western blotting.
Results
Flow dynamics, injury markers, and bile production were similar in both groups. Kruppel-like factor 2 expression was significantly higher in nonsteatotic livers. Western blotting analyses showed significantly higher levels of activated eNOS in nonsteatotic livers, consistent with an increase in NO production over time. Macrosteatotic livers showed decreased KLF2 upregulation, eNOS activity, and NO production during machine perfusion.
Conclusions
These results indicate a perturbed KLF2 sensing in steatotic livers, which aligns with perturbed microcirculatory state. This may indicate endothelial dysfunction and contribute to poor posttransplantation outcomes in fatty livers, and further studies to confirm by evaluation of flow and testing treatments are warranted.
With about 120 000 patients on the waiting list for organ transplantation in the United States, there is an enormous gap between the need for and availability of suitable organs for transplant. Because of this shortage, about 10% of the patients die while on the waiting list.1,2 To prevent patient death on the waiting list, there is a constant search for novel strategies to increase availability of organs for transplant. Over the past 2 decades, accepting liver grafts that were previously considered unsuitable has extended the donor pool significantly.3,4 Because of a serious shortage of donor organs suitable for transplant, livers with suboptimal quality, such as steatotic livers, are increasingly considered for transplantation. However, transplantation of such grafts is associated with a higher risk of posttransplantation complications, morbidity, and mortality.5-8
Hepatic steatosis, resulting from abnormal accumulation of triacylglycerol in the cytoplasm of hepatocytes, is an important factor affecting the function of donor livers. Steatosis is estimated to be present in up to 50% of organ donors. Because of the current epidemic of obesity, this is likely to continue to increase.9 Although steatosis can diminish within weeks after transplantation, early functional recovery is still impaired. Grafts with severe macrovesicular steatosis (>60% of hepatocytes involved) are often discarded because of the high incidence of primary nonfunction after transplantation. The use of grafts with moderate steatosis (30-60% of hepatocytes involved), however, remains a challenging question. Primary nonfunction rates in this group are as high as 15%, whereas delayed graft function rates may reach 35%.4-7 Collett et al10 recently developed a UK Donor Liver Index including steatosis as risk factor for short-term graft failure. Thus, although use of these marginal grafts may be an effective way to decrease the discrepancy between organ supply and demand, they still bear a considerable risk for the development of several severe complications and graft loss.11 Ischemia-reperfusion (I/R) injury is considered to be the main cause of liver graft dysfunction after transplantation, independent of liver basal characteristics.12,13 Steatotic livers are especially sensitive to I/R injury, in part due to alterations in the microcirculation, although the exact mechanism is unclear.14,15 Minimizing the adverse effects of I/R injury could increase the number of suitable steatotic grafts. This small-scale, pilot study aims to test if there are differences in shear stress sensing between macrosteatotic and nonsteatotic human donor livers. Such a sensory failure may lead to an endothelial dysfunction and contributing to poor posttransplantation outcomes. In particular, identifying such differences in human donor livers would indicate the likelihood that this is a clinically relevant target for further studies.
The liver sinusoidal endothelial cells are not only a physical barrier but also regulate hepatic vascular tone, contribute to hemostasis, thrombosis, inflammation, and angiogenesis.16 They are also one of the major targets of shear stress created by the blood flow. Shear stress affects many cell types in the liver, including hepatocytes and immune cells of the liver (like Kupffer cells, and intrahepatic leukocytes). As a result, mechanosensory stimulation may affect many systems while altering the endothelial response to shear stress.17 Targeting the Kruppel-like-factor (KLF)2 and endothelial nitric oxide synthase (eNOS) system, we tried to investigate a well-defined, narrow field of effects particularly altering flow dynamics and endothelial function. Endothelial cells are able to sense variations of flow in reaction to changing shear stress that in turn regulates endothelial gene expression and generate vasodilator agents to reestablish the blood pressure.18 Fat droplet accumulation in the hepatocytes, as in hepatic macrosteatosis, increases cell volume and results in partial or complete obstruction of the hepatic sinusoidal space and is known to subsequently lead to a reduced sinusoidal blood flow.15 The degree of fat accumulation in the cytoplasm of the hepatocytes is inversely related to both total hepatic blood flow and flow in the microcirculation and worsens after brain death in several animal models.19,20 Flow cessation or disturbed flow in the microcirculation triggers acute endothelial dysfunction in part due to a reduced expression of vasoprotective transcription factors.21
Evidence suggests that KLF2 acts as a key regulator of normal endothelium function and physiology.14,22 It has been estimated that flow-mediated KLF2 regulates 109 genes, representing 15.3% of the total number of genes regulated by flow.23 Kruppel-like factor 2 is expressed almost exclusively in endothelial cells and is selectively induced by a biomechanical stimulus caused by laminar flow (shear stress) through activation of the MEK5/ERK5 pathway which in turn activates myocyte enhancer factor 2, a transcription factor that upregulates KLF2 expression.23-25 Kruppel-like factor 2 induces expression of vasoprotective and anti thrombotic genes, such as thrombomodulin (TM) and eNOS.26 Besides transcriptional regulation by KLF2, regulation of eNOS activity is mediated by shear stress as well. One of the major activating posttranslational modifications for eNOS is phosphorylation of serine 1177 (ser-1177) via AKT and PKA.27-29 When ser-1177 is phosphorylated in response to increased shear stress, nitric oxide (NO) production increases twofold to threefold above baseline levels.27 Steady laminar flow enhances the expression of vasoprotective genes eNOS and TM through KLF2 and regulates the activation of eNOS. Conversely, disturbed flow or lack of flow, causing low to no shear stress, enhances the expression of proinflammatory, proapoptotic, and procoagulant genes and downregulates vasoprotective genes.30,31
Subnormothermic machine perfusion (SNMP) provides a good experimental model to test differences in shear stress sensing between macrosteatotic and nonsteatotic livers. As we demonstrated in prior studies,32,33 3 hours of SNMP is sufficient for many relevant recovery mechanisms in particular adenosine triphosphate recovery, and stabilization of arterial resistance. Similarly, NO producing sensory systems can be activated within minutes34,35 and the mediator of the response, NO has a very short half-life. Moreover, it is operationally practical, and does not require availability of type-matched, fresh human blood which a reperfusion model would need.36 To determine whether macrosteatosis impaired shear stress-responsive components of the NO pathway, we evaluated KLF2, eNOS, and TM gene expression, eNOS phosphorylation, and NO levels in macrosteatotic and nonsteatotic livers on SNMP.
MATERIALS AND METHODS
Organs
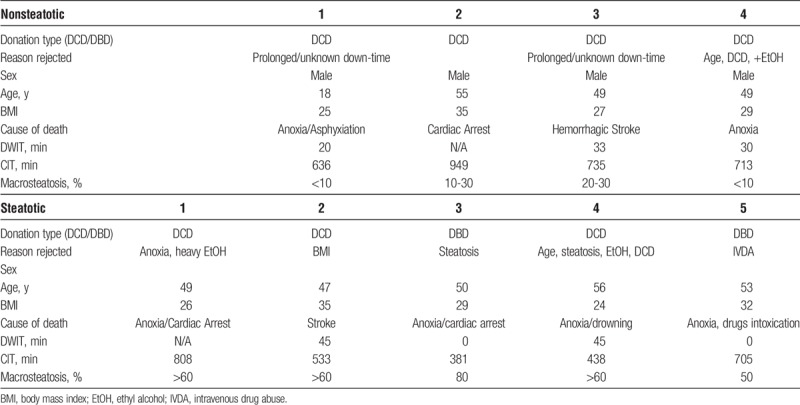
Steatotic and nonsteatotic donor human livers from donors aged 18 to 60 years that were declined for transplantation due to various reasons were obtained from the New England Donor Services with consent for research. Livers with viral infection or major surgical lacerations were excluded. In total, n = 9 livers were included to the study, consisting of n = 5 macro-steatotic and n = 4 nonsteatotic livers. This study was exempted by the institutional review board of Massachusetts General Hospital (protocol 2011P001496). Donor warm ischemia time (DWIT) was defined as the time from withdrawal of life support to cold flush. Cold ischemia time (CIT) was defined as the time from cold flush to reperfusion. Details of donor and graft characteristics can be found in Table 1.
TABLE 1.
Donor and graft characteristics
Perfusion and Tissue Sampling
We used the SNMP model described previously by our group (Figure 1).33 Briefly, we performed a 3-hour perfusion. Williams E-based supplemented media was been used for SNMP at 7 mm Hg portal, and 70 to 80 mm Hg hepatic artery (HA) pressures. Media was oxygenated continuously and routine biochemical and chemical screening was taken throughout the perfusion. Tissue samples were collected hourly, whereas perfusate samplings take place every 10 minutes for the first 30 minutes and then once in every 30 minutes. Further details can be found at SDC, Materials and Methods (http://links.lww.com/TXD/A80).
FIGURE 1.
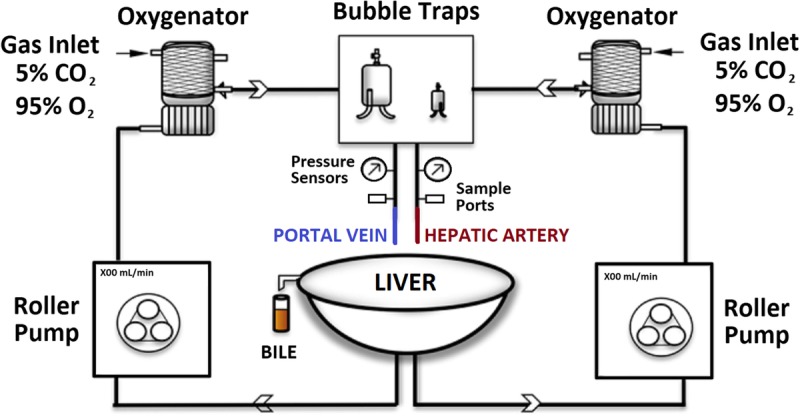
Schematic representation of perfusion system. The perfusion system incorporated 2 independent circulations: an arterial and a portal, each with their own pump, oxygenator using carbogen gas (95% O2, 5% CO2), bubble trap, pressure, and flow meter.
Scoring Steatosis
The percentage of steatosis was scored by 2 blinded experts (a pathologist and a liver transplant surgeon) by estimating the percent hepatocytes containing macrovesicular lipid droplets on hemotoxylin and eosin–stained sections using light microscopy. Steatosis was defined as ≥50% macro-steatosis, and only macro-steatotic livers are included into the analyses along with lean controls. Scoring results are provided in Table 2 and representative images of macrosteatotic and lean livers can be seen in Figure 2.
TABLE 2.
Macrosteatosis scores of each liver
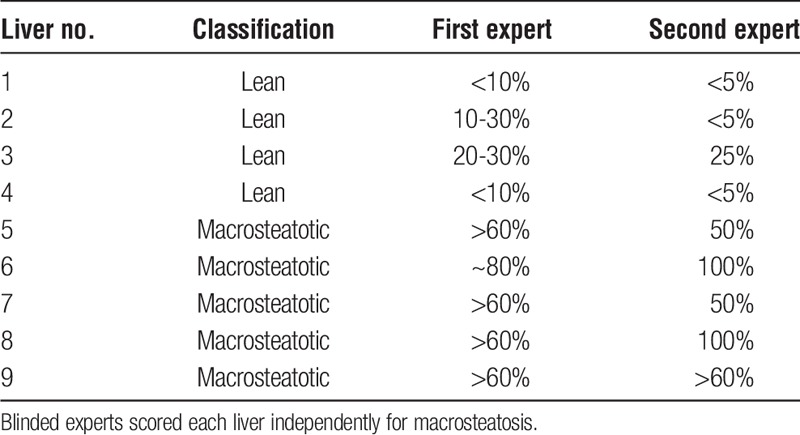
FIGURE 2.
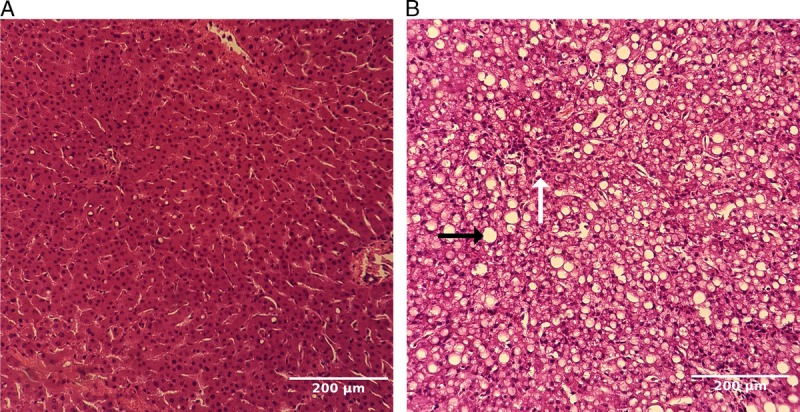
Representative images of lean (A) and macrosteatotic (B) livers at 20× objective magnification. Liver tissue samples were collected at the beginning and the end of perfusion for hemotoxylin and eosin staining scoring for macrosteatosis. Representative images are obtained from 1 lean (panel A) and 1 macrosteatotic (panel B) liver showing postperfusion state. Large fat droplets are prevailing in panel B (black arrow), whereas sinusoids can be barely visible (white arrow). No significant fibrosis has been detected in any of the samples.
Gene Expression Analyses, Western Blot Analyses and NO Measurements
Quantitative reverse transcription polymerase chain reaction was used to determine gene expression levels of KLF2, eNOS, and TM. To verify the presence of endothelial cells, CD31 was used as the marker gene. Specific primers with the sequences shown in Table 3 were synthesized by the Massachusetts General Hospital DNA Core. Endothelial nitric oxide synthase activation was evaluated by determining the serine phosphorylation at S-1177. Timewise collected tissue samples were directed to phospho S1177 specific p-eNOS Western blot analyses (Figure S1 http://links.lww.com/TXD/A81). Finally, NO levels were measured from the collected perfusate by a colorimetric assay. Further details can be found at SDC, Materials and Methods (http://links.lww.com/TXD/A80).
TABLE 3.
Primer sequences for qPCR analyses of targeted genes

Statistical Analysis
Continuous variables are presented as medians and interquartile range (IQR). As the data are not normally distributed in all cases, and there are only 2 groups, Mann-Whitney U test was used for continuous variables between the 2 experimental groups, and Wilcoxon Signed Rank was used for comparison within groups. Correlations between variables were tested with Spearman rho correlation, a nonparametric version of the Pearson test. Variables with a P value of 0.05 or less were considered to be significant. All statistical analyses were done with IBM SPSS version 23 for Mac, and graphs were made with Graphpad Prism 6 for Mac.
RESULTS
Donor and Graft Characteristics
A total of 4 nonsteatotic and 5 steatotic discarded human livers were perfused (Table 1). All the nonsteatotic livers were donated after circulatory death (DCD), with a median CIT of 713 (IQR, 674.5-724) minutes and a median DWIT of 30 (IQR, 25-31.5) minutes. Two of the steatotic livers were donated after brain death (DBD) and 3 were DCD with a median CIT of 485 (IQR, 438-533) minutes and a median warm ischemia time (WIT) of 22.5 (IQR, 0-46.5) minutes.
Functional Measurements on SNMP
Portal vein (PV) resistance was similar between steatotic and nonsteatotic livers (Figure 3A). Hepatic artery resistance trended higher in steatotic than nonsteatotic livers (Figure 3B), but this difference was not statistically significant because of the variability in resistances seen. Lactate clearance, bile production, and alanine transaminase levels were similar between steatotic and nonsteatotic livers (Table S1, http://links.lww.com/TXD/A82).
FIGURE 3.
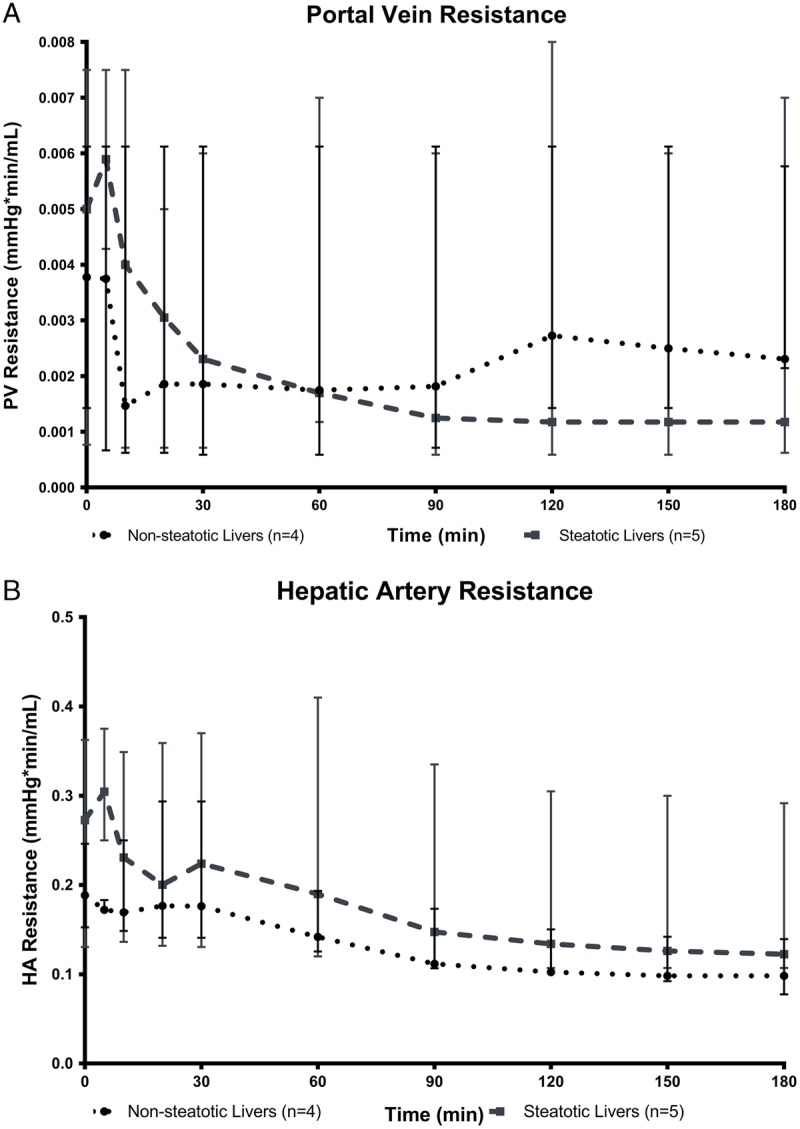
Resistance in the PV (A) and the HA (B) calculated as quotient of pressure and flow. Hepatic artery and PV resistance decreased gradually throughout perfusion in both nonsteatotic and steatotic livers. However, no statistically significant differences were observed between the 2 groups (data are presented as median ± IQR).
KLF2 Messenger Ribonucleic Acid Is Upregulated in Normal Livers, But Not in Steatotic Livers
Kruppel-like factor 2 was significantly upregulated in both groups at the end of SNMP compared with the start of SNMP (median fold change of 15.88 (P = 0.034) for nonsteatotic and 3.13 (P = 0.040) for steatotic livers). The degree of upregulation was significantly higher in nonsteatotic compared to steatotic livers (P = 0.032) (Figure 4A). Although messenger ribonucleic acid (mRNA) levels of KLF2 inducible genes eNOS and TM showed an increasing trend during perfusion, this was not statistically significant (Figure 4B and C, respectively).
FIGURE 4.
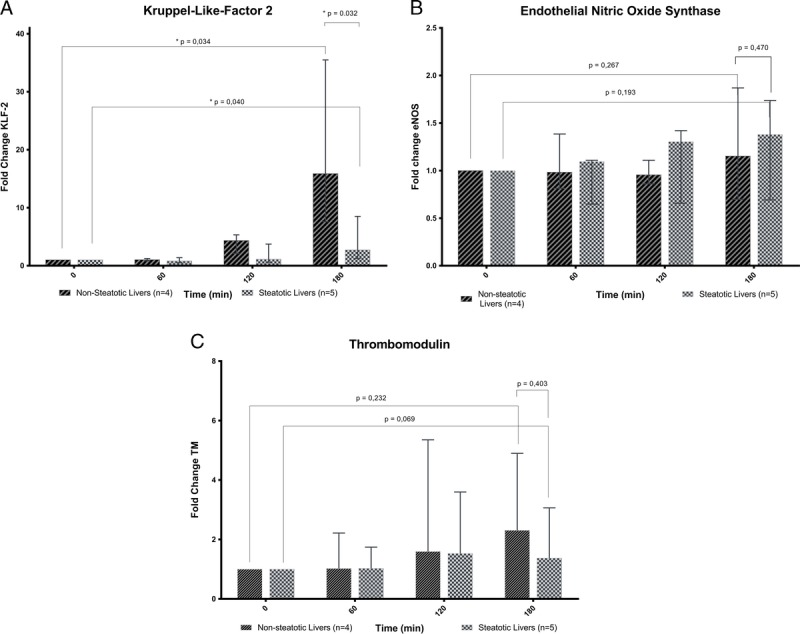
Gene expression analyses. SNMP induces upregulation of KLF2 in both steatotic and nonsteatotic livers although significantly more in the nonsteatotic livers (A). eNOS is not significantly more expressed at the end of SNMP in both steatotic and nonsteatotic livers (B). Thrombomodulin is not significantly more expressed at the end of SNMP in both steatotic and nonsteatotic livers (C). Fold change is calculated from T=0 for each liver (data are presented as median ± IQR).
NO Levels Increase in Normal Livers, But Not Steatotic Livers
Nitric oxide concentration in the nonsteatotic livers increased from a median of 5.37 μM at the beginning of perfusion to 7.12 μM after 3 hours of perfusion (P = 0.055). Nitric oxide levels in the steatotic group shows a decreasing trend from a median of 11.73 μM at the beginning of perfusion (T = 0) to 7.57 μM at the end of perfusion (T = 180) although this trend was not significant (P = 0.142). Even though the levels of NO had a decreasing trend in steatotic livers, it still remained higher at the end of SNMP compared with nonsteatotic livers but this was not statistically significant either (P = 0.365) (Figure 5A).
FIGURE 5.
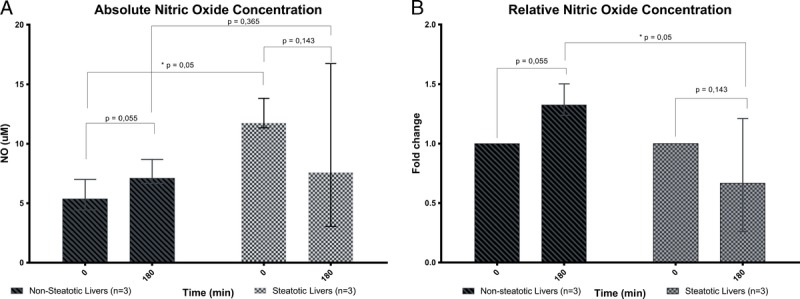
NO measurements. Absolute NO concentration increased in the nonsteatotic livers throughout SNMP but did not change in steatotic livers (A). Relative NO production calculated as fold change from T = 0 for each liver (B). Relative NO production fold change increased in the nonsteatotic livers but did not change in steatotic livers (data are presented as median ± IQR).
Nitric oxide levels in nonsteatotic livers had an increasing trend with a median fold change of 1.33 (P = 0.055) at the end of SNMP; while steatotic livers, although not significant, showed a decreasing trend with a median fold change of 0.67 at the end of SNMP (P = 0.143). Fold change between both groups did, however, alter significantly (P = 0.05) (Figure 5b).
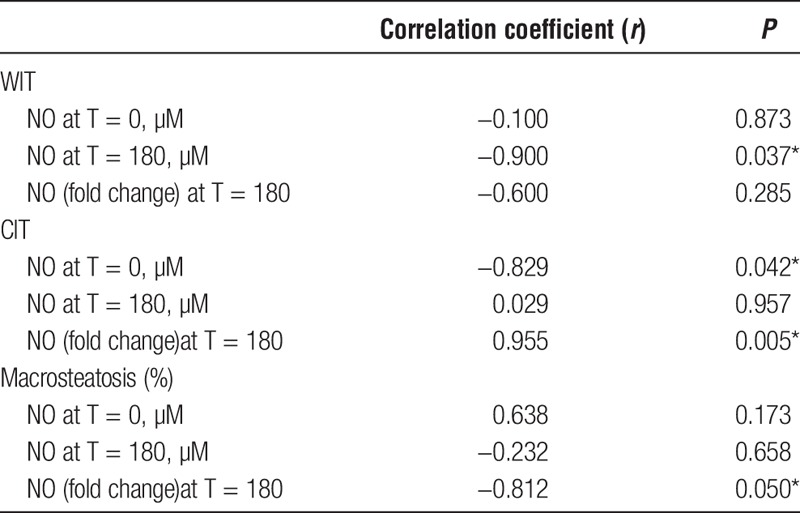
Other notable donor graft characteristics besides macrosteatosis are CIT and WIT. Increasing WIT was associated with lower tissue NO at T = 180 (P = 0.037). Increasing CIT, on the other hand, was associated with lower tissue NO at T = 0 (P = 0.042) and higher fold change (P = 0.005). Increasing macrosteatosis was associated with decrease in NO concentration over time (P = 0.05). Correlations between NO production and macrosteatosis, CIT and WIT are shown in Table 4.
TABLE 4.
Correlations between NO production and donor graph characteristics (data are presented with the Spearman rank correlation coefficient)
Activated eNOS Levels Higher in Nonsteatotic Livers
We used ser-1177 phosphorylated eNOS as a surrogate for eNOS activity in the organs. Although variability was much wider in nonsteatotic livers, every single nonsteatotic liver had higher p-eNOS levels than all the steatotic livers, which had uniformly low activated (ie, phosphorylated) eNOS levels at all time points (P < 0.005, Figure 6).
FIGURE 6.

Normalized levels of eNOS phosphorylation. Levels of eNOS phosphorylation are significantly higher in non-steatotic livers compared with steatotic livers (data are presented as median ± IQR).
DISCUSSION AND CONCLUSION
Elucidating the exact mechanisms of steatotic livers' poor transplantation outcomes is not a straightforward endeavor. Here, we investigated 1 potential contributor: perturbation of the microcirculation due to impingement of large lipid vacuoles into the sinusoids, attenuating vasoprotective signaling events. In a preliminary study of discarded human livers, we found evidence of impaired NO pathway activation in steatotic livers compared to nonsteatotic livers. During SNMP, endothelial cells in steatotic livers fail to upregulate KLF2, a transcription factor induced in response to shear stress, to the extent seen in nonsteatotic livers. Tissue levels of end-effector molecule NO increased only 40% the amount seen in nonsteatotic livers.
Interestingly, although KLF2 induces eNOS expression, there was no difference in eNOS and TM mRNA levels between steatotic and nonsteatotic livers seen during the period of SNMP in our study. It is possible that 3 hours is not long enough for KLF2-dependent gene transcription to increase, especially at subnormothermic temperatures when cellular metabolism and biochemistry are slower than at physiologic temperatures, and that longer perfusion times would allow us to detect differences. Thus, the lower NO levels in steatotic livers are likely the result of decreased eNOS phosphorylation and activity. Indeed, when comparing posttranslational modification in steatotic versus nonsteatotic livers, eNOS was phosphorylated to a much lesser extent in steatotic livers. Activation of eNOS through phosphorylation of Ser1177 increases in response to shear stress via KLF2-independent caveolin signaling pathways, which does not require new transcription or translation, and can therefore occur in a very short time frame.27-29 Heterogeneity in donor characteristics does make it difficult to interpret results in this small study. For example, little is known about warm ischemia derived endothelial damage, as most studies have focused on cold ischemia and reperfusion. However, increased cell death has been described in endothelial cell cultures exposed to warm ischemia and reoxygenation.37 Furthermore, Hide et al38 recently found a negative correlation between warm ischemia and NO production in a reperfusion animal model. We found a negative correlation between DWIT and NO concentrations at T = 0. Although the low number of replicates require caution, this correlation may explain the seemingly paradoxical higher levels of immediate postcold storage (T = 0) NO levels in steatotic livers compared with nonsteatotic livers. Two of five steatotic livers were DBD livers, and thus did not suffer from warm ischemia during withdrawal of life support, whereas all of the nonsteatotic livers were DCD livers with notable DWIT. The lower baseline values of NO in nonsteatotic livers, after a cold-storage period without perfusion, may reflect endothelial damage or reduction of NO to inorganic nitrite and nitrate during DWIT (hence results with lower readings) in the nonsteatotic livers, rather than any genuine response to shear stress. Notably, our steatotic livers had lower WIT than the nonsteatotic livers and still failed to increase NO tissue levels during SNMP, so our findings are not likely to be the result of WIT variation and more likely to be due to the steatosis. Endothelial cells may become dysfunctional due to warm ischemia, diminishing NO synthesis capacity, which is compounded in steatotic livers by the failure to upregulate NO synthesis because of disturbed shear stress patterns.
Another important donor graft characteristic was CIT. We found a negative correlation between NO levels at the start of SNMP and CIT (r = −0.829 P = 0.042), which is not surprising, as it is known that endothelial cells are more vulnerable to cold ischemia than hepatocytes. However, this correlation disappeared over the course of SNMP and instead the fold increase in NO was more pronounced with longer CIT. In vitro studies have shown endothelial cells can tolerate up to 9 hours of cold ischemia with reversible damage but deteriorate rapidly afterward. After 24 h of cold ischemia, the vast majority of endothelial cells are no longer viable.39-41 Because our average CIT was less than 12 hours, endothelial cells may still be largely viable. The question remains whether our first finding is a reflection of NO degradation during longer CIT or endothelial cell dysfunction. However, as endothelial cells recovered during SNMP, NO synthesis caught up and surpassed that of organs with shorter CIT to achieve similar NO levels at T = 180 (data not shown). This may be an indication that SNMP can repair cells and not just slow the decay that occurs in cold storage. Furthermore, various animal models were used to study the effects of cold ischemia, mostly lacking the influence of prior warm ischemia. Again, our steatotic livers also had shorter CIT than the nonsteatotic controls, and thus the failure of steatotic livers to produce NO would likely have been even more pronounced if we had equalized CIT. Better elucidation of endothelial injury during warm and cold ischemia may be better accomplished in an animal where precise control of ischemic time is possible, and the data we present should be taken as a motivation and clinically relevant basis for designing such studies.
It is important to note that the response to shear stress occurs of a broad time frame. Mechanosensory signal on the cell surface activates caveolin bound eNOS by phosphorylation on Ser1177,34,42 increasing NO concentrations before any changes in gene transcription or protein production can occur. The potent gaseous hormone effects smooth muscle relaxation within seconds.42-45 At the same time, a different set of adaptor molecules lead to myocyte enhancer factor 2 activation and KLF2 transcription,23,34,46 itself a transcription factor inducing eNOS and TM, as well as inhibiting endothelial inflammation.23 The need for 1 to 2 rounds of transcription and translation extend the effects of shear stress to hours, and modulation of coagulation and inflammation influence long-term microvascular modeling. We evaluated only short-term events with our 3-hour SNMP model,33 and will use our experience with perfusing livers up to 24 hours to further study medium-term events. In addition, we have developed a reperfusion simulation model on pump that will be useful for evaluating livers at physiologic conditions after SNMP. Small animal transplantation models will also prove useful in observing long-term effects of the impaired NO response in steatotic livers.
Finally, it is worth mentioning that steatotic livers may respond differently to shear stress for reasons other than simple topology. For example, NO production also responds to inflammatory stimuli via inducible nitric oxide synthase activity, and Gehrau et al47,48 showed that proinflammatory cytokines were significantly increased in steatotic grafts after reperfusion. As a result, it is crucial to investigate the activity levels of different NOS isoforms to assess the overall involvement of different NO production processes. In our study, the NO concentrations trended fairly closely with eNOS phosphorylation and presumably, activity levels. Additionally, there is relatively little inducible nitric oxide synthase expression in the liver49 (gtexportal.org and proteinatlas.org). Thus, we suspect that the majority of the difference in NO levels was generated by endothelial eNOS.
This study used SNMP as a method to assess the differences between steatotic and nonsteatotic livers during preservation. Machine perfusion not only provides an opportunity to assess the quality of grafts ex situ but also helps to improve grafts.33 Strategies considered to improve endothelial function for steatotic livers to reduce tissue injury may include chemically increasing NO levels by adding NO donors into the perfusion fluid. The use of statins may be promising too. Statins were originally designed to decrease cholesterol levels but they have also been found to be potent KLF2 activators with the potential to enhance eNOS levels and subsequently maintain NO bioavailability. Gracia-Sancho et al have shown the beneficial effects of statins as prophylactic treatment to prevent damage due to I/R injury in steatotic rat livers.50 Administering statins to a potential donor is however impractical and unethical. The same group has also shown that the use of statins in cold storage solution improved microcirculation in rat livers and reduced I/R injury compared with grafts preserved in cold storage solution without statins.51 The addition of statins to perfusion fluid in human livers has not been examined yet but is worth investigating especially for grafts with moderate/severe macrosteatosis to reduce primary graft dysfunction rate that cannot be reduced by means of machine perfusion alone.
In conclusion, donor WIT, CIT, and macrosteatosis all appear to have an influence on NO production or availability, and outcome variables may depend on a variety of interactions of time and type of ischemia (warm or cold) and type of liver (steatotic or nonsteatotic). While the heterogeneity of deceased donor livers and relatively small groups makes it difficult to interpret results, the pilot study data presented here indicate that steatosis in human livers results in endothelial dysfunction indicated by decreased upregulation of KLF2 and, less activation of eNOS and NO production. These results indicate research on this pathway, either with larger sample sizes or better controlled animal models, as a profitable direction.
Supplementary Material
ACKNOWLEDGMENTS
Funding from the US National Institutes of Health (grants R01DK096075, R01DK107875 and R21EB020819) and the Shriners Hospitals for Children is gratefully acknowledged.
The authors would like to gratefully acknowledge the New England Donor Services (NEDS), National Institute of Health (NIH) and Shriners Hospitals for Children-Boston for supporting this work.
Footnotes
Published online 23 April, 2018.
Funding from the US National Institutes of Health (grants R01DK096075, R01DK107875 and R21EB020819) and the Shriners Hospitals for Children is gratefully acknowledged.
K.U. is inventor on pending patents relevant to this study (WO/2011/002926; WO/2011/35223) and has a provisional patent application relevant to this study (Massachusetts General Hospital 22743). K.U. has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. K.U.'s interests are managed by the Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
K.U., R.J.P., J.F.M., and H.Y. supervised the study and experimental design. I.B., S.M., H.Y., and K.U. wrote the article. I.B. and S.M. performed biochemical experiments and relevant data analysis. V.H., N.K., I.B., S.G., and S.M. performed surgical experiments, subnormothermic machine perfusions and relevant data analysis. All authors contributed to the final editing of the draft manuscript and various aspects of research design.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. . 2006;43: 345–351. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 annual data report: liver. . 2015;15(Suppl 2):1–28. [DOI] [PubMed] [Google Scholar]
- 3.Saidi RF. Utilization of expanded criteria donors in liver transplantation. . 2013;4:46–59. [PMC free article] [PubMed] [Google Scholar]
- 4.Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. . 2008;14:1694–1707. [DOI] [PubMed] [Google Scholar]
- 5.Verran D, Kusyk T, Painter D, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. . 2003;9:500–505. [DOI] [PubMed] [Google Scholar]
- 6.Urena MA, Moreno Gonzalez E, Romero CJ, et al. An approach to the rational use of steatotic donor livers in liver transplantation. . 1999;46:1164–1173. [PubMed] [Google Scholar]
- 7.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. . 2010;16: 874–884. [DOI] [PubMed] [Google Scholar]
- 8.Chu MJ, Dare AJ, Phillips AR, et al. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. . 2015;19:1713–1724. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. . 2006;12:523–534. [DOI] [PubMed] [Google Scholar]
- 10.Collett D, Friend PJ, Watson CJ. Factors associated with short- and long-term liver graft survival in the United Kingdom: development of a UK donor liver index. . 2017;101:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerkamp AC, de Boer MT, van den Berg AP, et al. Similar outcome after transplantation of moderate macrovesicular steatotic and nonsteatotic livers when the cold ischemia time is kept very short. . 2015;28: 319–329. [DOI] [PubMed] [Google Scholar]
- 12.Tashiro H, Kuroda S, Mikuriya Y, et al. Ischemia-reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. . 2014;44:1611–1625. [DOI] [PubMed] [Google Scholar]
- 13.Selzner N, Selzner M, Jochum W, et al. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. . 2006;44:694–701. [DOI] [PubMed] [Google Scholar]
- 14.Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. . 2013;59:1094–1106. [DOI] [PubMed] [Google Scholar]
- 15.Ijaz S, Yang W, Winslet MC, et al. Impairment of hepatic microcirculation in fatty liver. . 2003;10:447–456. [DOI] [PubMed] [Google Scholar]
- 16.Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. . 2017;66:212–227. [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. . 1999;29:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. . 2011;15:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifalian AM, Chidambaram V, Rolles K, et al. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. . 1998;4:71–77. [DOI] [PubMed] [Google Scholar]
- 20.Yamagami K, Hutter J, Yamamoto Y, et al. Synergistic effects of brain death and liver steatosis on the hepatic microcirculation. . 2005;80:500–505. [DOI] [PubMed] [Google Scholar]
- 21.Marrone G, Russo L, Rosado E, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. . 2013;58:98–103. [DOI] [PubMed] [Google Scholar]
- 22.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. . 2009;29:39–40, 41–3. [PubMed] [Google Scholar]
- 23.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. . 2006;116, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar KM, Nambudiri V, Dai G, et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. . 2005;280:26714–26719. [DOI] [PubMed] [Google Scholar]
- 25.Gimbrone MA, Jr, García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. . 2013;22:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. . 2005;96:e48–e57. [DOI] [PubMed] [Google Scholar]
- 27.McCabe TJ, Fulton D, Roman LJ, et al. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. . 2000;275:6123–6128. [DOI] [PubMed] [Google Scholar]
- 28.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. . 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- 29.Koo A, Nordsletten D, Umeton R, et al. In silico modeling of shear-stress-induced nitric oxide production in endothelial cells through systems biology. . 2013;104:2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, et al. Flow cessation triggers endothelial dysfunction during organ cold storage conditions: strategies for pharmacologic intervention. . 2010;90:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin T, Gongol B, Chen Z, et al. Mechanosensitive microRNAs—role in endothelial responses to shear stress and redox state. . 2013;64:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruinsma BG, Sridharan GV, Weeder PD, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. . 2016;6: 22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. . 2014;14:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. . 2009;89:481–534. [DOI] [PubMed] [Google Scholar]
- 35.Browning EA, Chatterjee S, Fisher AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. . 2012;74:403–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruinsma BG, Avruch JH, Sridharan GV, et al. Peritransplant energy changes and their correlation to outcome after human liver transplantation. . 2017;101:1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banga NR, Prasad KR, Burn JL, et al. An in vitro model of warm hypoxia-reoxygenation injury in human liver endothelial cells. . 2012;178:e35–e41. [DOI] [PubMed] [Google Scholar]
- 38.Hide D, Ortega-Ribera M, Garcia-Pagan JC, et al. Effects of warm ischemia and reperfusion on the liver microcirculatory phenotype of rats: underlying mechanisms and pharmacological therapy. . 2016;6:22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauen U, Hanssen M, Lauchart W, et al. Energy-dependent injury to cultured sinusoidal endothelial cells of the rat liver in UW solution. . 1993;55:469–473. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell-Kenkel JC, Currin RT, Tanaka Y, et al. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. . 1989;10:292–299. [DOI] [PubMed] [Google Scholar]
- 41.Huet PM, Nagaoka MR, Desbiens G, et al. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. . 2004;39:1110–1119. [DOI] [PubMed] [Google Scholar]
- 42.Bredt DS. Nitric oxide signaling specificity—the heart of the problem. . 2003;116:9–15. [DOI] [PubMed] [Google Scholar]
- 43.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. . 1991;43:109–142. [PubMed] [Google Scholar]
- 44.Grange RW, Isotani E, Lau KS, et al. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. . 2001;5:35–44. [DOI] [PubMed] [Google Scholar]
- 45.Thomas DD, Liu X, Kantrow SP, et al. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. . 2001;98:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dekker RJ, van Soest S, Fontijn RD, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). . 2002;100:1689–1698. [DOI] [PubMed] [Google Scholar]
- 47.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. . 2006;113:1708–1714. [DOI] [PubMed] [Google Scholar]
- 48.Gehrau RC, Mas VR, Dumur CI, et al. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. . 2015;99:2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halpern KB, Shenhav R, Matcovitch-Natan O, et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. . 2017;542:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gracia-Sancho J, García-Calderó H, Hide D, et al. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. . 2013;58:1140–1146. [DOI] [PubMed] [Google Scholar]
- 51.Russo L, Gracia-Sancho J, García-Calderó H, et al. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. . 2012;55:921–930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


