Supplemental Digital Content is available in the text
Keywords: disease-free survival, meta-analysis, obesity, overall survival, triple-negative breast cancer
Abstract
Objective:
To investigate the relationship between obesity and disease-free survival (DFS) and overall survival (OS) of triple-negative breast cancer.
Methods:
Citations were searched in PubMed, Cochrane Library, and Web of Science. Random effect model meta-analysis was conducted by using Revman software version 5.0, and publication bias was evaluated by creating Egger regression with STATA software version 12.
Results:
Nine studies (4412 patients) were included for DFS meta-analysis, 8 studies (4392 patients) include for OS meta-analysis. There were no statistical significances between obesity with DFS (P = .60) and OS (P = .71) in triple-negative breast cancer (TNBC) patients.
Conclusion:
Obesity has no impact on DFS and OS in patients with TNBC.
1. Introduction
Breast cancer is a multiple kinds of extremely heterogeneous diseases with the same location of origin, each kind displays distinctive etiology, clinical pathological features, molecular characteristics, and therapeutic response.[1] The prognosis of breast tumors with different biological and molecular characteristics is quite diverse.[2] Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) and other molecular markers are usually evaluated on breast cancer specimens, which play an important role in the development of optimal treatment strategies for patients with breast cancer.[3] Nevertheless, breast cancer is frequently sorted as receptor positive, HER2 overexpression, and triple-negative breast cancer (TNBC) due to modern technologies and these assortments are not yet routinely used in clinical practice.[4]
There is not enough expression of ER, PR, and HER-2 in patients with TNBC.[5] Compared with other subtypes of breast tumors, TNBC has a shorter recurrence time and more possibilities metastasize to distance by hematogenic channel.[6,7] The key drivers of TNBC involve as following: lability of genome, such as p53 functional deletion,[8–10] sensitization of pivotal signaling networks,[11–16] the function of progenitor cells in driving the transition between epithelium with mesenchyme and plasticity of phenotype,[17] and the microenvironment of obesity.[18,19]
In accordance with The Centers for Disease Control and Prevention, obesity is defined as body mass index (BMI) ≥30 kg/m2.[2] The prevalence of obesity (35.5%–40.4%) and severe obesity (BMI >40 g/m2 [7.4%–9.9%]) is found more in women than in men.[20,21] It is discovered that overweight and obesity are the potential causes carcinoma in esophagus, gastric cardia, thyroid, pancreas, colon, rectum, endometrium, prostate, gallbladder, ovary, and breast by recent researches.[22]
The women with higher BMI are more likely to suffer breast cancer, that has been demonstrated by some scientific evidence.[23] Recent studies have suggested that obesity is still related with poor outcomes (i.e., decline in overall survival [OS] and disease-free survival [DFS], OS is computed by the time from diagnosis to death or final follow-up, and DFS is computed by the time from diagnosis to recurrence) in patients with breast cancer being received doxorubicin chemotherapy.[23] By contrast, some studies have stated that BMI affects DFS, and merely has a significant impact on OS.[24] Some studies show that there is no significant difference in OS and DFS between obese and nonobese patients with breast cancer,[25–28] and a study find that there is significant difference in DFS but not in OS.[29] Noteworthily, there is a key article justifies that for hormone receptor-positive breast cancer, patients with obesity, when compare with no obesity, display inferior outcomes in OS and DFS, but not for hormone receptor-negative breast cancer.[30] Paradoxically, Liu et al[31] come to conclude that people with obesity tend to predict worse outcomes for DFS and OS in a study with 44 TNBC patients. There is no comprehensive conclusion in the field at present. The second major reason of carcinoma death in women is mammary cancer, which is the most usually developing one yet, and approximately 12% to 17% of women with breast cancer are diagnosed as TNBC.[5,32] Therefore, it is greatly essential to investigate whether obesity is an aggravating factor influencing the DFS and OS in TNBC.
2. Methods
2.1. Search strategy
In November 2017, 2 authors (LH and MX) independently carried out comprehensive literature searches in PubMed, Web of Science, and Cochrane Library using the predefined keywords ([triple negative breast cancer] and [BMI or body mass index or overweight or underweight or obesity or body weight]). We included the articles with no restrictions on publication language (English or non-English), publication year, geographical location, and the age of the participants. In addition, we augmented the searches with the subject heading terms option as much as possible.
2.2. Inclusion criteria and study selection
In order to qualify for meta-analysis, studies needed to content the following criteria: publication language in English, exposure factor was obesity, patients diagnosed with TNBC, and both reported the OS and DFS outcomes.
Two authors (LH and MX prudentially filtered the retrieved citations, and selected potential researches according to titles, abstracts, and full-texts. If there were some disagreements, the 2 authors resolved them by discussion until reached a consensus.
2.3. Data abstraction
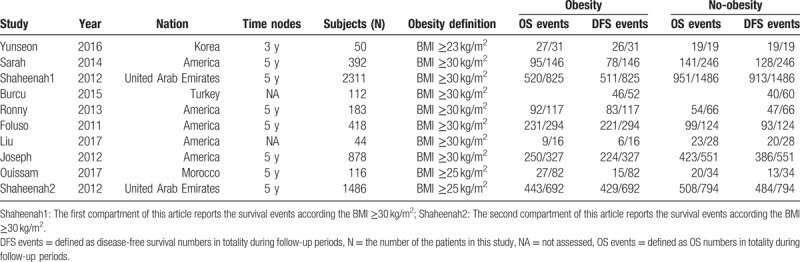
In order to collect the data, the 2 authors (LH and MX) used Excel 2016 and abstracted the following components: the first author, publication year, nation, numbers of subjects, obesity definition, OS events (OS numbers vs totality during follow-up periods) in obesity or no-obesity, DFS events (DFS numbers vs totality during follow-up periods) in obesity or no-obesity. If there were any inconsistency, that was resolved by discussion. The details are described in Table 1.
Table 1.
Characteristic details of the studies involved in meta-analysis.
2.4. Data analysis
We implemented random-effect meta-analysis and used the odds ratio (OR) value and its 95% confidence interval (CI) to express the data with the Rveman software version 5.3. It was thought to be statistically significant when the P < .05. Heterogeneity in different group was tested by I2 test, and we conducted subgroup analysis in terms of different BMI definition. Publication bias was evaluated by Egger regression using the STATA software version 12.
3. Results
3.1. Search results
There were 612 citations retrieved after comprehensive literature searches, and 368 were excluded due to patently no pertinent. And we removed 158 studies because they did not meet inclusion criteria, when screening the title and abstract, 58 citations were excluded. The further exclusion via perusing full text led 19 studies to be debarred. Finally, 9 studies were included for DFS meta-analysis, and 8 studies were included for OS meta-analysis (Fig. 1). One of these articles[26] reports the OS and DFS events of TNBC patients according to BMI ≥25 kg/m2 and BMI ≥30 kg/m2 cut-off points, respectively; thus, it can be analyzed by dividing into 2 compartments on the basis of the 2 cut-off points. Overall, we included 5898 patients with triple negative breast tumors who were reported DFS outcomes for obesity or no-obesity, and 5878 patients with TNBC who were reported OS outcomes for obesity or no-obesity. Although all the retrospective studies were from institutional databases, their quality was satisfactory.
Figure 1.
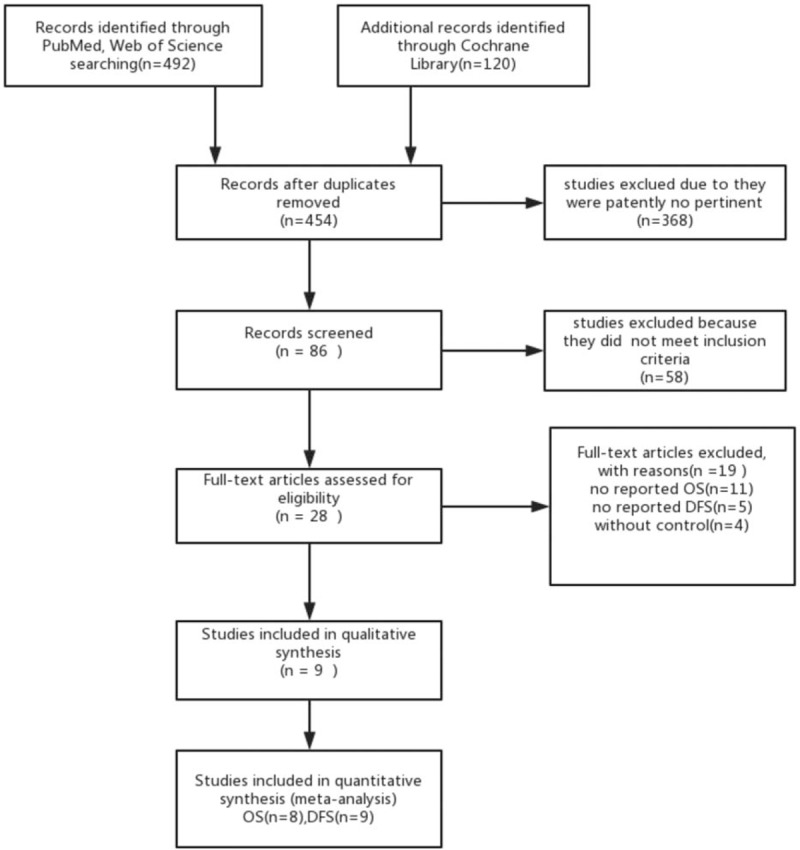
The process of the study selection. DFS = disease-free survival, OS = overall survival.
3.2. Obesity vs no-obesity for OS
As can be observed in Figure 2, the combination of obesity was no significantly associated with a higher risk of OS than no-obesity in patients with TNBC.
Figure 2.
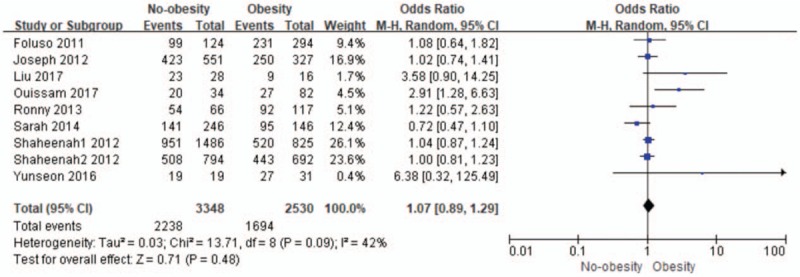
The relationship between obesity and no-obesity for overall survival (OS). CI = confidence interval.
Pooled odds ratio for OS was 1.07 (95% CI: 0.89–1.29; I2 = 42%; n = 5878) for no-obesity, and there was no significant difference of the obesity and no-obesity on impact of OS in TNBC (P = .48).
We continued to conduct subgroup analysis with the obesity definition (BMI ≥30 kg/m2 group vs BMI <30 kg/m2 group) which is shown in Figure 3. In the BMI ≥30 mg/m2 group, pooled odds ratio for OS was 1.01 (95% CI: 0.85–1.21; I2 = 18%; n = 4226) for no-obesity, and there was no significant difference of the obesity and no-obesity on impact of OS in TNBC (P = .87). In the BMI <30 mg/m2, pooled odds ratio for OS was 1.79 (95% CI: 0.67–4.82; I2 = 73%; n = 1652) for no-obesity, the pooled result was no statistically significant yet (P = .25).
Figure 3.
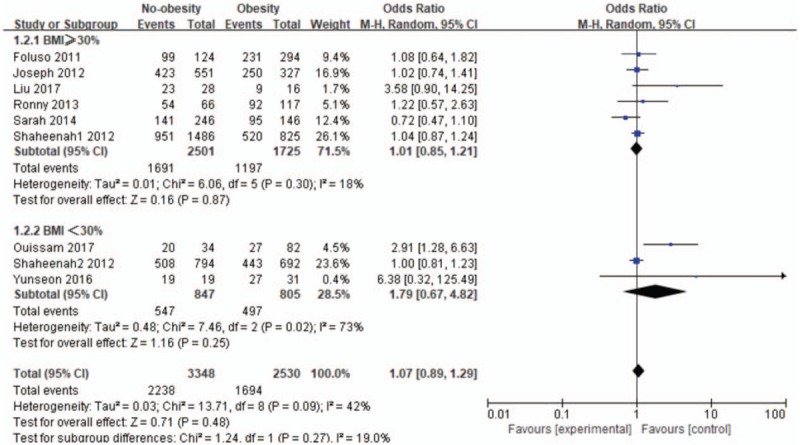
The subgroup analysis in the relationship between obesity and no-obesity for overall survival (OS). CI = confidence interval.
3.3. Obesity vs no-obesity for disease-free survival
As shown in Figure 4, the combination of obesity was not related significantly with a greater risk of DFS than no-obesity in patients with TNBC, either.
Figure 4.
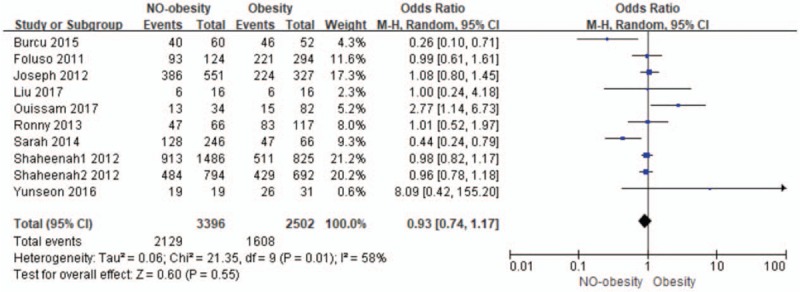
The relationship between obesity and no-obesity for DFS.
Pooled odds ratio for DFS was 0.93 (95% CI: 0.74–1.17; I2 = 58%; n = 5898) for no-obesity, and there was not statistically significant of the obesity or no-obesity on impact of DFS in TNBC (P = .55).
We consequently conducted subgroup analysis with the obesity definition (BMI ≥30 kg/m2 group vs BMI <30 kg/m2 group), which is shown in Figure 5. In the BMI ≥30 mg/m2 group, pooled odds ratio for DFS was 0.98 (95% CI: 0.77–1.24; I2 = 49%; n = 4338) for no-obesity, and there was no significant difference of the obesity and no-obesity on impact of OS in TNBC (P = .87). In the BMI <30 mg/m2, pooled odds ratio for DFS was 1.76 (95% CI: 0.64–4.86; I2 = 71%; n = 1650) for no-obesity, and the pooled result was no statistically significant (P = .27).
Figure 5.
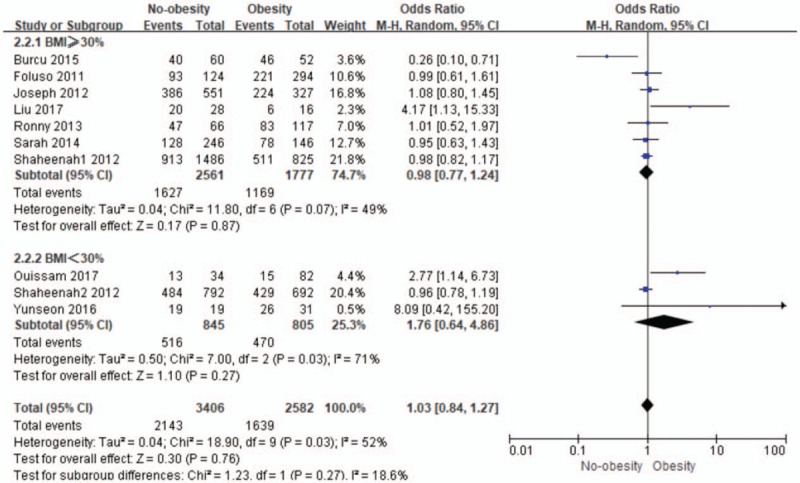
The subgroup analysis in relationship between obesity with no-obesity for disease-free survival (DFS). CI = confidence interval.
3.4. Heterogeneity
In this meta-analysis, moderate-level heterogeneity (I2 = 42%, P = .09; Fig. 2) and substantial-level heterogeneity (I2 = 58%, P = .01; Fig. 4) was estimated in the involved studies on the association between obesity with no-obesity for OS and DFS in TNBC. When we narrowed down the group according to different BMI definition and conducted subgroup meta-analysis, the heterogeneity had polarized into 2 diversifications. For the OS in TNBC, there was no heterogeneity (P = .30) in BMI ≥30% subgroup and substantial heterogeneity (I2 = 73%, P = .02) in BMI <30%, respectively. It had moderate heterogeneity (I2 = 49%, P = .07) in BMI ≥30% subgroup and substantial heterogeneity (I2 = 71%, P = .03) in BMI <30% for the DFS in TNBC.
3.5. Publication bias
It is indicated that no publication bias was observed, and there were no statistical differences (both P > .05) that is shown in the Egger regression table (appendix).
4. Discussion
Considering the absence from therapeutic target, TNBC usually leads to worse prognosis, so it is worth to explore the controllable factor, such as obesity. In recent years, a great volume of studies had tested the association of outcomes in TNBC with obesity; however, the conclusions during each other are controversial. Epidemiological studies have proven that obesity increases the risk of breast cancer, and performs an independent risk factor for poor outcome in it.[31]
Opposite to hormone receptor-positive breast tumors, obesity has a lower risk of mortality for hormone receptor-negative breast cancer. A key study involving 1238 women with breast cancer, Maehle and Tretli[33] examined the impact of BMI on breast cancer mortality, and found that the risk of mortality in women with obesity was generally 49% higher. When using receptor status to check this impact, the risk of death in obese women with hormone receptor-positive tumors was 3 times higher than that in lean women. However, in women with hormone receptor-negative tumors, this effect of obesity was the inverse situation.
There were a large amount of studies concerning the relationship between obesity with the clinical prognosis of TNBC, yet the conclusions of them are controversial.
In a study involved 44 patients with TNBC, Liu et al[31] reported that obesity was related to worse DFS (HR 2.62, 95% CI: 1.03–6.66, P = .04) and a tendency toward worse OS (HR 3.00, 95% CI 0.95–9.51, P = .06). In another study with 2041 TNBC patients, Fontanella et al[34] suggested that the mean DFS in obesity and very obesity (68.0 months P = .043 and 42.3 months P = .010, respectively) and mean OS in obesity and severe obesity (74.5 months P = .018 and 48.0 months P = .003, respectively) were significantly less than those in normal weight (DFS 77.7 months; OS 85.3 months). Both the studies indicated that obesity had a huge negative impact on DFS and OS in patients with triple negative breast tumors.
Nevertheless, there are different standpoints. In a study by Ronny et al[28] in 2013, which contained 183 patients with TNBC, it is concluded that the OS amount of normal patients was higher than that of overweight or obese patients, and there was no significant difference (P = .29), and there was no statistically significant difference in disease-free survival (P = .91). Another study that included 418 TNBC patients, Foluso et al[22] pointed out that compared with normal or underweight patients, the HR for OS in obesity was 0.94 (95% CI: 0.54–1.64; P = .825), and in overweight was 0.60 (95% CI: 0.32–1.14; P = .120), as well as the HR for DFS was 0.81 (95% CI: 0.49–1.34; P = .416) for patients with obesity and 0.74 (95% CI: 0.43–1.27; P = .274) for overweight patients, there was still not significant difference between them, which meant that this single study did not find any clear relationship between higher BMI with OS or DFS in patients with TNBC. Both of these conclusions were consistent with the results of our meta-analysis. When conducting the meta-analysis, we found that the association between obesity and DFS or OS in patients with TNBC was not statistically significant, and the included studies were more than moderate-level heterogeneity. Secondarily, we conducted the meta-analysis based on the obesity definition (BMI ≥30 kg/m2 group or BMI <30 kg/m2 group that is equivalent to the occidental race or nonoccidental race, Table 1), and found that the relationship between obesity and DFS or OS in patients with TNBC remained not statistically significant in both subgroups.
This was the first meta-analysis that focused on the obesity as a predictor affecting the DFS and OS events in patients with TNBC, as well as conducted the subgroup meta-analysis according to different obesity definition. Our study still had its limitation as followings: first, we chose only English literatures which could lead to selection bias and measurement bias, although we did not find publication bias; second, in the involved studies, there might be clinical heterogeneity, such as different chemotherapy regimens, demographic baseline, pathological stage, histology, menopausal status, lymphovascular invasion, and median follow-up, this was why we using the random effect model for the purpose to merge and reduce the impact of heterogeneity; third, the number of included literatures was too small to provide effective information for meta-analysis, and might lead to the results bias of meta-analysis, although we did not find statistically significant publication bias in the Egger’ regression; finally, the survival analysis was calculated by OR value that might lead to a unauthentic conclusion.
In the clinical treatment, in addition to considering the obesity as an impact factor on the survival of patients with TNBC, there are amounts of non-negligible factors needed to be taken into account, incorporating the patients age, clinical stage, tumor size, histological type, lymphatic node positivity, chemotherapy administration (adjuvant chemotherapy or neoadjuvant chemotherapy), etc. Therefore, we intend to analyze these factors in the oncoming future.
5. Conclusion
The result of this meta-analysis study indicates that there is no statistical significance between obesity with DFS and OS in patients with TNBC. However, due to the limited number of literature review, further analysis will be necessary to confirm this conclusion.
Author contributions
Conceptualization: Mengmeng Xu.
Data curation: Lin He, Mengmeng Xu.
Formal analysis: Lin He, Mengmeng Xu.
Funding acquisition: Lin Mei.
Investigation: Lin He, Yang Lv.
Methodology: Yang Lv.
Resources: Lin He, Fengxi Hao.
Software: Lin He.
Supervision: Lin He, Yuhua Song, Lijiu Zhang.
Validation: Lin He.
Writing – original draft: Lin Mei.
Writing – review and editing: Lin Mei.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, DFS = disease-free survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, OR = odds ratio, OS = overall survival, PR = progesterone receptor, TNBC = triple-negative breast cancer.
LM, LH, and YS contributed equally to this work.
Compliance with Ethical Standards: The authors have no potential conflicts of interest in this manuscript, and this article does not contain any studies with human participants or animals performed by any of the authors.
Supplemental Digital Content is available for this article.
References
- [1].Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology 2009;41:40–7. [DOI] [PubMed] [Google Scholar]
- [2].Vona-Davis L, Rose DP, Hazard H, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev 2008;17:3319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol 2010;33:637–45. [DOI] [PubMed] [Google Scholar]
- [4].Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137:307–14. [DOI] [PubMed] [Google Scholar]
- [5].Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938–48. [DOI] [PubMed] [Google Scholar]
- [6].Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. [DOI] [PubMed] [Google Scholar]
- [7].Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006;24:5652–7. [DOI] [PubMed] [Google Scholar]
- [8].Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 2014;232:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu H, Eirew P, Mullaly SC, et al. The omics of triple-negative breast cancers. Clin Chem 2014;60:122–33. [DOI] [PubMed] [Google Scholar]
- [10].Shaver TM, Lehmann BD, Beeler JS, et al. Diverse, biologically relevant, and targetable gene rearrangements in triple-negative breast cancer and other malignancies. Cancer Res 2016;76:4850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wend P, Runke S, Wend K, et al. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med 2013;5:264–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].King TD, Suto MJ, Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem 2012;113:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kriegsmann M, Endris V, Wolf T, et al. Mutational profiles in triple-negative breast cancer defined by ultradeep multigene sequencing show high rates of PI3K pathway alterations and clinically relevant entity subgroup specific differences. Oncotarget 2014;5:9952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gordon V, Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clin Cancer Res 2013;19:3738–44. [DOI] [PubMed] [Google Scholar]
- [15].Massihnia D, Galvano A, Fanale D, et al. Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 2016;7:60712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McDaniel JM, Varley KE, Gertz J, et al. Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget 2017;8:8226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rangel MC, Bertolette D, Castro NP, et al. Developmental signaling pathways regulating mammary stem cells and contributing to the etiology of triple-negative breast cancer. Breast Cancer Res Treat 2016;156:211–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: links to cancer. J Carcinog 2013;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].D’Esposito V, Liguoro D, Ambrosio MR, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016;7:24495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heo M, Kabat GC, Gallagher D, et al. Optimal scaling of weight and waist circumference to height for maximal association with DXA-measured total body fat mass by sex, age and race/ethnicity. Int J Obes (Lond) 2013;37:1154–60. [DOI] [PubMed] [Google Scholar]
- [22].Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention 2017;67:378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat 2010;119:145–53. [DOI] [PubMed] [Google Scholar]
- [24].Herman DR, Ganz PA, Petersen L, et al. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast Cancer Res Treat 2005;93:13–23. [DOI] [PubMed] [Google Scholar]
- [25].Cakar B, Muslu U, Erdogan AP, et al. The Role of Body Mass Index in Triple Negative Breast Cancer. Oncol Res Treat 2015;38:518–22. [DOI] [PubMed] [Google Scholar]
- [26].Dawood S, Lei X, Litton JK, et al. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer 2012;12:364–72. [DOI] [PubMed] [Google Scholar]
- [27].Ademuyiwa FO, Groman A, O’Connor T, et al. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 2011;117:4132–40. [DOI] [PubMed] [Google Scholar]
- [28].Mowad R, Chu QD, Li BD, et al. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res 2013;184:253–9. [DOI] [PubMed] [Google Scholar]
- [29].Choi Y, Park SK. Being Overweight or Obese Increases the Risk of Progression in Triple-Negative Breast Cancer after Surgical Resection. J Korean Med Sci 2016;31:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 2012;118:5937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu YL, Saraf A, Catanese B, et al. Obesity and survival in the neoadjuvant breast cancer setting: role of tumor subtype in an ethnically diverse population. Breast Cancer Res Treat 2018;25:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [33].Maehle BO, Tretli S. Pre-morbid body-mass-index in breast cancer: reversed effect on survival in hormone receptor negative patients. Breast Cancer Res Treat 1996;41:123–30. [DOI] [PubMed] [Google Scholar]
- [34].Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat 2015;150:127–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


