Abstract
Background:
This study explored the feasible efficacy and safety of the Spore Powder of Ganoderma Lucidum (SPGL) for treating patients with Alzheimer disease (AD).
Methods:
Forty-two eligible patients with AD were recruited. These patients were randomly allocated to an intervention group and a control group equally. The patients in the intervention group underwent SPGL, whereas the subjects in the control received placebo. All patients were treated for a total of 6 weeks. The primary outcome was measured by Alzheimer's disease Assessment Scale-Cognitive (ADAS-cog). The secondary outcomes were measured by the World Health Organization Quality of Life questionnaire (WHOQOL-BREF) and Neuropsychiatric Index (NPI). The adverse events were also recorded during the treatment period.
Results:
At the end of the treatment, GLSP did not show more encouraging outcomes in symptoms improvement, measured by the ADAS-cog (P = .31), and NPI (P = .79); and quality of life enhancement, measured by the WHOQOL-BREF (physical, P = .62; psychological, P = .69; social relationships, P = .75; environment, P = .82; overall quality of life, P = .74), compared with the control group. In addition, all adverse events were mild, and no significant differences were found between 2 groups.
Conclusion:
The results of this study did not find the promising efficacy of SPGL for the treatment of AD after 6-week treatment. It may be because of the relative short-term of intervention. Future clinical trials with larger sample size and longer treatment period are urgently needed.
Keywords: Alzheimer disease, Spore Powder of Ganoderma Lucidum, efficacy
1. Introduction
Alzheimer disease (AD) is a chronic progressive neurodegenerative disease among elderly population.[1–3] It often manifests with losing of reasoning, memory, language, and personality changes.[4,5] It has been reported that AD accounts for 60% to 70% of all dementia cases.[6] It has been estimated that the worldwide prevalence increases from 26.6 million in 2006 to the quadruple numbers with 1 in 85 persons worldwide by 2050.[7] The health care costs $172 billion per year for patients with AD in the United States alone.[8] Thus, it brings a severer public health challenge for the society, especially with the rapid increase of the aging population worldwide.
Treatment mainly consists of cognition enhancing medications. These medications include donepezil,[9–11] galantamine,[12–13] memantine,[14–16] and rivastigmine.[17,18] However, all these medications can not cure AD completely. They just temporarily relieve symptoms, such as mental function, lowers blood pressure, and balance mood for patients with AD.[9–18] Most importantly, if patients received these medications for a long term, they also bring lots of adverse events for the patients.[19–21]
Alternative treatment is urgently for patients with AD. Spore Powder of Ganoderma Lucidum (SPGL) is a potential intriguing candidate. Animal studies have support the efficacy of SPGL for the treatment of AD.[22–29] However, there is still no clinical study that investigates the efficacy and safety of SPGL for treating patients with AD. In this pilot study, we evaluated the feasible efficacy and safety of SPGL in Chinese patients with AD.
2. Methods/design
2.1. Objective
The primary objective of this pilot study is to evaluate the feasible efficacy of SPGL for treating patients with AD.
2.2. Design
This pilot-randomized controlled trial included a 6-week treatment period. Forty-two eligible patients with AD were equally and randomly allocated to an intervention group and a control group. After randomization, patients in the intervention group underwent SPGL, whereas patients in the control group received placebo over a period of 6 weeks. All outcomes were measured and evaluated at baseline and at the end of 6-week treatment.
This clinical study was conducted based on the principles of the Declaration of Helsinki (version Seoul, 2008). It was approved by the Ethical Committee of First Affiliated Hospital of Jiamusi University. It was conducted at the same hospital from January 2016 to December 2017. All patients provided written informed consent.
2.3. Participants
Patients were recruited through advertisements and posted notices. The recruitment period was from January 2016 to December 2017. The study inclusion criteria of patients were as follows: confirmed diagnosed as AD according to the criteria of Diagnostic and Statistical Manual of Mental Disorders[30]; age between 50 to 86 years; a Minimental State Examination (MMSE) score: 10≤MMSE≤23,[31] and a Hamilton depression scale(HAMD) score: HAMD <7. Patients were excluded if they had asthma or chronic obstructive pulmonary disease; alcohol or drugs abuse; abnormal functions of liver and kidney; psychiatric or severe neurologic diseases, such as brain cancer; and medications to improve the cognitive function during the past 1 month before the recruitment of this study. In addition, patients were also excluded if they had received SPGL within 1 month before the study.
2.4. Randomization allocations and blinding
After the baseline evaluation, all eligible patients with AD were randomly allocated into either an intervention group or a control group in a ratio of 1:1. The randomization was conducted by a professional statistician using SPSS Statistics 17.0 (IBM Corp., Armonk, NY) with computer-generated, randomly location. After randomization, the random number table was sealed in an opaque envelope. All patients, researchers, outcome assessors, and data analysts were masked to the allocation of this study.
2.5. Intervention schedule
All patients in the intervention group received SPGL (Provided by Beijing Great Wall Pharmaceutical Factory with Batch number of B20050008), 4 capsules of 1000 mg each time (250 mg/capsule), 3 times daily, and 7 days weekly for a total of 6 weeks. The patients in the control group received placebo, the same dose, size, color, flavor as the SPGL, as well as the same provider.
2.6. Outcome measurements
The primary outcome was measured by Alzheimer's disease Assessment Scale-Cognitive (ADAS-cog). This tool covers 11 items to evaluate the multiple cognitive domains, including memory, language, praxis, and orientation. It varies from 0 to 70. The higher scores indicate the greater impairment. The secondary outcomes were measured by the World Health Organization Quality of Life questionnaire (WHOQOL-BREF)[32] and Neuropsychiatric Index (NPI).[33] WHOQOL-BREF was used to assess the quality of life.[32] It consists of the domains of physical, psychological, social relationships, environment, and overall quality of life, with the higher scores indicating the greater quality of life. NPI scale was to evaluate the frequency and severity of behavioral symptoms.[33] It includes 10 neuropsychic symptoms and 2 autonomic nervous symptoms. The scores range from 0 to 144, with the higher scores indicating severer behavioral disturbance.[33] The adverse events were also documented during the treatment period.
2.7. Sample size
This study is a pilot study for the assessment of GLSP for patients with AD and the feasibility of a further larger clinical trial. The desired sample size of this pilot study is 42 patients because of the short duration with 6 weeks. Each group had 21 patients with 15% dropout rate, which is the minimum sample size necessary to assess the efficacy of GLSP for AD.[34]
2.8. Statistical analysis
All variables were analyzed by using SPSS Statistics 17.0 (IBM Corp., Armonk, NY). Chi-square test was used to analyze the count data. Student t test was used to analyze measurable data. All the outcome data were analyzed based on the intent-to-treat principle. All safety related data were summarized by using the descriptions if there were not significant differences between the 2 groups after the treatment. P < .05 was considered as the statistical significance.
3. Results
In this pilot study, 71 patients were recruited for eligibility assessment initially (Fig. 1). Of these patients, 29 were excluded because they did not meet the inclusion and exclusion criteria. The remaining 42 patients equally assigned to the intervention group and the control group. All patients in both groups completed the treatment.
Figure 1.
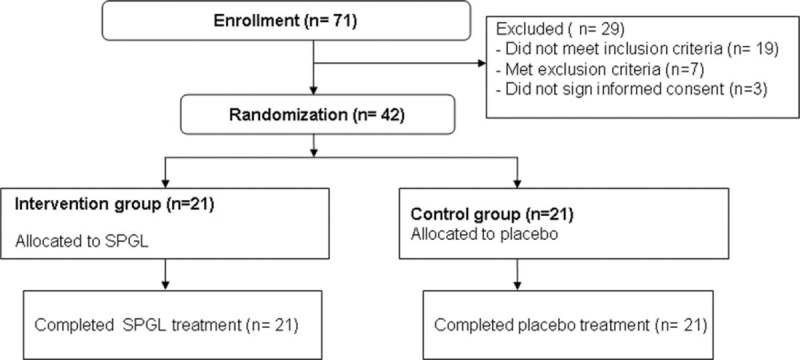
Flowchart of patient selection.
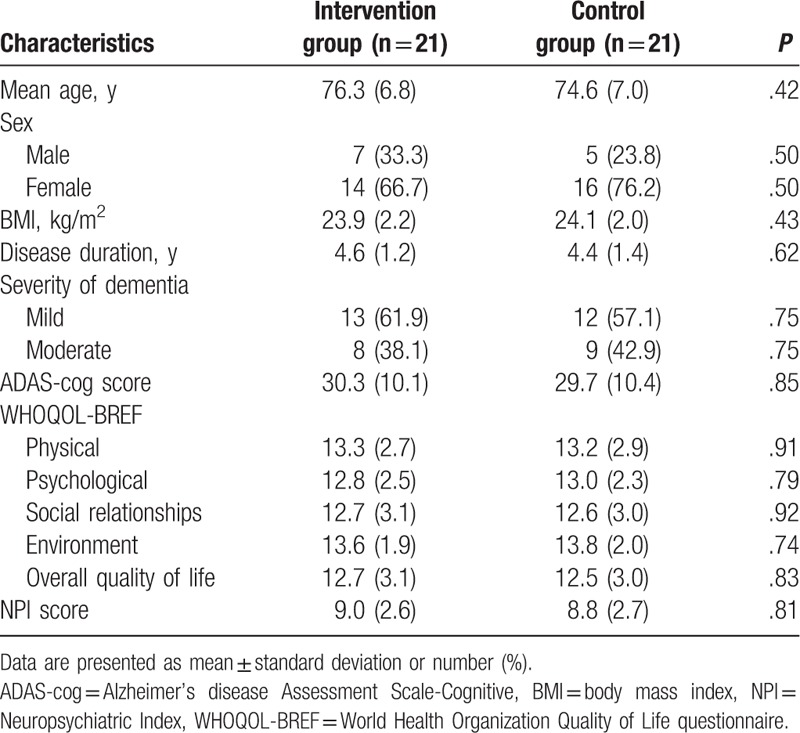
The characteristics of all included patients at baseline are listed in Table 1. There were not significant differences of all patients’ characteristics between 2 groups.
Table 1.
Baseline characteristics of included patients.
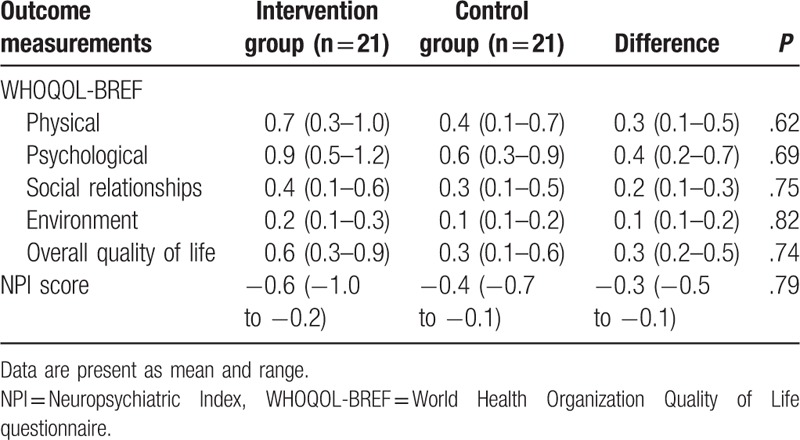
At the end of the treatment, patients in the intervention group did not achieve better symptoms improvements, measured by the ADAS-cog (P = .31; Table 2), and NPI (P = .79; Table 3), compared with those patients in the control group. In addition, patients in the intervention group also did not enhance their quality of life more than patients in the control group, measured by the WHOQOL-BREF (physical, P = .62; psychological, P = .69; social relationships, P = .75; environment, P = .82; overall quality of life, P = .74; Table 3).
Table 2.
Primary outcome measurements at the end of the 6-week treatment (change from baseline).
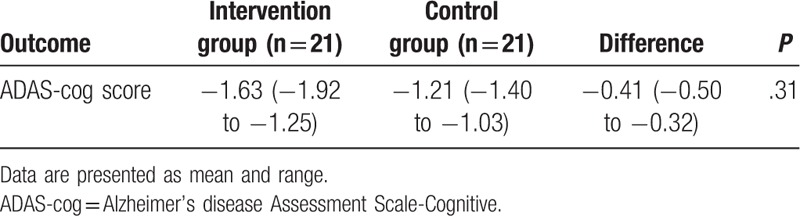
Table 3.
Secondary outcome measurements at the end of the 6-week treatment (change from baseline).
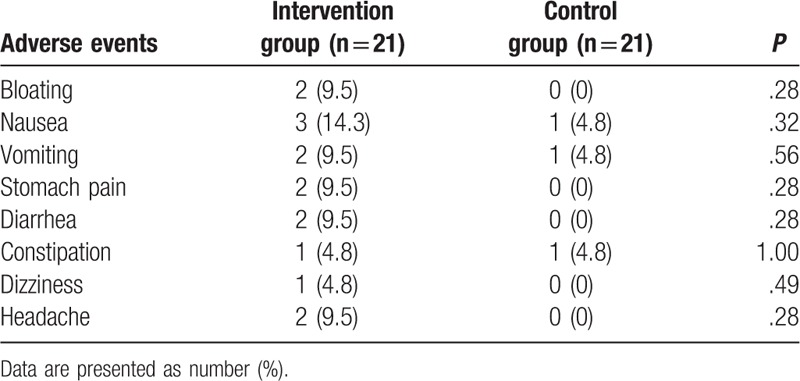
All adverse events related to the treatment are showed in Table 4. No significant differences of adverse events were found between 2 groups. All adverse events were mild, and no severe adverse events occurred.
Table 4.
Adverse events occurred between 2 groups.
4. Discussion
Previous related animal studies found that Ganoderma lucidum polysacchraride peptide (GLPP) can make the senile plaques and neurofibrillary degeneration in the brain, and reduce amyloid vascular lesions.[22] It has been reported that GLPP can also decrease the levels of Aβ, 3,4-methylenedioxyamphetamine,[28] and synaptophysin/synapse, reducing the expression of FasL and caspase-3 in hippocampus, inhibiting tau hyperphosphorylation, alleviating the ultrastructure damage and improving the spatial memory ability of rats with AD.[23,25–26] Moreover, GLPP can enhance the learning and memory ability and antioxidant capacity in the Aβ-induced AD model rats,[24,28] and improve neuronal degeneration in the hippocampal CA1 area.[26,28] The other study has also reported that GLPP has certain protective effect on hippocampal degenerative neurons induced by Aβ25 to 35 in rat model of AD and can also reduce neuroinflammation in brain tissue.[29] However, no clinical study of GLSP for treating AD has been reported.
To our best knowledge, this pilot clinical trial first explored the feasible efficacy and safety of SPGL for the treatment of patients with AD. The results did not show encouraging efficacy of SPGL in ADAS-cog, WHOQOL-BREF, and NPI, when compared with the placebo. It may be because of the relative short period of GLSP therapy. Within such 6-week treatment, GLSP may start to work, but it still did not achieve the statistical significance.
This pilot study has several limitations. First, this study is a pilot study, and it just assesses the feasible efficacy and safety of SPGL for treating patients with AD. Then, it has a pretty small sample size. Finally, this study only included 6-week period of treatment. Thus, all those limitations may affect the results of this study.
5. Conclusion
The results of this study demonstrated that SPGL may not benefit to patients with AD after 6-week treatment. It may be because of the too short treatment period. Thus, further studies should extend the treatment duration, as well as enlarge the sample sizes of the patients with AD.
Author contributions
Conceptualization: Li-hong Qin, Guo-hui Wang.
Data curation: Li-hong Qin, Guo-hui Wang.
Formal analysis: Guo-hui Wang.
Investigation: Guo-hui Wang.
Methodology: Chen Wang.
Project administration: Li-hong Qin, Chen Wang.
Resources: Li-hua Wang.
Software: Li-hong Qin, Li-hua Wang, Chen Wang.
Supervision: Chen Wang.
Validation: Li-hua Wang.
Visualization: Li-hua Wang, Chen Wang.
Writing – original draft: Li-hong Qin, Guo-hui Wang, Li-hua Wang, Chen Wang.
Writing – review and editing: Li-hong Qin, Guo-hui Wang, Li-hua Wang, Chen Wang.
Footnotes
Abbreviations: AD = Alzheimer disease, ADAS-cog = Alzheimer's disease Assessment Scale-Cognitive, GLPP = Ganoderma lucidum polysacchraride peptide, HAMD = Hamilton depression scale, MMSE = minimental state examination, NPI = Neuropsychiatric Index, SPGL = Spore Powder of Ganoderma Lucidum, WHOQOL-BREF = World Health Organization Quality of Life questionnaire.
This study was supported by the Dr. Start Project of Jiamusi University (22Zb201510).
The authors have no conflicts of interest to disclose.
References
- [1].Jiang H, Liu Y, Wei Y, et al. Impaired retinal microcirculation in patients with Alzheimer's disease. PLoS One 2018;13:e0192154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hadjichrysanthou C, Ower AK, de Wolf F, et al. The development of a stochastic mathematical model of Alzheimer's disease to help improve the design of clinical trials of potential treatments. PLoS One 2018;13:e0190615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tang Y, Lin X, Lin XJ, et al. Therapeutic efficacy of neuromuscular electrical stimulation and electromyographic biofeedback on Alzheimer's disease patients with dysphagia. Medicine (Baltimore) 2017;96:e8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peng X, Xing P, Li X, et al. Towards Personalized Intervention for Alzheimer's Disease. Genomics Proteomics Bioinformatics 2016;14:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol 2018;25:59–70. [DOI] [PubMed] [Google Scholar]
- [6].Jindal H, Bhatt B, Sk S, et al. Alzheimer disease immunotherapeutics: then and now. Hum Vaccin Immunother 2014;10:2741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007;3:186–91. [DOI] [PubMed] [Google Scholar]
- [8].Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014;88:640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ma Y, Ji J, Li G, et al. Effects of donepezil on cognitive functions and the expression level of β-amyloid in peripheral blood of patients with Alzheimer's disease. Exp Ther Med 2018;15:1875–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Batarseh YS, Kaddoumi A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer's disease. J Nutr Biochem 2017;55:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hardy CJD, Hwang YT, Bond RL, et al. Donepezil enhances understanding of degraded speech in Alzheimer's disease. Ann Clin Transl Neurol 2017;4:835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim JK, Park SU. Pharmacological aspects of galantamine for the treatment of Alzheimer's disease. EXCLI J 2017;16:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tokuchi R, Hishikawa N, Matsuzono K, et al. Cognitive and affective benefits of combination therapy with galantamine plus cognitive rehabilitation for Alzheimer's disease. Geriatr Gerontol Int 2016;16:440–5. [DOI] [PubMed] [Google Scholar]
- [14].Kishi T, Matsunaga S, Iwata N. The effects of memantine on behavioral disturbances in patients with Alzheimer's disease: a meta-analysis. Neuropsychiatr Dis Treat 2017;13:1909–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kishi T, Matsunaga S, Oya K, et al. Memantine for Alzheimer's disease: an updated systematic review and meta-analysis. J Alzheimers Dis 2017;60:401–25. [DOI] [PubMed] [Google Scholar]
- [16].Dong H, Wu S, Hu N, et al. Efficacy of tenuigenin and β-asarone as augmentations for memantine in the treatment of Alzheimer's disease. Neuroreport 2018;29:203–7. [DOI] [PubMed] [Google Scholar]
- [17].Birks JS, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev 2015;CD001191. [DOI] [PubMed] [Google Scholar]
- [18].Servello A, Ettorre E, Cacciafesta M. Improvement in behavioral and psychological symptoms of dementia by rivastigmine patch in a group of Italian elderly patients with Alzheimer's disease. Panminerva Med 2017;59:275–7. [DOI] [PubMed] [Google Scholar]
- [19].Götz J, Eckert A, Matamales M, et al. Modes of Aβ toxicity in Alzheimer's disease. Cell Mol Life Sci 2011;68:3359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Drolle E, Negoda A, Hammond K, et al. Changes in lipid membranes may trigger amyloid toxicity in Alzheimer's disease. PLoS One 2017;12:e0182194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ntsapi C, Lumkwana D, Swart C, et al. New insights into autophagy dysfunction related to amyloid beta toxicity and neuropathology in Alzheimer's disease. Int Rev Cell Mol Biol 2018;336:321–61. [DOI] [PubMed] [Google Scholar]
- [22].Qin C, Wu SQ, Chen BS, et al. Pathology of ganoderma lucidum in treating APP/PS-1 Alzheimer's disease transgenic mice model. Chin Acad Med Sci 2017;39:552–61. [DOI] [PubMed] [Google Scholar]
- [23].Li YP, Wang XY, Chen J, et al. Effects of Ganoderma lucidum polysaccharide peptide on β-amyloid protein and hyperphosphorylation of tau protein in Alzheimer's disease rats. Chin J Pract Diagn Treat 2015;29:862–5. [Google Scholar]
- [24].Yan T, Chen SB, Xu L, et al. Ganoderma lucidum polysaccharide on Alzheimer's disease in rats learning and memory and oxidative stress. Shaanxi Med J 2011;40:387–9. [Google Scholar]
- [25].Yuan DJ, Zhang YF, Yao CX. Effects of Ganoderma lucidum polysaccharides on synaptic and synaptophysin expression in the hippocampus of Alzheimer's disease model rats. Acta Metallurgica Sinica 2011;17:151–5. [Google Scholar]
- [26].Zhang YP, Yuan H, Li L, et al. Effects of Ganoderma lucidum polysaccharides on the expression of Caspase-3 and FasL in hippocampus of AD model rats. Chin J Histochem Cytochem 2008;19:484–9. [Google Scholar]
- [27].Guo YJ, LI P, Yuan H. Effects of Ganoderma lucidum polysaccharides on learning and memory in rats with Alzheimer's disease and its relationship with apoptosis of hippocampal cells. Anality Tribune 2008;44:376–8. [Google Scholar]
- [28].Guo YJ, Yuan H, Zhang LN, et al. Effects of Ganoderma lucidum polysaccharides on the histopathology and antioxidant capacity of hippocampus in Alzheimer's disease rats. J Anatomy 2006;53:509–13. [Google Scholar]
- [29].Guo YJ, Yuan H, Wen SW, et al. Ganoderma lucidum polysaccharide on Aβ25-35-induced Alzheimer's disease in rats brain tissue protection. Chin J Histochem Cytochem 2006;17:447–51. [Google Scholar]
- [30].McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [31].Porsteinsson AP, Grossberg GT, Mintzer J, et al. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double blind, placebo-controlled trial. Curr Alzheimer Res 2008;5:83–9. [DOI] [PubMed] [Google Scholar]
- [32].Fleck MPA. Desenvolvimento da versão em português do instrumento de avaliação de qualidade de vida da OMS (WHOQOL-100). Rev Bras Psiquiatr 1999;21:19–28. [Google Scholar]
- [33].Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–14. [DOI] [PubMed] [Google Scholar]
- [34].Johanson GA, Brooks GP. Initial scale development: sample size for pilot studies. Educ Psychol Meas 2010;70:394–400. [Google Scholar]


