Abstract
Percutaneous cholecystostomy (PC) is a well-established treatment for acute cholecystitis. We investigate the performance and role of PC in managing acute cholangitis.
Retrospective review on all patients who underwent PC for acute cholangitis between January 2012 and June 2017 at a major regional hospital in Hong Kong.
Thirty-two patients were included. The median age was 84 years and median American Society of Anaesthesiologists (ASA) physical status was Class III (severe systemic disease). All fulfilled Tokyo Guidelines 2013 (TG13) diagnostic criteria for moderate or severe cholangitis. Eighty-four percent of the patients were shown to have lower common bile duct stones on imaging. The majority had previously failed intervention by endoscopic retrograde cholangiopancreatography (38%), percutaneous transhepatic biliary drainage (38%), or both (13%)
The technical success rate for PC was 100% with no procedure-related mortality. The overall 30-day mortality was 9%. Rest of the patients (91%) had significant improvement in clinical symptoms and could be discharged with median length of stay of 14 days. Significant postprocedural biochemical improvement was observed in terms of white cell count (P < .001), serum bilirubin (P < .001), alkaline phosphatase (P = .001), and alanine transaminase levels (P < .001). Time from admission to PC was associated with excess mortality (P = .002).
PC is an effective treatment for acute cholangitis in high-risk elderly patients. Early intervention is associated with lower mortality. PC is particularly valuable as a temporising measure before definitive treatment in critical patients or as salvage therapy where other methods endoscopic retrograde cholangiopancreatography/percutaneous transhepatic biliary drainage (ERCP/PTBD) have failed.
Keywords: cholangitis, critical care, geriatrics, percutaneous cholecystostomy
1. Introduction
Acute cholangitis is a life-threatening illness characterized by ascending infection and inflammation of the biliary tree. Unlike acute cholecystitis, many patients suffer from systemic disturbance such as cardiorespiratory failure or disseminated intravascular coagulation. Even with modern treatment, the mortality of acute cholangitis can be up to 27%. [1]
Cholangitis is facilitated by biliary stasis and stones. The acute treatment of severe acute cholangitis therefore involves drainage of bile by endoscopic intervention (endoscopic retrograde cholangiopancreatography [ERCP]) or percutaneous intervention (percutaneous transhepatic biliary drainage [PTBD]), in addition to parenteral antibiotics and supportive care.[2]
ERCP and PTBD are considered difficult procedures with failure rates up to 11% and 19% respectively.[3,4] This is especially the case in frail elderly patients who are unable to cooperate during procedures. Furthermore, elderly patients are more likely to have pre-existent comorbidities and severe grade cholangitis. [5]
Percutaneous cholecystostomy (PC) is a minimally invasive method routinely employed to treat acute cholecystitis. We postulate that it may also be effective for acute cholangitis in selected patient groups and conducted a retrospective review on cases where PC is applied in such a manner.
2. Methods
This is a retrospective study performed by analyzing electronic patient records. The local institution review board approved of the study. The setting is a major hospital in Hong Kong with radiology, medicine, and surgery departments.
All patients diagnosed of acute cholangitis who underwent PC between January 1, 2012 and June 30, 2017 were included. Patients with acute cholecystitis, underlying malignant biliary obstruction and those who successfully underwent other forms of treatment were excluded.
All PCs were performed by 6 interventional radiologists under local anaesthesia using a standard trocar technique.[6] After a small skin incision, a 7 French (Fr) pigtail catheter with an internal metallic trocar and locking system (Mermaid Medical A/S, Copenhagen, Denmark) was inserted under sonographic guidance. The transhepatic approach was adopted. After the deployment of the pigtail loop within the gallbladder, small amount of contrast medium (iohexol 300 mg iodine/mL, Omnipaque 300, GE Healthcare, Shanghai, China, diluted to half strength) was injected under fluoroscopy to confirm placement. The catheter was anchored to skin with stitches (Figs. 1–3).
Figure 1.
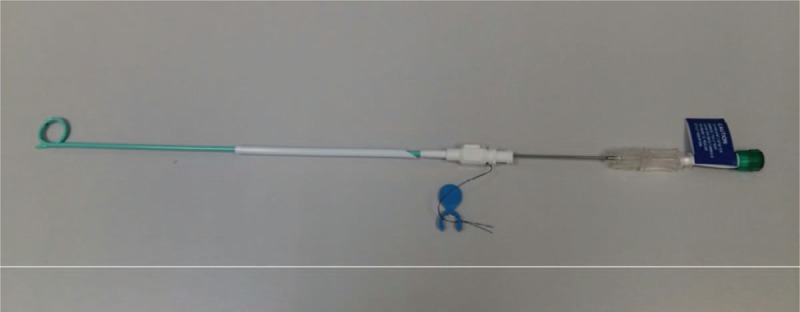
A 7Fr single-step locking pigtail catheter used for percutaneous cholecystostomy in our institution. (Mermaid Medical A/S, Copenhagen, Denmark).
Figure 3.

Cholangiogram showing gallstones and an obstructing lower common bile duct stone with upstream biliary dilation.
Figure 2.

Ultrasound image demonstrating the catheter entering the gallbladder.
Patient data including demographics, comorbidities, presentation, imaging findings, biochemistry results, procedural records, discharge, and follow-up data were analyzed with IBM SPSS Statistics for Windows Version 24.0 (IBM Corp, Armonk, NY). Paired t test was used to analyze the change in biochemical parameters at presentation and prior to discharge. Mann–Whitney U test, Pearson correlation, and Spearman correlation were used to ascertain the relationship between patient features with outcome. Results with P value less than or equal to.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
Thirty-two patients underwent PC for acute cholangitis. Most were elderly patients (median age of 84) with multiple comorbidities (median American Society of Anaesthesiologists (ASA) class III). Baseline patient characteristics are presented at Table 1.
Table 1.
Patient baseline characteristics.

3.2. Diagnosis of acute cholangitis
All patients presented with fever, abdominal pain, and liver function derangement. The median serum bilirubin level was 68 μmol/L (reference range: 4−23 μmol/L). The median white cell count was 18.1 × 109/L (reference range: 3.89−9.93 × 109/L). Biochemical parameters at presentation are shown in Table 2.
Table 2.
Comparison of biochemical parameters at presentation and at discharge.

All patients fulfilled the diagnosis of acute cholangitis under Tokyo “TG13” criteria.[7] Twenty-one patients had severe (Grade III) acute cholangitis while the remaining 11 had moderate (Grade II) acute cholangitis.
Twenty-eight patients had bile samples sent for culture and all yielded positive growth. The most common causative microbe was E coli (23/32, 72%) followed by Enterococcus faecalis (9/32, 28%), and Klebsiella spp. (8/32, 25%).
Most patients underwent ultrasound (56%) for diagnostic evaluation, followed by computed tomography (47%) and magnetic resonance cholangiopancreatography (22%). The mean maximal diameter of the common bile duct (CBD) was 12.5 mm. Intrahepatic ducts were dilated in 5 patients (16%). Most patients had stones visualized at the distal CBD (27/32, 84%). The CBD was obscured in 3 patients who underwent ultrasound. The imaging details of the patients were summarized in Table 3.
Table 3.
Imaging characteristics of patients at presentation.

3.3. Previous interventions
Twelve patients (38%) received PC as upfront therapy as they were deemed not suitable for ERCP by the clinical team. Others had prior failed interventions for the same episode. Twelve patients (38%) failed ERCP with the most common reason being failure to cannulate the CBD (9/12, 75%). Twelve patients (38%) failed PTBD with the most common reason being failure to puncture the nondilated intrahepatic ducts. Details of prior interventions were tabulated in Table 4.
Table 4.
Prior interventions.

3.4. Percutaneous cholecystostomy
All PCs were performed in accordance with the technique as described. The median time from admission to procedure was 63.5 hours (range: 1−123 hours). Drains were successfully placed with 1 pass for all cases. No immediate complication was observed within 24 hours after procedure. There was 1 procedure-related complication: biliary peritonitis occurred 4 days after procedure of a patient who subsequently made an uneventful recovery after laparotomy.
3.5. Clinical outcome
In the biochemical aspect, there was significant drop in white cell count, serum bilirubin, alkaline phosphatase (ALP), alanine transaminase (ALT) levels after procedure (Table 2).
Twenty-nine patients (91%) made a good recovery with improvement of clinical symptoms and signs leading to discharge from hospital. The median length of stay for surviving patients was 14 days. Three patients (9%) died during the same admission with cause attributed to biliary sepsis. The 30-day postprocedural mortality rate was 9%, of which the median survival after the procedure was 14 days. A longer time from admission to PC was associated with mortality during the same episode (P = .002) (Table 5).
Table 5.
Relationship between time from admission to intervention and outcome.

History of failed intervention (ERCP or PTBD) before PC was not associated with mortality (P = .24). There was also no significant association of CBD diameter (P = .30), preprocedural bilirubin level (P = .80), or white cell count (P = .21), with mortality.
Twenty-one patients (65%) underwent elective ERCP after PC in the same admission episode. This was successful in 18 patients (86%) in whom stone clearance was confirmed. This included 7 of 12 patients (58%) who initially failed ERCP. Four patients (13%) underwent delayed cholecystectomy. Four patients (13%) developed recurrent episodes of biliary sepsis which occurred between 1 and 13 months after discharge.
4. Discussion
Ultrasound-guided PC was first described by Elyaderani and Gabriele [8] and first applied to patients with acute cholecystitis in 1982 by Radder.[9] It has subsequently gained popularity in the treatment of acute cholecystitis in patients who are too frail to undergo emergency cholecystectomy. In a systematic review performed in 2009, 53 studies have been published regarding PC in the treatment of acute cholecystitis.[10] In contrast, the evidence for using PC in treating cholangitis is scanty. There are only 2 retrospective cohorts examining 6 and 8 patients respectively in peer-reviewed literature.[11,12] To our knowledge, this is the largest cohort (32 patients) presented.
Mild acute cholangitis may subside with supportive care and antibiotics. However, biliary drainage is often necessary for moderate to severe cholangitis.[13] ERCP-based treatments such as papillotomy, placement of drainage catheters, and stone removal have traditionally been favored as the modality of biliary drainage[14,15] PTBD is also accepted as an alternative. [16]
ERCP is a difficult and high-risk procedure that may not be suitable or successful for the frail elderly who are acutely unwell from severe cholangitis. First, it requires sedation and access to the upper aerodigestive tract which may cause hypotension or desaturation in patients who already have cardiorespiratory compromise. Second, it requires patient cooperation that may not be present in disoriented elderly patients. Third, some patients may have unfavorable anatomy rendering CBD cannulation difficult, which is further hampered by hesitancy to proceed to papillotomy in these high-risk patients. Overall, the failure rate of ERCP in our cohort (38%) is substantially higher than that published in the literature (up to 11%). Other ERCP-related complications including bleeding, pancreatitis, perforation, and worsened infection cannot be overlooked in these critically ill patients as well.[17]
PTBD is also a potentially high risk and difficult procedure. The initial step of PTBD entails puncture of an intrahepatic duct (typically few millimeters in diameter) with a fine needle under ultrasound or fluoroscopy. This can fail if the patient cannot control their respiration, or engender additional risk of puncturing adjacent structures such as blood vessels and the lung bases. Nondilated intrahepatic ducts were common (84%) in our cohort, and further added difficulty. This likely explained the higher failure rate for PTBD (38%) observed in our cohort than that quoted in the literature (up to 19%). Lastly, other PTBD-related complications such as worsened sepsis, biliary peritonitis, hemorrhage can also have drastic consequences. [18–20]
On the contrary, PC is better suited for these critical patients. The procedure itself is more straightforward than ERCP and PTBD. The distended gallbladder is a bigger target than the intrahepatic duct and easier to puncture even without full patient cooperation. Therefore, less puncture attempts are needed in PC than in PTBD. It can be completed in 5 to 10 minutes in experienced hands. In certain institutions, PC can also be performed in the ward or intensive care unit with a portable ultrasound machine without necessitating risky transfer to the endoscopy or interventional radiology suite.[6] In our series, 100% technical success was obtained which was consistent with the experience published in large series in the literature. [21]
PC is also a safer procedure with a lower rate of complications. In our series, there was only a single major complication of biliary peritonitis which necessitated laparotomy for lavage and cholecystectomy after which the patient made an uneventful recovery. The procedure-related morbidity is 3% and mortality is 0%. In related literature examining complications of PC in treating cholecystitis, the morbidity and mortality rates were 4% and 0.4% respectively, consistent with our experience and significantly lower than those quoted for ERCP and PTBD in the literature. [10,17–19,22]
Some of the major risks of PTBD are massive hemorrhage (hemobilia or intraperitoneal haemorrhage) and worsened sepsis due to bacteria translocating from the bile ducts into the systemic circulation. PC also avoids ERCP-specific risks such as duodenal perforation and pancreatitis. Lastly, PC can be performed with local anesthesia and obviates the additional risk of sedation or general anaesthesia in ERCP/ PTBD.
Clinically, PC produces robust improvement in symptomatology and biochemical parameters to a point that most patients are well enough to be discharged. Our overall 30-day mortality of 9% compared favorably with that quoted for acute cholangitis in the literature which ranged from 7% to 50%. [1]
Most patients (65%) in our cohort subsequently underwent elective ERCP after PC. Interestingly, 7 out of 12 patients (58%) who initially failed emergency ERCP underwent successful elective ERCP. The overall success rate (86%) was higher than that of the initial cohort (62%). A longer period elapsed between admission to PC was also associated with mortality (P = .02). These findings highlighted the role of PC as a temporising measure to stabilize critical patients before definitive treatment was attempted.
5. Conclusion
PC is an effective treatment for acute cholangitis in high-risk elderly patients with multiple comorbidities. It has high success and low complication rates and can be performed in a matter of minutes to produce robust clinical improvement. Early intervention is associated with lower mortality. PC is particularly valuable as a temporising measure before definitive treatment in critical patients or as a salvage therapy where other methods have failed.
Author contributions
Conceptualization: Yan-Lin Li, Kin-Hoi Wong.
Data curation: Yan-Lin Li, Kin-Hoi Wong, Andrew Kai-Chun Cheng, Ronald Kin-On Cheung, Max Kai Ho Yam, Angie Lok-Chi Chan.
Formal analysis: Yan-Lin Li, Kin-Hoi Wong.
Funding acquisition: Keith Wan-Hang Chiu.
Investigation: Yan-Lin Li, Kin-Hoi Wong.
Methodology: Yan-Lin Li, Kin-Hoi Wong.
Project administration: Yan-Lin Li, Kin-Hoi Wong, Andrew Kai-Chun Cheng, Ronald Kin-On Cheung, Max Kai Ho Yam, Angie Lok-Chi Chan.
Resources: Yan-Lin Li, Kin-Hoi Wong.
Software: Yan-Lin Li, Kin-Hoi Wong.
Supervision: Keith Wan-Hang Chiu, Victor Siang-Hua Chan, Martin Wai-Ling Law, Paul Sing-Fun Lee.
Validation: Yan-Lin Li, Kin-Hoi Wong.
Visualization: Kin-Hoi Wong.
Writing – original draft: Yan-Lin Li.
Writing – review & editing: Yan-Lin Li, Kin-Hoi Wong, Keith Wan-Hang Chiu.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine transaminase, ASA = American Society of Anaesthesiologists, CBD = common bile duct, ERCP = endoscopic retrograde cholangiopancreatography, PC = percutaneous cholecystostomy, PTBD = percutaneous transhepatic biliary drainage, TG13 = Tokyo guidelines 2013.
Ethical approval: The study was approved by the local institutional approval board (Joint CUHK-NTEC CREC).
The authors have no conflicts of interest to disclose.
References
- [1].Lan Cheong Wah D, Christophi C, Muralidharan V. Acute cholangitis: current concepts. ANZ J Surg 2017;87:554–9. [DOI] [PubMed] [Google Scholar]
- [2].Takada T, Strasberg SM, Solomkin JS, et al. TG13: updated Tokyo guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2013;20:1–7. [DOI] [PubMed] [Google Scholar]
- [3].Kühn JP, Busemann A, Lerch MM, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol 2010;195:851–7. [DOI] [PubMed] [Google Scholar]
- [4].DeBenedet AT, Elmunzer BJ, McCarthy ST, et al. Intraprocedural quality in endoscopic retrograde cholangiopancreatography: a meta-analysis. Am J Gastroenterol 2013;108:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sugiyama M, Atomi Y. Treatment of acute cholangitis due to choledocholithiasis in elderly and younger patients. Arch Surg 1997;132:1129–33. [DOI] [PubMed] [Google Scholar]
- [6].Gulati MS. Percutaneous Cholecystostomy and Cholecystolithotomy. Interventional Radiology Procedures in Biopsy and Drainage [Internet]. London: Springer; 2010. 155–63. (Techniques in Interventional Radiology). [Google Scholar]
- [7].Kiriyama S, Takada T, Strasberg SM, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci 2013;20:24–34. [DOI] [PubMed] [Google Scholar]
- [8].Elyaderani M, Gabriele OF. Percutaneous cholecystostomy and cholangiography in patients with obstructive jaundice. Radiology 1979;130:601–2. [DOI] [PubMed] [Google Scholar]
- [9].Radder RW. Percutaneous cholecystostomy. AJR Am J Roentgenol 1982;139:1240–1. [DOI] [PubMed] [Google Scholar]
- [10].Winbladh A, Gullstrand P, Svanvik J, et al. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB 2009;11:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ren Z, Xu Y, Zhu S. Percutaneous transhepatic cholecystostomy for choledocholithiasis with acute cholangitis in high-risk patients. Hepatogastroenterology 2012;59:329–31. [DOI] [PubMed] [Google Scholar]
- [12].Shitrit AB-G, Braverman D. Interval percutaneous cholecystostomy is effective for decompression of the common bile duct in high-risk elderly patients prior to endoscopic retrograde cholangiopancreatography. Gerontology 2008;54:144–7. [DOI] [PubMed] [Google Scholar]
- [13].Okamoto K, Takada T, Strasberg SM, et al. TG13 management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2013;20:55–9. [DOI] [PubMed] [Google Scholar]
- [14].Leung JW, Chung SC, Sung JJ, et al. Urgent endoscopic drainage for acute suppurative cholangitis. Lancet Lond Engl 1989;1:1307–9. [DOI] [PubMed] [Google Scholar]
- [15].Boender J, Nix GA, de Ridder MA, et al. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol 1995;90:233–8. [PubMed] [Google Scholar]
- [16].Pessa ME, Hawkins IF, Vogel SB. The treatment of acute cholangitis. Percutaneous transhepatic biliary drainage before definitive therapy. Ann Surg 1987;205:389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781–2. [DOI] [PubMed] [Google Scholar]
- [18].Hamlin JA, Friedman M, Stein MG, et al. Percutaneous biliary drainage: complications of 118 consecutive catheterizations. Radiology 1986;158:199–202. [DOI] [PubMed] [Google Scholar]
- [19].Mueller P, van Sonnenberg E, Ferrucci J. Percutaneous biliary drainage: technical and catheter-related problems in 200 procedures. Am J Roentgenol 1982;138:17–23. [DOI] [PubMed] [Google Scholar]
- [20].Ginat D, Saad WEA, Davies MG, et al. Incidence of cholangitis and sepsis associated with percutaneous transhepatic biliary drain cholangiography and exchange: a comparison between liver transplant and native liver patients. AJR Am J Roentgenol 2011;196:W73–77. [DOI] [PubMed] [Google Scholar]
- [21].Tseng LJ, Tsai CC, Mo LR, et al. Palliative percutaneous transhepatic gallbladder drainage of gallbladder empyema before laparoscopic cholecystectomy. Hepatogastroenterology 2000;47:932–6. [PubMed] [Google Scholar]
- [22].Kim D, Iqbal SI, Ahari HK, et al. Expanding role of percutaneous cholecystostomy and interventional radiology for the management of acute cholecystitis: an analysis of 144 patients. Diagn Interv Imaging 2018;99:15–21. [DOI] [PubMed] [Google Scholar]


