Abstract
To assess the association of plasma cytokines and growth factor levels with clinical characteristics and inflammatory indices in patients with gastric cancer.
Plasma samples derived from 99 gastric cancer patients were used for analysis. Levels of interferon (IFN)-γ, tumor growth factor (TGF)-β1, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, and vascular endothelial growth factor (VEGF) were measured by Luminex suspension array technology. The association between cytokine/growth factor levels and demographic/clinical characteristics was assessed. Correlation between cytokines and growth factor levels was assessed by Pearson's correlation analysis.
Male patients had significant higher levels of plasma TNF-α, IL-12p70, IL-4, IL-10, and VEGF as compared with those in women (P < .05). Plasma levels of TNF-α in older patients with gastric cancer (≥60 years) were higher than those in young patients (P < .05). Elevated plasma levels of IL-8 and IL-10 were identified as risk factors for increased tumor size (diameter ≥5 cm). Higher plasma levels of TGF-β1 were associated with increased risk of vascular or nerve invasion and advanced tumor stage. The levels of systemic inflammatory markers, including white blood cell counts, neutrophil/lymphocyte proportion, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio (PLR), C-reactive protein and modified Glasgow prognostic score (mGPS) were closely associated with a series of plasma cytokines. A prominent correlation was observed between the plasma IL-12p70 and IFN-γ levels (r = 0.729, P < .01).
Our findings suggest that plasma cytokines and growth factor levels may help predict the development and progression of gastric cancer. Our findings need to be validated by larger studies.
Keywords: cytokine, gastric cancer, growth factor, inflammation, plasma
1. Introduction
According to cancer statistics for 2015,[1] gastric cancer was the second leading cause of cancer related death in China, next only to lung cancer. Although the potential mechanisms of carcinogenesis and progression of gastric cancer have not yet been fully understood, results of recent studies suggest that inflammatory changes in the tumor microenvironment play a vital role in carcinogenesis.[2] Moreover, in addition to surgical resection and chemotherapy, immunotherapy has emerged as a promising therapeutic strategy for clinical management of gastric cancer.[3]
In the tumor-associated inflammatory microenvironment, immune cells communicate with each other through cytokines, chemokines, and growth factors, which may in turn promote or suppress tumor progression.[4] Different types of immune cells have been shown to respond to a diverse range of biochemical signals. For instance, T helper (Th) 1 lymphocytes were shown to be activated by interferon (IFN)-γ and interleukin (IL)-12, both of which were generated mainly by dendritic cells.[5] After induction, these cells generate proinflammatory cytokines including tumor necrosis factor-α (TNF-α) and IFN-γ.[2] Th2 cells could be induced by IL-4, subsequently leading to a secretion of IL-10 which was an immunosuppressive cytokine.[2] Epplein et al[6] reported that high level of IL-8 was associated with increased risk of gastric cancer. Nevertheless, the association of plasma cytokines and growth factors with clinical characteristics and inflammatory indices of gastric cancer remains unexplored.
In this study, we assessed the plasma levels of 12 cytokines and growth factors in patients with gastric cancer using Luminex suspension array technology. An analysis was made to investigate the association of plasma cytokine and growth factor levels with clinical characteristics and inflammatory indices of gastric cancer patients. Our findings may provide important evidence of the potential involvement of these cytokines and growth factors in oncogenesis and development of gastric cancer.
2. Materials and methods
2.1. Subjects
A total of 105 individuals with pathologically confirmed gastric cancer who were admitted to the department of general surgery in Nanjing Drum Tower Hospital (NDTH) between March 2014 and September 2014, were enrolled. None of the patients had received prior surgical treatment, chemotherapy, radiotherapy, or any other anti-cancer therapy. Informed consent was obtained from all participants. The study was approved by NDTH Institutional Ethics Review Board.
Three days prior to surgery, fasting peripheral blood samples (2 mL) were collected from each patient. After centrifugation at 1500 rpm for 15 minutes, 100 μL of the supernatant was collected into a tube and stored at –80 °C for further use.
2.2. Inclusion criteria and exclusion criteria
One hundred five patients diagnosed with pathologically confirmed gastric cancer were eligible for initial inclusion. Subjects who met any of the following criteria were excluded from the study: history of previous surgical treatment, chemotherapy, radiotherapy, or other anti-cancer therapies; patients with infection, chronic inflammation, or autoimmune disease at the time of blood collection; history of malignancy other than gastric cancer; presence of other severe disorders; evidence of hemolysis or other abnormalities in the collected blood samples. Six patients with gastric cancer were excluded, and a total of 99 patients were finally included in the analysis (Fig. 1).
Figure 1.
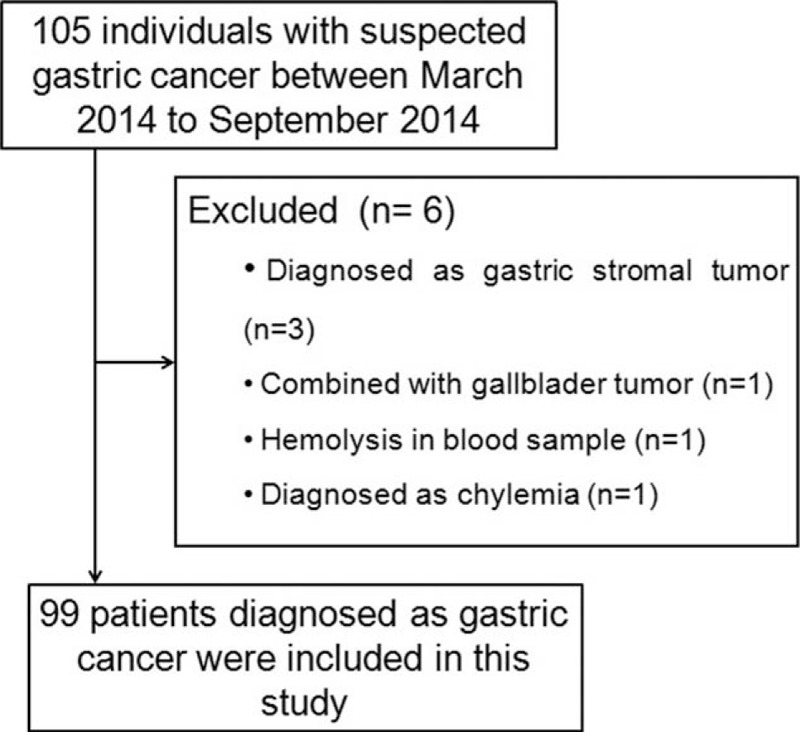
Schematic illustration of the selection criteria for patients with gastric cancer.
2.3. Data collection
We collected the demographics of patients such as sex and age. The clinicopathological characteristics including tumor type, differentiation status, tumor diameter, vascular invasion, nerve invasion, tumor node metastasis (TNM) stage, T stage, N stage, were assessed by independent pathologists. The TNM stage was classified based on the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging system.[7] To make further analysis in the present study, inflammatory indices in peripheral blood including the white blood cell counts, differential neutrophil counts, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), C reactive protein (CRP), and modified Glasgow prognostic score (mGPS) were also tested or calculated.
2.4. Luminex suspension array technology
Plasma levels of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, TNF-α, IFN-γ, TGF-β1, and vascular endothelial growth factor (VEGF) were measured by Luminex suspension array using a specific kit, according to manufacturer's instructions (Merck Millipore, Germany). In brief, 25 μL of plasma sample was mixed with 25 μL of Assay Buffer and 25 μL of beads and the mixture was loaded on 96-well plate and incubated at 4 °C in dark with shaking overnight. On the next day, the liquids in the 96-well plate were removed, and the plate was washed twice with 200 μL Wash Buffer. Subsequently, 25 μL of antibodies were added into each well and were incubated with 25 μL of PE-conjugated Streptavidin at room temperature for 30 minutes with shaking. The liquids were removed and the plate was washed twice with 200 μL Wash Buffer. Then, 100 μL of driving liquid was added into the plate and samples were analyzed with MagPlex instrument (Merck Millipore, Germany).
2.5. Statistical analysis
Statistical analysis was performed using SPSS 20.0 software. Data were expressed as median ± interquartile range. Non-parametric test was used to assess between-group differences. Correlation among different cytokines was evaluated by Pearson correlation analysis. Two-tailed P value <.05 was considered as statistically significant.
3. Results
3.1. Association of plasma cytokine and growth factor levels with demographic characteristics
In the whole population included in this study, plasma levels of TGF-β1 and VEGF were higher than those of the other cytokines. As shown in Table 1, plasma levels of TNF-α, IL-12p70, IL-4, IL-10, and VEGF in male patients (n = 75) were significantly higher than those in female patients (n = 24) (P < .05 for all). Patients aged ≥60 years had significantly higher plasma levels of TNF-α than those <60 years old (P < .05).
Table 1.
Profile of the plasma cytokines, growth factor levels with the demographic and clinical characteristics of patients with gastric cancer. Data are presented as median ± interquartile range (IQR).
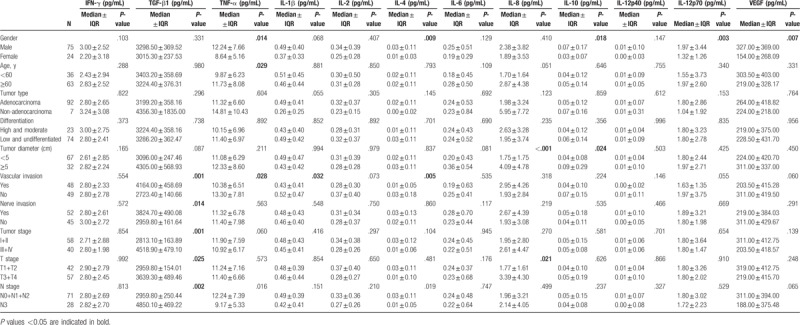
3.2. Association of plasma cytokine and growth factor levels with clinicopathological parameters
We observed that plasma levels of cytokines and growth factors had no any association with tumor type and differentiation status (P > .05 for all) (Table 1). Patients with tumor diameter ≥5 cm had significantly higher plasma levels of IL-8 and IL-10 as compared with those with tumor diameter <5 cm (P < .05 for all). In addition, patients with vascular or nerve invasion showed higher plasma levels of TGF-β1, and lower plasma levels of TNF-α, IL-1β, and IL-4 as compared with those in patients with no invasion (P < .05 for all). Of note, plasma levels of TGF-β1 in patients with advanced T stage and N stage were higher than those in patients with early stage cancer; plasma IL-8 levels were also higher in patients with advanced T stage (P < .05).
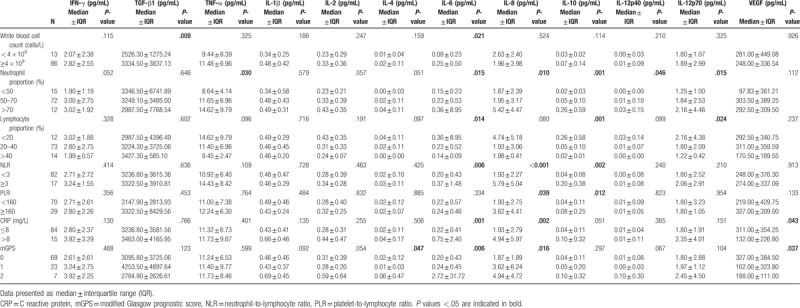
3.3. Association of plasma cytokine and growth factor levels with inflammatory indices
We observed an increase in plasma levels of TGF-β1 and IL-6 with increase in the white blood cell count (≥4 × 109 cells/L) (P < .05 for all) (Table 2). Likewise, a similar tendency was observed between the proportion of neutrophils and plasma levels of TNF-α, IL-6, IL-8, IL-10, IL-12p40, and IL-12p70, whereas the proportion of lymphocytes was inversely associated with plasma levels of IL-6, IL-10, and IL-12p70 (P < .05 for all). Plasma concentrations of IL-6, IL-8, IL-10 were found to be higher in patients with NLR value ≥3, and higher concentrations of IL-8 and IL-10 were also found in patients with PLR value ≥160 (P < .05 for all). Elevated CRP level (>8 mg/L) was found to be an indicator for increased plasma levels of IL-6, IL-8, and VEGF (P < .05 for all). Increased level of IL-4, IL-6, and IL-8 was also detected in the presence of higher plasma level of mGPS (P < .05).
Table 2.
Profile of the plasma cytokines, growth factor levels with the inflammatory indices of gastric cancer.
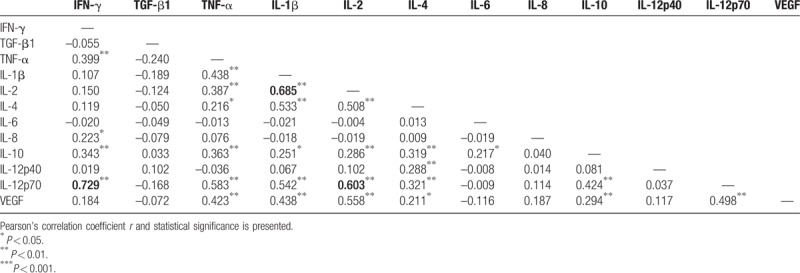
3.4. Correlation analysis for plasma cytokines and growth factors
Pearson's correlation analysis revealed a significant correlation of plasma IL-2 level with IL-1β (r = 0.685, P < .01) and IL-12p70 (r = 0.603, P < .05) levels (Table 3). A more prominent positive correlation was observed between plasma IL-12p70 and IFN-γ levels (r = 0.729, P < .01).
Table 3.
Correlation analysis of plasma levels of cytokines and growth factors in gastric cancer patients.
4. Discussion
In this study, we found that plasma levels of cytokines and growth factors had a close association with demographic and clinicopathological features including the inflammatory indices in patients with gastric cancer.
Plasma levels of TNF-α tended to vary by sex and age: male or old patients (≥60 years) had higher plasma TNF-α expression when compared with female and young patients, respectively. TNF-α is a well-characterized proinflammatory cytokine whose high expression might be a driver for carcinogenesis of gastric cancer.[8] Increased TNF-α expression, possibly generated by the activated macrophages, was shown to be associated with inflammation and infection of gastric mucosa.[9] Higher plasma levels of TNF-α in men as compared with that in women might be a potential mechanism underlying the observed 2-fold higher risk of gastric cancer among men.[10] Moreover, the prevalence of abnormalities in gastric mucosa which were mostly induced by Helicobacter pylori (HP) infection, tended to increase with age, leading to an increased expression of IL-6, likely as an inflammatory response to HP infection.[11] Therefore, men and older patients with gastric cancer might have increased circulating TNF-α levels induced by inflammatory response.
Among the various plasma cytokines, we observed the strongest correlation between plasma TGF-β1 and progression of gastric cancer. For instance, plasma TGF-β1 concentration was increased in patients with vascular invasion, nerve invasion, or advanced tumor stage. In line with our observations, TGF-β1 has been implicated in the development of epithelial-mesenchymal transition (EMT), tumor progression, and metastasis in the context of gastric cancer.[12,13] Suda et al[14] reported higher expressions of TGF-β1 in both plasma and tumor tissues with increase in tumor grade, which is consistent with the findings in the present study.
NLR, PLR, CRP, and mGPS values are well-accepted indices of systemic inflammatory response, all of which have been shown to have prognostic value for patients with gastric cancer.[15,16] Gastric cancer patients with higher NLR level (≥3) were shown to have a poor prognosis[17] and increased plasma levels of IL-6, IL-8, IL-10. Likewise, an unfavorable prognosis and higher plasma level of IL-8, IL-10 levels was also observed in the presence of higher PLR levels (≥160)[15] in gastric cancer patients. The mGPS, assessed based on plasma level of CRP and albumin, was found to modulate the postoperative mortality risk in patients with gastric cancer.[18] On the other hand, increased mGPS was also associated with advanced tumor stage of gastric cancer.[18] In the present study, we found that higher mGPS was involved in increased plasma levels of IL-4, IL-6, IL-8. Increased levels of IL-4 and IL-8 may also be induced by HP infection, and therefore might play a role in the inflammatory response according to previous studies.[19,20] In this study, 35 patients received HP examination. Among these, 12 were diagnosed as HP-positive, while others were negative for HP. None of the HP-positive patients received anti-bacterial or other treatment. Considering the close association between cytokine generation and HP infection in gastric cancer, future study will be conducted by recruiting a larger sample to address this issue. IL-6 is a key mediator of cancer-related inflammation[21];high plasma levels of IL-6 were shown to predict a poor prognosis in patients with gastric cancer.[4] Collecting these evidences, the important role in inflammatory response might be the potential mechanism for the observed association of cytokines with gastric cancer progression in the present study.
There are some limitations in this study. First, the retrospective study design is liable to introduce an element of bias. Second, although a series of plasma cytokines and growth factor levels were included in the analysis, some other important cytokines also likely to be involved in inflammatory responses may have been missed. Third, lifestyle-related variables, such as smoking, which may affect the levels of these cytokines, were not factored in the present study. Lastly, subjects included in this study were sourced from a single hospital and may not be representative of the whole population.
5. Conclusion
High plasma level of IL-6, IL-8, and TGF-β1 were predictors of gastric cancer progression. There was a close relationship between systemic inflammatory markers and plasma cytokines. Given the limitations of this study, large prospective studies are warranted to validate our findings.
Author contributions
Conceptualization: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Data curation: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Formal analysis: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Funding acquisition: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Wenxian Guan, Baorui Liu.
Investigation: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Methodology: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Project administration: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu.
Resources: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Software: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Baorui Liu.
Supervision: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Xiangshan Fan.
Validation: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Visualization: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Writing – original draft: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Writing – review & editing: Zhengyun Zou, Lianjun Zhao, Shu Su, Qin Liu, Lixia Yu, Jia Wei, Yang Yang, Juan Du, Jie Shen, Xiaoping Qian, Xiangshan Fan, Wenxian Guan, Baorui Liu.
Footnotes
Abbreviations: CRP = C reactive protein, IL = interleukin, mGPS = modified Glasgow prognostic score, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, TGF = tumor growth factor, TNF-α = tumor necrosis factor-α, TNM = tumor node metastasis, VEGF = vascular endothelial growth factor.
ZZ and LZ have contributed equally to this study.
This study was supported by the Key Programs of the Nanjing Medical Science and Technology Development Foundation (Grant No. ZKX15012).
The authors declare that they have no conflict of interest.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Lee K, Hwang H, Nam KT. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut Liver 2014;8:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol 2014;20:1657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chang WJ, Du Y, Zhao X, et al. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol 2014;20:4586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int 2015;2015:327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Epplein M, Xiang YB, Cai Q, et al. Circulating cytokines and gastric cancer risk. Cancer Causes Control 2013;24:2245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [8].Fujiki H, Sueoka E, Suganuma M. Tumor promoters: from chemicals to inflammatory proteins. J Cancer Res Clin Oncol 2013;139:1603–14. [DOI] [PubMed] [Google Scholar]
- [9].Oguma K, Oshima H, Oshima M. Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol 2010;6:515–26. [DOI] [PubMed] [Google Scholar]
- [10].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [11].Sonnenberg A, Genta RM. Changes in the gastric mucosa with aging. Clin Gastroenterol Hepatol 2015;13:2276–81. [DOI] [PubMed] [Google Scholar]
- [12].Choi YJ, Kim N, Chang H, et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015;36:553–63. [DOI] [PubMed] [Google Scholar]
- [13].Fu H, Hu Z, Wen J, et al. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim Biophys Sin (Shanghai) 2009;41:648–56. [DOI] [PubMed] [Google Scholar]
- [14].Suda A, Saito N, Seshimo A, et al. Examination of transforming growth factor beta1 expression in the serum and tumor tissue of gastric cancer. Int Surg 2009;94:182–8. [PubMed] [Google Scholar]
- [15].Kim EY, Lee JW, Yoo HM, et al. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol 2015;22:4363–70. [DOI] [PubMed] [Google Scholar]
- [16].Gao Y, Huang D. The value of the systematic inflammation-based Glasgow Prognostic Score in patients with gastric cancer: a literature review. J Cancer Res Ther 2014;10:799–804. [DOI] [PubMed] [Google Scholar]
- [17].Ock CY, Nam AR, Lee J, et al. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer 2017;20:799–804. [DOI] [PubMed] [Google Scholar]
- [18].Jiang X, Hiki N, Nunobe S, et al. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer 2012;107:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang XY, Chan WY, Whitney BM, et al. Changes of interleukin expression correlate with Helicobacter pylori infection and lymph node metastases in gastric carcinoma. Diagn Mol Pathol 2002;11:135–9. [DOI] [PubMed] [Google Scholar]
- [20].Kido M, Tanaka J, Aoki N, et al. Helicobacter pylori promotes the production of thymic stromal lymphopoietin by gastric epithelial cells and induces dendritic cell-mediated inflammatory Th2 responses. Infect Immun 2010;78:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]


