Supplemental Digital Content is available in the text
Keywords: minimally invasive, pancreaticoduodenectomy, robotic surgical systems
Abstract
Rationale:
Synchronous double malignancies, including carcinoma of the ampulla of Vater and rectal carcinoma, are generally uncommon occurrences in the gastrointestinal tract.
Patient concerns:
The present study report a case of a 37-year-old man who was incidentally found to suffer from carcinoma of the ampulla of Vater and rectal carcinoma.
Diagnoses:
The duodenoscopy was performed and revealed an ulcerated and bulky ampulla of Vater, the biopsy from which revealed a moderate-differentiated adenocarcinoma, A local hospital colonoscopy confirmed a tumor located in rectal 7 cm from the anal margin and biopsy-confirmed poorly differentiated adenocarcinoma.
Interventions:
About such patient treatment, both open and laparoscopic surgery are restricted because of operation complexity, large injury, and poor cosmetic effect. surgery performed using Da Vinci robotic surgical system (DVSS).
Outcomes:
No evidence of recurrence or relapses was found in the first year after surgery.
Lessons:
Although sporadic double malignancies are uncommon, they should be considered when evaluating cancer patients. Complex surgery performed by robotic surgery may became surgeon's preferred treatment modality.
1. Introduction
Pancreaticoduodenectomy (PD) performed by minimally invasive approach has been the exploration direction because of its less traumatic, rapid recovery, and cosmetic appearance. However, the procedure is still one of the most difficult and challenging abdominal surgical operation. Although the first laparscopic PD was described by Gagner in 1994,[1] attributed to the retroperitoneal location of the pancreas and difficult 3 separate anastomoses reconstruction, it has not gained widespread popularity.[2,3] Emergence of DVSS could improve such situations.
Carcinoma of the ampulla of Vater (CAV) is a malignancy involving the papilla of Vater, accounting for about 1% in gastrointestinal malignant tumor,[4] while rectal carcinoma (RC) is one of the most common gastrointestinal malignancies. Although CAV are reported to occur in the presence of familial adenomatous polyposis (FAP), hereditary nonpolyposis colon cancer (HNPCC), and Gardner syndrome, sporadic double malignancies involving the ampulla of Vater and rectal, are extremely uncommon.[5] In the present report, a case of multisource cancer (CAV and RC) in a young man without hereditary disease is reported. Available diagnostic and therapeutic strategies for surgical treatment are also discussed in relation to the experience of the present case and previously reported cases Supplementary video 1.
2. Case report
On January 11, 2016, a 37-year-old man was admitted to the Department of Gastrointestinal Surgery, The First Affiliated Hospital of NanChang University (NanChang, JiangXi province, China). The patient presented with blood in the stool and tenesmus over the course of 3 months and complained of reduced appetite in combination with significant weight loss during the same period. There was no significant past or family history of malignancy of the bowel or the biliary tract to the patient. Abdominal examination produced unremarkable results, However, a hard mass was detected by rectal examination and yield a blood-stained finger. A local hospital colonoscopy confirmed a tumor located in rectal 7 cm from the anal margin and biopsy-confirmed poorly differentiated adenocarcinoma. Hematological and biochemical investigations showed impaired liver function with alanine aminotransferase 408 U/L, total bilirubin 23.4 μmol/L, direct bilirubin 12.5 μmol/L, alkaline phosphatase 783 U/L, and γ-glutamyltransferase 1062 U/L; besides, elevated alpha fetoprotein and cancer antigen 19-9 levels were 111.50 ng/mL and 31.07 U/mL, respectively. Contrast-enhanced computed tomography (CT) scan of the abdomen showed bilateral intrahepatic biliary radical dilatation, the dilated common bile duct up to the lower end, and local thickening of the low rectal (Fig. 1A–D). No regional lymph node swelling, metastases, or direct invasion to adjacent organs was detected on both chest and abdominal CT images. Gastroduodenoscopy was performed and revealed an ulcerated and bulky ampulla of Vater, the biopsy from which revealed a moderate-differentiated adenocarcinoma (Fig. 2A, B). A diagnosis of synchronous carcinoma of the colon and ampulla of Vater was performed. PD combined with rectal cancer anterior resection should be performed according to his conditions. Considering the complexity of such surgery, DVSS was decided as the optimal method. The procedure was approved by institutional ethic committee of the First Affiliated Hospital of Nanchang University; also, the consent for publication was informed and signed. Histopathology revealed a poorly differentiated adenocarcinoma of the rectum, which invade all of the intestinal wall (pT3) and 1 lymph node metastasis (pN1). The resected specimen of PD revealed a moderately differentiated adenocarcinoma of the ampulla with all the intestinal wall, neural, and pancreas peplos invasion (pT3), but without lymph node metastases (pN0). All the resection margins were free. Overall, the procedure lasted 8 hours and the patient was discharged on the eighth day with good recovery. After 1-year follow-up period, the patient was well and no relapses in their health were recorded.
Figure 1.
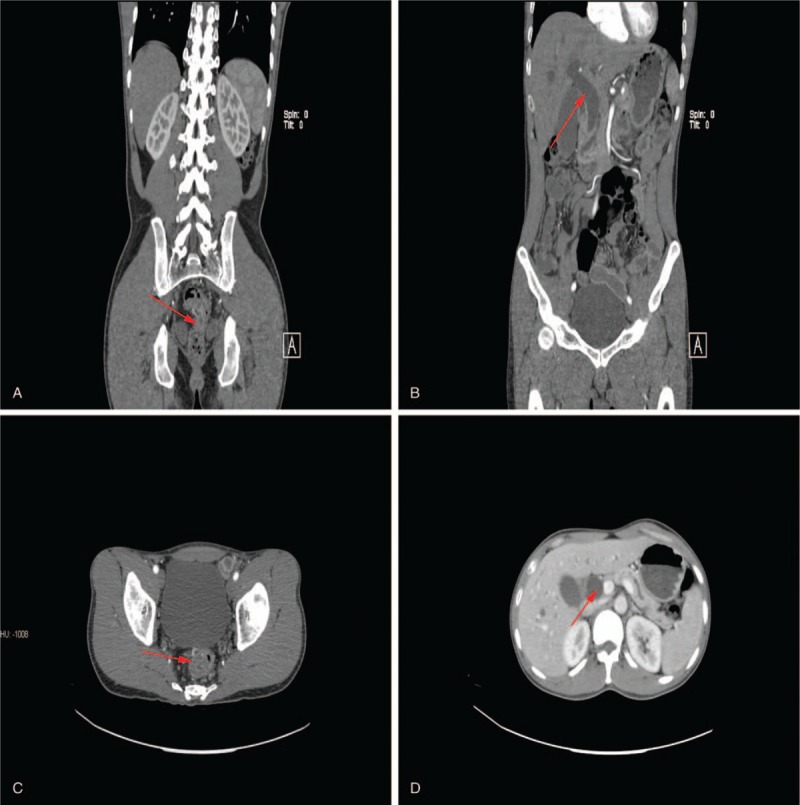
(A, B) Local thickening of the low rectal; (C, D) the dilated common bile duct (red arrow).
Figure 2.
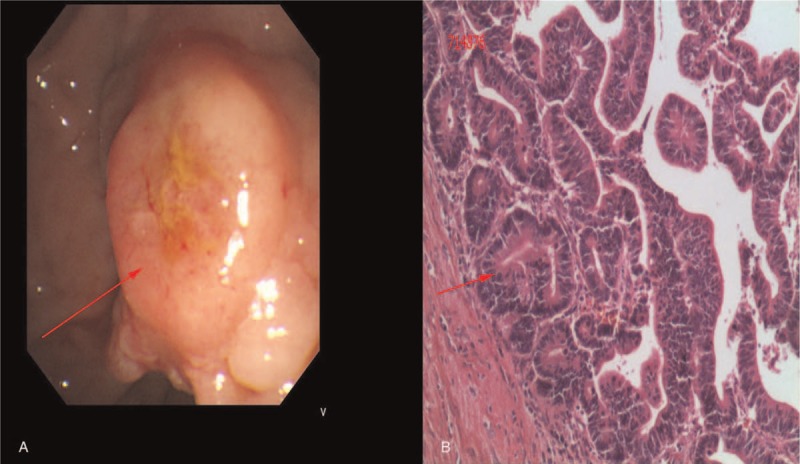
(A) An ulcerated and bulky ampulla of Vater; (B) biopsy showed a moderate differentiated adenocarcinoma (red arrow).
3. Technical notes
Due to the complexity of such operation, the port location (Fig. 3) was designed. The general steps are as follows (Fig. 4):
-
(1)
The abdominal cavity was explored to determine the existence of distant or peritoneal metastasis.
-
(2)
A Kocher maneuver was performed whereby the duodenum and the head of pancreas were mobilized. The portal triad was visualized and dissected. The nodal tissue of the hepatic artery, portal vein, and the common bile duct were sent for pathological examination.
-
(3)
The right colon was mobilized, and the superior mesenteric vein (SMV) was identified. The gastric colon ligament was incised, and the lesser sac was pierced. The right colon was then mobilized up to the origin of the gastroepiploic vessels.
-
(4)
The common bile duct was dissected. The gallbladder was removed and dissected, and ligation of the gastroduodenal artery was performed. The hepatic duct was incised from the upper part of the merger of the cystic duct and the common bile duct.
Figure 3.
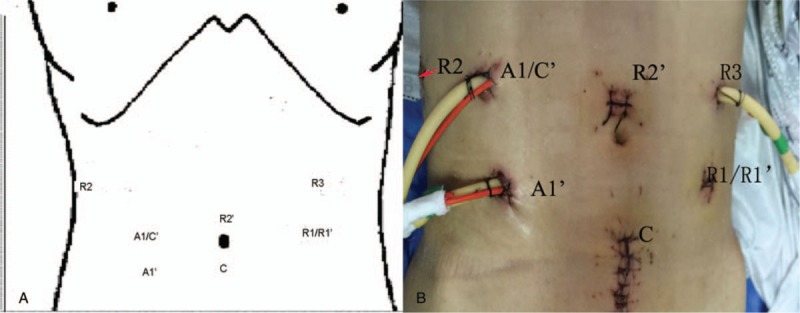
Port positions. C stands for DVSS camera port, R for DVSS instrument arm, and A for assistant port. C’, A1’, R1’, R2’ for rectal cancer anterior resection.
Figure 4.
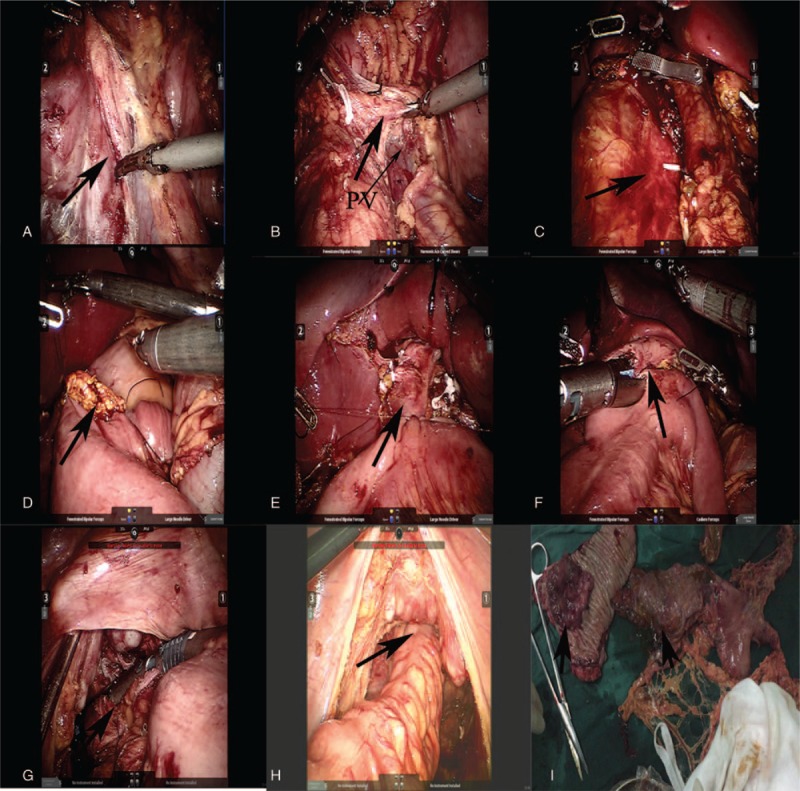
(A) Kocher maneuver is performed, (B) complete dissection of the pancreatic uncinate, (C) removal of specimen, (D) anastomosis of pancreas and intestine, (E) biliary enteric anastomosis, (F) gastrointestinal anastomosis, (G) cut off the rectum, (H) anastomosis of rectum and sigmoid colon, (I) specimen (black arrow).
3.1. Pancreas exploration
The retro cavity was explored, the pancreas was exposed, and the neck was identified.
-
(5)
The retropancreatic tunnel is created.
-
(6)
The stomach and jejunum were transected. Approximately 15 cm of the jejunum was removed with a 45-mm cartridge endostapler (blue load) (Ethicon Endo-Surgery, Cincinnati, OH). A 45-mm cartridge endostapler (blue load) incised the cross-sectional junction of the gastric antrum and the body. This maneuver later facilitated the exposure of the superior mesenteric artery and vein.
-
(7)
The neck of the pancreas was removed at the left side of the portal vein, which identified the pancreatic duct.
-
(8)
The pancreatic uncinate membrane along the right side of the superior mesenteric artery was completely dissected. The stability and magnification of the robotic instruments and cameras was allowed to be performed in a very precise and controlled fashion, with minimal blood loss.
-
(9)
Three separate anastomoses reconstructions were performed by DVSS.
-
(10)
The specimen was removed and placed in a bag inside the abdominal cavity.
-
(11)
The patient's position was altered to allow for the insertion of a Trocar to perform transabdominal anterior resection of the rectal cancer.
-
(12)
Small bowel loops were retracted from the pelvic area into the right upper quadrant.
-
(13)
Primary vascular control was achieved by first dividing the inferior mesenteric artery (IMA) and the inferior mesenteric vein (IMV). Alternatively, the IMV was divided after mobilizing the colon mesentery.
-
(14)
The extent of the dissection was defined as the inferior border of the pancreas superiorly, followed by Gerota fascia laterally and the psoas muscle inferiorly.
-
(15)
The splenic flexure was mobilized.
-
(16)
Rectal dissection was performed by using an elliptical dissection pattern of the posterior (1) continuing laterally to the right (2) and to the left side (3) and finally to the anterior side of the rectum.
-
(17)
Rectum-descending colon anastomosis was performed by DVSS.
4. Discussion
Multisource cancer involving CAV and RC are generally uncommon occurrences in the gastrointestinal tract. According to a review by Minni et al,[6] most patients with such synchronous double malignancies are more likely to have cancers such as FAP, and HNPCC. Interesting, older age, environmental factors, and genetic mutations may also be partially responsible for such synchronous double malignancies.[7] The patient reported in the present study patient was young and had no significant past or family history. Accordingly, it was reasonable to assume that genetic mutations of oncogenes (K-ras, Her-2/neu) and tumor suppressor genes (APC, DCC, p53, DPC4, BRCA2) may have been the main cause.[8]
Compared with the laparoscopy, the robot offered several advantages, including 3D vision, dexterity, and ergonomy. As to such patients, the robotic approach was observed to be suitable in their case. Since the first described case in 2003, robotic PD has been proven to be safe and feasible.[9,10] Specifically, sharp dissection along the superior mesenteric artery and portal vein, and hand sewing of all three anastomoses performed by DVSS nearly identical to those performed during open surgery. In the absence of literature on how to manage such situation, a 6-port approach involving the Robot was designed to perform such complicated operations.
To conclude, although sporadic double malignancies are uncommon, they should be considered when evaluating cancer patients. Complex surgery performed by robotic surgery may became surgeon's preferred treatment modality.
Acknowledgment
Thanks due to Jiang Qunguang and Tang Cheng for assistance with the surgery and to Liu Dong Ning for valuable discussion.
Author contributions
Conceptualization: TaiYuan Li.
Data curation: TaiYuan Li.
Formal analysis: TaiYuan Li.
Funding acquisition: QunGuang Jiang.
Investigation: QunGuang Jiang, DongNing Liu.
Methodology: QunGuang Jiang, Cheng Tang.
Writing – original draft: QunGuang Jiang.
Writing – review & editing: QunGuang Jiang.
Supplementary Material
Footnotes
Abbreviations: CAV = carcinoma of the ampulla of Vater, CT = computed tomography, DVSS = Da Vinci robotic surgical system, FAP = familial adenomatous polyposis, HNPCC = hereditary nonpolyposis colon cancer, IMA = inferior mesenteric artery, IMV = inferior mesenteric vein, PD = pancreaticoduodenectomy, RC = rectal carcinoma, SMV = superior mesenteric vein.
LT and other coauthors have no conflict of interest.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408–10. [DOI] [PubMed] [Google Scholar]
- [2].Gumbs AA, Rodriguez Rivera AM, Milone L, et al. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol 2011;18:1335–41. [DOI] [PubMed] [Google Scholar]
- [3].Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015;29:9–23. [DOI] [PubMed] [Google Scholar]
- [4].Bouvet M, Gamagami RA, Gilpin EA, et al. Factors influencing survival after resection for periampullary neoplasms. Am J Surg 2000;180:13–7. [DOI] [PubMed] [Google Scholar]
- [5].Björk J, Akerbrant H, Iselius L, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology 2001;121:1127–35. [DOI] [PubMed] [Google Scholar]
- [6].Minni F, Casadei R, Marrano N, et al. Second tumours in patients with malignant neoplasms of the digestive apparatus. A retrospective study on 2406 cases. Ann Ital Chir 2005;76:467–72. [PubMed] [Google Scholar]
- [7].Esposito I, Friess H, Büchler MW. Carcinogenesis of cancer of the papilla and ampulla: pathophysiological facts and molecular biological mechanisms. Langenbecks Arch Surg 2001;386:163–71. [DOI] [PubMed] [Google Scholar]
- [8].Fearon ER. Vogelstein B: a genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- [9].Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777–84. [DOI] [PubMed] [Google Scholar]
- [10].Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554–9. discussion 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


