Abstract
Epidemiological studies exploring the role of flavonoids intake in preventing type 2 diabetes mellitus (T2DM) showed inconsistent results. Therefore, we performed a meta-analysis of relevant studies to examine the relationship between flavonoids intake and risk of T2DM. We hypothesized that flavonoids intake may decrease the risk of developing T2DM.
A systematical search in PubMed and Embase until September 2017 was performed to identify eligible prospective cohort studies. The summary relative risks (RRs) and 95% confidence intervals (CIs) were calculated using random-effect models. Dose-response pattern between total flavonoids intake and T2DM risk was also estimated.
Eight prospective studies were included with 312,015 participants, of whom 19,953 developed T2DM during the follow-up periods of 4 to 28 years. Compared with lower consumption, high intake of total flavonoids was associated with a decreased risk of T2DM (RR: 0.89, 95% CI: 0.82–0.96). Among flavonoid subclasses, inverse correlations with T2DM were achieved for intakes of anthocyanidins, flavan-3-ols, flavonols, and isoflavones. Dose-response meta-analysis indicated a curvilinear relationship between total flavonoids intake and incident T2DM (P for nonlinearity = .042), with a significant risk reduction at an intake of ≥550 mg/day. When assuming a linear pattern, the risk of T2DM was decreased by 5% for each 300-mg/day increment in total flavonoids intake (RR: 0.95, 95% CI: 0.93–0.97).
Our study suggests that higher intakes of total flavonoids and subclasses (anthocyanidins, flavan-3-ols, flavonols, and isoflavones) are associated with lower risk of T2DM.
Keywords: flavonoids, meta-analysis, prospective Studies, type 2 diabetes
1. Introduction
Type 2 diabetes mellitus (T2DM) is a burdensome chronic disorder with increasing prevalence worldwide. It was estimated that nearly 415 million people had T2DM in the year 2015, and this number is expected to increase to 642 million by 2040.[1] Persons who developed T2DM are also at greater risks for cardiovascular disease, diabetic retinopathy, renal dysfunction, and leg ulcers, imposing a tremendous burden on health care system.[2] Thus, improving the primary prevention of T2DM has become imperative. Among the preventive strategies against T2DM, an adoption of a healthier diet has been widely accepted to play a critical role. Data from epidemiological studies suggest that dietary intake of fruits, vegetables, whole-grains, fish, and dairy products may reduce the risk of T2DM, whereas consuming sugar-sweetened beverages and red or processed meats increases the risk.[3–5]
Dietary flavonoids represent a large group of polyphenolic compounds, which are abundant in various plant-root foods, including commonly-consumed vegetables and fruits, tea, grains, and cocoa.[6] On the basis of their chemical structure, flavonoids can be mainly categorized as flavonols, flavones, flavan-3-ols, anthocyanins, flavanones, and isoflavones. In addition to the potent antioxidant properties, dietary flavonoids also improve glucose and lipid metabolism and exhibit anti-inflammation effects,[7,8] pointing to a protective role in the development of cardiometabolic diseases. Accordingly, clinical evidences have suggested that flavonoids intake significantly decreases the risk of cardiovascular disease.[9] For T2DM, however, the findings regarding dietary flavonoids are to date inconclusive. This may be attributed to the fact that the number of prospective cohort studies is limited and their results are inconsistent. Herein, we carried out a meta-analysis of prospective cohort studies to comprehensively evaluate the role of flavonoids and its subclasses intake in the prevention of T2DM, and the dose-response relationship between total flavonoids intake and T2DM was also evaluated. We hypothesized that higher intake of flavonoids may be associated, in a dose-response manner, with a lower risk of incident T2DM.
2. Materials and methods
2.1. Search strategy
The present work was conducted according to the Meta-analysis Of Observational Studies in Epidemiology guideline.[10] We identified eligible publications by performing a manual literature search in PubMed and Embase through September 2017, with the use of the following search terms: “flavonoids,” “flavonols,”,“flavones,” “flavanones,” “flavan-3-ols,” “flavanols,” “isoflavones,” “anthocyanidins,” “proanthocyanidins,” or “phytoestrogens,” and “diabetes” or “T2DM”. Besides, the references of retrieved articles and reviews were manually checked for inclusion of potential complements. Only full-length articles published in English were considered.
2.2. Eligibility criteria
To be included, the studies needed to meet 4 specific conditions: prospective cohort study; the exposure of interest was intake of total flavonoids or flavonoid subclasses, including anthocyanidins, flavan-3-ols (flavanols), flavanones, flavones, flavonols, isoflavones, or proanthocyanidins; the outcome of interest was incident T2DM, with a follow-up duration ≥1 year; the risk estimates of T2DM, such as relative risks (RRs) or hazard ratios, were available. In dose-response meta-analysis, the included studies should report the risk estimates for at least 3 quantitative categories of total flavonoids intake, and the data of cases and person-years were also provided (or with sufficient information to calculate them). Animal studies, reviews, editorials, abstracts, and unpublished results were excluded from this meta-analysis. If reports pertained to overlapping populations, only the study with the largest sample size was retained.
2.3. Details abstraction and quality assessment
A predesigned Excel (Microsoft Corporation) file was used to extract the baseline characteristics of the included studies, including study author, publication year, population origin, number of cases and total participants, baseline age, country, ascertainments of exposure and outcome, follow-up duration, and factors adjusted in multivariate models. We also recorded the median intake levels of flavonoids and the maximally-adjusted risk estimates for aggregated analyses. When necessary, the corresponding authors of the original studies were contacted for missing data. The methodological quality of studies was evaluated by the Newcastle-Ottawa Scale (NOS) score.[11] With this scoring system, each study can score up to 9 points based on 3 major respects: selection of populations (0–4 points), comparability between groups (0–2 points), and assessment of outcomes (0–3 points). The high-quality study was defined as a study with a NOS score of ≥ 7.[12,13] All data extraction and quality evaluation were carried out by 2 independent investigators, with disagreements settled by consulting with a third investigator.
2.4. Statistical methods
In this meta-analysis, we used RR and its 95% confidence interval (CI) to present the summary results. The study-specific risk estimates of T2DM for the highest versus lowest intake of total flavonoids or flavonoid subclasses were pooled by using random effect models. The heterogeneity between studies was explored by Cochrane Q test and I2 statistic. For the Cochrane Q test, a P value of <.1 indicated the presence of heterogeneity; and for the I2 metric, the following cutoff values were applied: <25% (no or low heterogeneity), 25% to 75% (moderate heterogeneity), and >75% (high heterogeneity).[14] Subgroup analyses were conducted according to sample size, sex, follow-up duration, and study location, and the Altman and Bland test [15] was used to confirm the difference between subsets. To evaluate the stability of results, we also performed a sensitivity analysis by excluding study one at a time. Potential publication bias was detected by funnel plots and by Egger test.
To assess the quantified dose-response relationship between total flavonoids intake and risk of T2DM, we firstly assigned the median or mean level of flavonoid intake for each category to each corresponding RR. Then, a 2-staged random-effect dose-response meta-analysis was performed. In the first stage, we established a restricted cubic spline model with 3 knots at the 10th, 50th, and 90th percentiles of flavonoids intake by using generalized least-square regression, taking into account the correlation within each set of published RRs as reported by Orsini et al.[16] In the second stage, the restricted maximum likelihood method in a random-effect meta-analysis was used to aggregate the study-specific RRs.[17] A P value for nonlinearity was obtained from the test of the null hypothesis that the coefficient of the second spline is equal to zero.
All data analyses were implemented using STATA 13.0 (StataCorp, College Station, TX) and R 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria) software, and 2-sided P values of <.05 were treated as of significance.
2.5. Ethics statement
This study was a secondary analysis of human subject data published in the public domain, thus no ethical approval was required.
3. Results
3.1. Study search
The preliminary search identified 3509 studies, of which 662 duplicated publications and 2817 irrelevant reports were removed. After full-text review of the retained articles, 22 studies were eliminated because of failure to meet the eligibility criteria. As a result, 8 prospective studies [18–25] with 10 independent cohorts (Wedick study [24] consisted of 3 independent cohorts) were included in this meta-analysis (Fig. 1).
Figure 1.

Flow diagram of study selection process. T2DM = type 2 diabetes mellitus.
3.2. Characteristics of studies
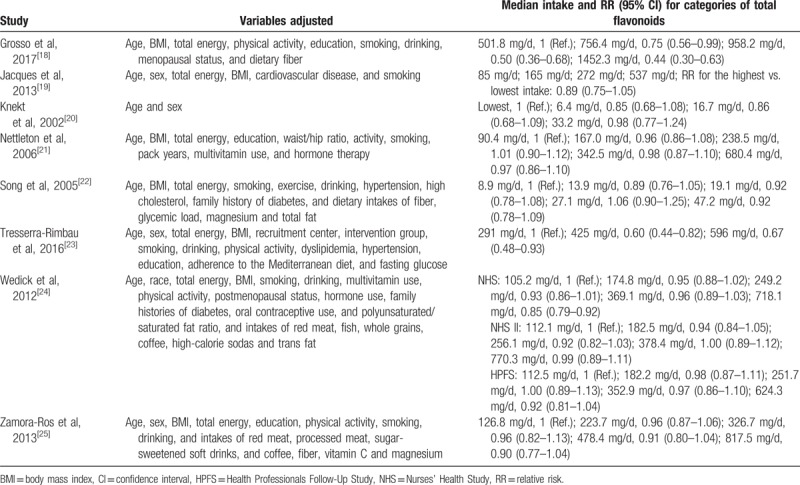
The main details of the included studies are listed in Table 1. These prospective studies were published between 2002 and 2017 with a total of 312,015 participants, of which 19,953 T2DM cases were identified during the follow-up periods from 4 to 28 years. Six studies were conducted in the United States, and the remaining 4 studies were from European countries. All participants were free of T2DM at baseline, with age ranging from 28 to 75 years. Dietary flavonoids intake was evaluated using food-frequency questionnaire, and T2DM was frequently ascertained through medical records. In the original studies, the median intake level of total flavonoids was 8.9 to 501.8 mg/day for the lowest categories and 33.2 to 1452.3 mg/day for the highest categories, and the most commonly adjusted variables included age, sex, total energy intake, body mass index, smoking, alcohol consumption, and physical activity (Table 2). All included studies had a NOS score of ≥7, with an average score of 7.9, indicating the presence of high methodological quality.
Table 1.
Baseline characteristics of studies.
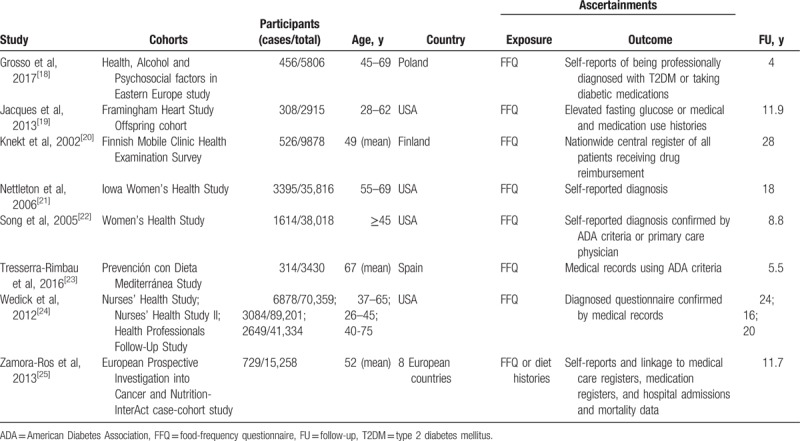
Table 2.
The confounders and risk estimates in the included studies.
3.3. Total flavonoids intake and T2DM
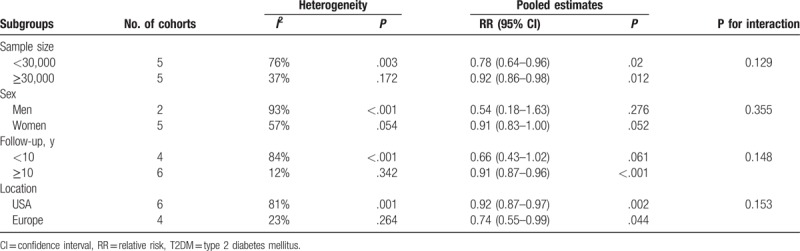
Compared with the lowest intake, the highest intake of total flavonoids was associated with a decreased risk of T2DM (RR: 0.89, 95% CI: 0.82–0.96; Fig. 2), with moderate heterogeneity across the studies (I2 = 63%, P = .004). After removal of Grosso study,[18] the heterogeneity was distinctly reduced (I2 = 21%, P = .257), and the pooled result of the remaining studies was still significant (RR: 0.91, 95% CI: 0.86–0.96). In stratified analyses, the pooled RR for total flavonoids intake was not modified by sample size, sex, follow-up duration, and study location (Table 3). Leave-one-out sensitivity analysis showed that no individual study had a significant influence on the final result. There was no indication of publication bias from visual inspection of the funnel plot, which was further confirmed by Egger test (P = .253).
Figure 2.
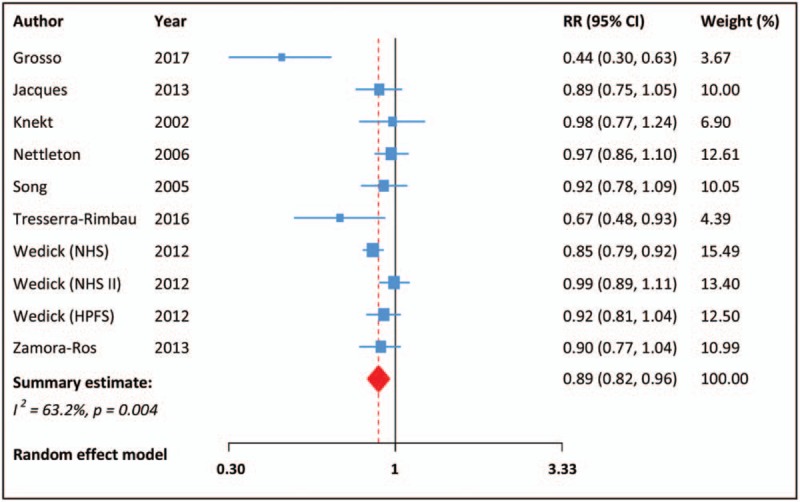
Meta-analysis for total flavonoids intake and risk of type 2 diabetes mellitus. CI = confidence interval, RR = relative risk.
Table 3.
Subgoup analyses for total flavonoids intake and risk of T2DM.
3.4. Flavonoids subclasses intake and T2DM
With regard to flavonoid subclasses, low-to-moderate heterogeneities were found among the included studies. We obtained a decreased risk of developing T2DM for the highest versus lowest intake of anthocyanidins, flavan-3-ols, flavonols, and isoflavones. For other flavonoids subclasses, however, no preventive effect against T2DM was identified (Fig. 3).
Figure 3.
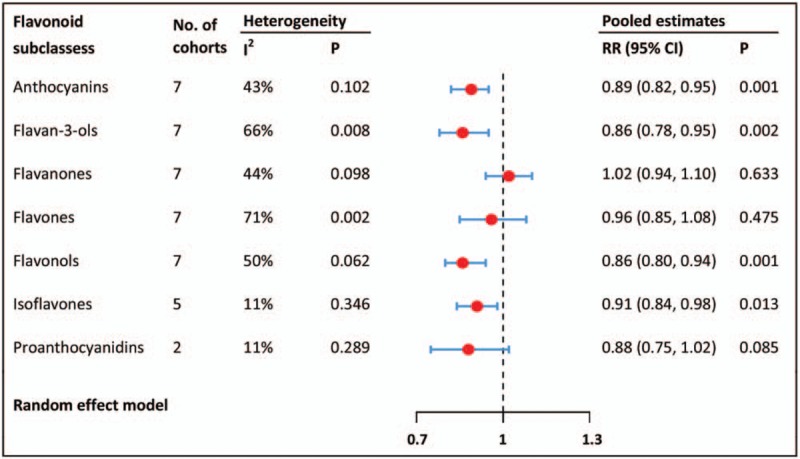
Meta-analyses for flavonoids subclasses intake and risk of type 2 diabetes mellitus. CI = confidence interval, RR = relative risk.
3.5. Dose-response meta-analysis
Seven studies [18,20–25] with 9 cohorts were included in the dose-response meta-analysis, and we found a curvilinear relationship between total flavonoids intake and T2DM risk (P for nonlinearity = .042; Fig. 4), with a significant risk reduction at an intake of ≥550 mg/day. When assuming a linear association, the risk of T2DM was reduced by 5% (RR: 0.95, 95% CI: 0.93–0.97) for each 300-mg/day increment in total flavonoids intake.
Figure 4.
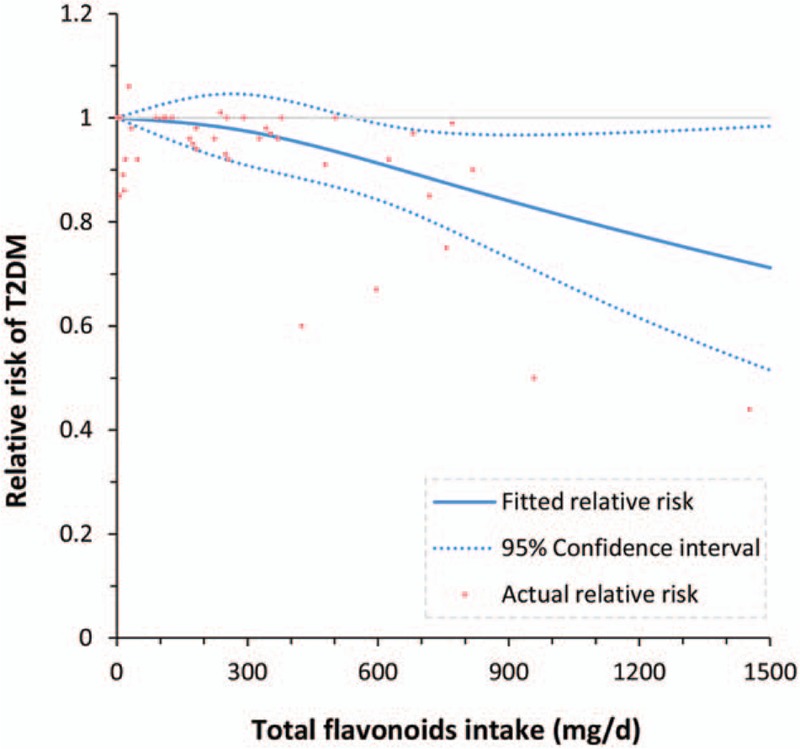
Dose-response meta-analysis for total flavonoids intake and risk of T2DM. T2DM = type 2 diabetes mellitus.
4. Discussion
Dietary flavonoids are natural substances with potential salutary effects on human health. The results from our study demonstrated that intakes of total flavonoids, anthocyanidins, flavan-3-ols, flavonols, and isoflavones were associated with significantly decreased risks of T2DM in the highest versus lowest analyses. Additionally, the dose-response analysis suggested a curvilinear relationship of total flavonoids intake with T2DM risk; when assuming a linear manner, the risk of T2DM was reduced by 5% for an increment of 300 mg/day in total flavonoids intake.
The antidiabetic effects of flavonoids-rich food have been reported in many previous studies. In a recent meta-analysis, Yuan et al[26] found a J-shaped association of cocoa products (chocolate) intakes with incident T2DM, and, consuming chocolate of 6 servings/week or lower may decrease the risk of T2DM. Likewise, a cohort study involving 8 European countries showed that tea consumption was inversely related to T2DM incidence, with a significant benefit observed at an intake of ≥4 cups/day.[27] As to dietary flavonoids, the meta-analysis by Liu et al[28] also suggested that intake of these nutrients significantly decreased the risk of T2DM. However, that analysis only included 4 prospective reports,[18–20,22] and the effects of flavonoids subclasses were not measured. The present work of 8 longitudinal cohort studies has evaluated the role of different flavonoid subclasses, thus providing more comprehensive and reliable insights into the benefits of flavonoids in preventing T2DM.
Here, we proposed several possible mechanisms responsible for the observed associations. By their antioxidant contents, flavonoids could protect tissues against free oxygen radicals and lipid peroxidation,[29] thus contributing to the prevention of T2DM. Emerging evidences have also suggested the ability of dietary flavonoids to regulate endothelial nitric oxide status, activity of NADPH oxidase, and inflammation response, which are linked to the risk of T2DM.[30–32] Besides, mechanistic studies pointed out that flavonoids intake may improve insulin resistance, glycemia metabolism, and pancreatic β-cell dysfunction, with particular interests in the subclasses including anthocyanins, flavonols, and flavan-3-ols.[33] In addition, recent viewpoints have highlighted the role of gut microbiome in the pathophysiological process of T2DM;[34] and, specific flavonoids intake may favorably affect the phylogenetic community and function of gut microbiome.[35,36] Therefore, the preventive effects of flavonoids intake may, at least partly, be mediated through their modulation of gut microbiome.
Some remaining points need to be addressed. Among flavonoids subclasses, we observed no significant benefits for high intakes of flavanones and flavones. Consistent with this, Bertoia et al[37] summarized the data of 3 prospective cohorts and revealed that consumption of flavanones and flavones were not associated with weight change. The potential reasons remain unknown, but it is deserved to note that a large part of these flavonoid subclasses come from consuming citrus fruits or juices.[38] Previous studies have shown that consumption of citrus had no effect on the risk of T2DM,[39] whereas higher intake of fruit juices may increase this risk.[40] Thus, as none of the included studies has adjusted fruits or juices intake, our findings on flavanones and flavones appear to involve bias. Future studies concerning this aspect are warranted. In addition, moderate heterogeneity is present in the highest versus lowest analyses, which is likely to have resulted from the different levels of intake categories between studies. To overcome this limitation, we carried out a dose-response meta-analysis and found that a curvilinear relationship between total flavonoids intake and incidence of T2DM. Considering the statistical significance for the nonlinear manner is marginal, we also fitted a linear model and the result indicated that every 300-mg/day increase in flavonoids intake is related to a decreased risk of T2DM, further confirming the dose-dependent effects of these nutrients.
There are some limitations that should not be ignored. First, because our study is a pooled analysis of observational studies, the recall and selection bias cannot be avoided. However, we only combined the maximally adjusted risk estimates in prospective cohort studies that could largely reduce the probability of biases. Second, stratified analyses by age, body mass index, and other important factors cannot be performed because of the lack of data. Also, there are limited studies available for the analyses of flavonoid subclasses (e.g., proanthocyanidins), which may influence the stability of our results. Third, geographical restriction is present in this meta-analysis, as all of the original studies were from the United States and European countries. Therefore, generalization of our findings to other ethnic groups should be considered with caution.
5. Conclusion
In summary, our meta-analysis indicates that high intake of total flavonoids is correlated, in a dose-response pattern, with a reduced risk of T2DM. Among flavonoids subclasses, anthocyanidins, flavan-3-ols, flavonols, and isoflavones—but not other subclasses—may be salutary in the prevention of developing T2DM. Future prospective studies are still required to confirm this association in other ethnic populations and to further elucidate the role of different flavonoid subclasses.
Author contributions
Conceptualization: Jia Luo.
Data curation: Hui Xu, Jia Huang.
Formal analysis: Hui Xu.
Investigation: Hui Xu, Jia Huang.
Methodology: Hui Xu, Jia Huang.
Resources: Hui Xu, Jia Huang.
Software: Hui Xu, Jia Huang.
Supervision: Jia Luo, Qian Wen.
Validation: Hui Xu, Jia Luo, Jia Huang, Qian Wen.
Writing – original draft: Hui Xu.
Writing – review & editing: Jia Luo.
Footnotes
Abbreviations: CIs = confidence intervals, NOS = Newcastle-Ottawa Scale, RRs = relative risks, T2DM = type 2 diabetes mellitus.
The authors report no conflicts of interest.
References
- [1].International Diabetes Federation.. IDF Diabetes Atlas. 7th edBrussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- [2].DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- [3].Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017;32:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barnard N, Levin S, Trapp C. Meat consumption as a risk factor for type 2 diabetes. Nutrients 2014;6:897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gijsbers L, Ding EL, Malik VS, et al. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–24. [DOI] [PubMed] [Google Scholar]
- [6].Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Testa R, Bonfigli AR, Genovese S, et al. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016;8:E310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond) 2015;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 2014;111:1–1. [DOI] [PubMed] [Google Scholar]
- [10].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [11].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 20, 2017. [Google Scholar]
- [12].Shang X, Lu R, Liu M, et al. Heart rate and outcomes in patients with heart failure with preserved ejection fraction: a dose-response meta-analysis. Medicine 2017;96:e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fan C, Yu S, Zhang S, et al. Association between folate intake and risk of head and neck squamous cell carcinoma: AN overall and dose-response PRISMA meta-analysis. Medicine 2017;96:e8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Larsson SC, Crippa A, Orsini N, et al. Milk consumption and mortality from all causes, cardiovascular disease, and cancer: a systematic review and meta-analysis. Nutrients 2015;7:7749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. [DOI] [PubMed] [Google Scholar]
- [18].Grosso G, Stepaniak U, Micek A, et al. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br J Nutr 2017;118:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jacques PF, Cassidy A, Rogers G, et al. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Knekt P, Kumpulainen J, Jarvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–8. [DOI] [PubMed] [Google Scholar]
- [21].Nettleton JA, Harnack LJ, Scrafford CG, et al. Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J Nutr 2006;136:3039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Song Y, Manson JE, Buring JE, et al. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr 2005;24:376–84. [DOI] [PubMed] [Google Scholar]
- [23].Tresserra-Rimbau A, Guasch-Ferré M, Salas-Salvadó J, et al. Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J Nutr 2016;146:767–77. [DOI] [PubMed] [Google Scholar]
- [24].Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zamora-Ros R, Forouhi NG, Sharp SJ, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care 2013;36:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yuan S, Li X, Jin Y, et al. Chocolate consumption and risk of coronary heart disease, stroke, and diabetes: a meta-analysis of prospective studies. Nutrients 2017;9:E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].InterAct Consortium. Tea consumption and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. PLoS One 2012;7:e36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu YJ, Zhan J, Liu XL, et al. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr 2014;33:59–63. [DOI] [PubMed] [Google Scholar]
- [29].de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 2010;11:1679–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suganya N, Bhakkiyalakshmi E, Sarada DV, et al. Reversibility of endothelial dysfunction in diabetes: role of polyphenols. Br J Nutr 2016;116:223–46. [DOI] [PubMed] [Google Scholar]
- [31].Bondonno CP, Croft KD, Ward N, et al. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev 2015;73:216–35. [DOI] [PubMed] [Google Scholar]
- [32].Mena P, Domínguez-Perles R, Gironés-Vilaplana A, et al. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014;66:745–58. [DOI] [PubMed] [Google Scholar]
- [33].Hanhineva K, Törrönen R, Bondia-Pons I, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 2010;11:1365–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sohail MU, Althani A, Anwar H, et al. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res 2017;2017:9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Duda-Chodak A, Tarko T, Satora P, et al. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 2015;54:325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gil-Cardoso K, Ginés I, Pinent M, et al. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr Res Rev 2016;29:234–48. [DOI] [PubMed] [Google Scholar]
- [37].Bertoia ML, Rimm EB, Mukamal KJ, et al. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016;doi: 10.1136/bmj.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tresserra-Rimbau A, Medina-Remón A, Pérez-Jiménez J, et al. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. Nutr Metab Cardiovasc Dis 2013;23:953–9.23332727 [Google Scholar]
- [39].Jia X, Zhong L, Song Y, et al. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim Care Diabetes 2016;10:272–80. [DOI] [PubMed] [Google Scholar]
- [40].Bazzano LA, Li TY, Joshipura KJ, et al. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008;31:1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


