Abstract
Introduction:
Fecal microbiota transplantation (FMT) is currently being explored as a potential therapy for ulcerative colitis (UC). Here, we report the first case of a UC patient with allergy to 5-aminosalicylic acid (5-ASA) who underwent FMT and achieved clinical remission.
Case presentation:
This patient had a 9-year history of UC and was allergic to 5-ASA. He suffered from gradually aggravated abdominal pain and frequent bloody diarrhea. There was a continuous distribution of superficial erosion and ulceration by colonoscopy. After steroid therapy failed, he underwent FMT. The donated fecal microbes were purified in laboratory and then transplanted into the terminal ileum and right colon of the patient by colonoscopy. During the 9 months’ follow-up, FMT has proved its efficacy in inducing and maintaining clinical and endoscopic remission of the patient.
Conclusion:
The choice of treatment for refractory UC patients who are allergic to 5-ASA is relatively limited. In our case, we highlight the specific role of FMT for refractory UC with absence of 5-ASA through intestinal microbiota reconstruction.
Keywords: 5-aminosalicylic acid, allergy, fecal microbiota transplantation, ulcerative colitis
1. Introduction
5-aminosalicylic acid (5-ASA) preparations have been used as first-line drugs for treatment of ulcerative colitis (UC).[1] However, a few patients with UC are allergic to 5-ASA. For these patients, effective induction and maintenance treatment appears to be weak. Although the etiology of UC has not been fully understood, it is well-known that abnormal intestinal flora participate in the development of UC.[2] Current theories postulate that the alteration of the intestinal microbiome, also known as intestinal dysbiosis, triggers abnormal mucosal immune response and leads to the development of chronic intestinal inflammatory disease.[3] Differences have been documented in the composition of the intestinal flora between UC patients and healthy individuals with a decrease in gut microbial diversity and a shift in the balance of intestinal flora. The unbalanced coexistence of gut microorganisms possibly contributed to the development of UC by immune changes and microbial metabolite alterations.[4]
Manipulation of the gut microbiota, therefore, may represent a target therapy for UC. Fecal microbiota transplantation (FMT), the most effective strategy for reconstruction of the gut microbiota,[5,6] is currently being explored as a potential therapy for UC.[7–12] However, the clinical results have been varied.[13,14] The reason for the failure of FMT is not clear and there is no reliable way to predict who may benefit from FMT. There is also no report whether FMT is effective for UC patients with allergy to 5-ASA. Considering that the intestinal flora in patient with allergic diseases was unbalanced, we speculate that FMT shows potential treatment for allergic diseases, including allergy to drugs. Here, we report the first case that a patient with refractory UC with allergy to 5-ASA underwent FMT as a treatment.
2. Case report
A 47-year-old man, with a 9-year history of UC was referred to Huai’an First People's Hospital, Nanjing Medical University in March 2017. It was not the first time for him to our hospital for medical help. During the past 9 years, he had received 5-ASA including sulfasalazine and mesalazine and irregular oral steroid therapy. However, administration of 5-ASA led to red itchy rash on his face and torso, although the rash disappeared after 5-ASA was stopped. But it appeared again if 5-ASA was re-used, suggesting that he was allergic to 5-ASA. Owing to reluctant use of immunosuppressive agents and tumor necrosis factor inhibitors, he routinely received intravenous steroid therapy every 2 to 3months because of worsening condition. On March 25th, he was hospitalized again because of suffering from gradually aggravated abdominal pain and frequent bloody diarrhea with an average of 8 times daily. Colonoscopy showed that a continuous distribution of superficial erosion and ulceration (Fig. 1A). As previous, we gave him intravenous steroid therapy (methyl-prednisolone, 60 mg daily). Unlike before, his symptoms did not improve after 3-day use of steroid. The following treatment was limited. Considering all that, after informed consent, FMT was performed after stopping steroid treatment.
Figure 1.
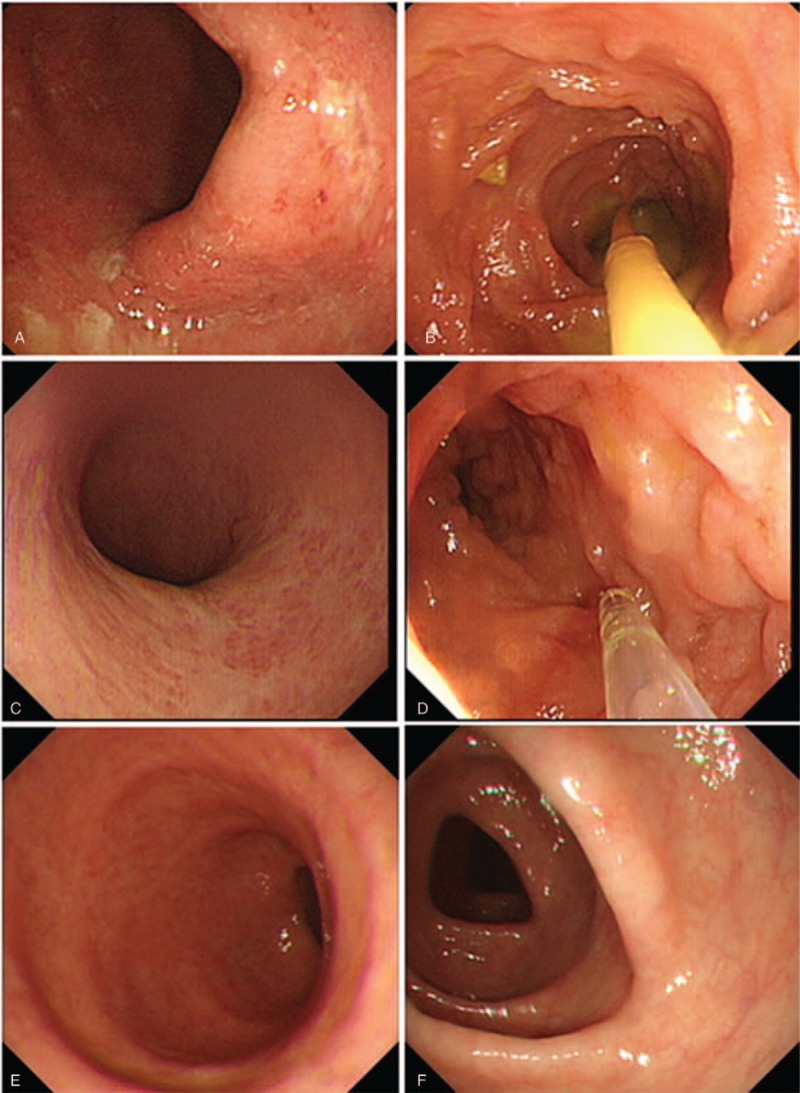
The endoscopic findings before and after fecal microbiota transplantation (FMT). (A) The endoscopic findings in the ulcerative colitis patient before FMT. (B) The 1st FMT was taken and the fresh fecal microbiota suspension was transplanted into the terminal ileum. (C) The endoscopic findings of the patient 1 month after the 1st FMT. (D) At the same time, the 2nd FMT was performed. (E, F) The endoscopic findings of this patient 3 months after the 2nd FMT.
The fecal microbes for FMT were obtained from a healthy 13-year-old boy according to the selection criteria described in our previous report.[15] Fecal microbes were purified in our laboratory according to Filtration Plus Centrifugation method.[15] The donor stool (about 200 g) was dissolved in 1000 mL of sterile saline, then the suspension was poured into several filters with different apertures to remove the scum. The filtered liquid was separated into several 50-mL tubes for centrifugation (2000 rpm/min, 3 minutes), and then we removed the supernatant, added sterile saline, and mixed for centrifugation again. This process was repeated 3 times. Finally, the sediment was obtained and diluted with 200-mL sterile saline. The whole processing time was about 1 hour. Thus, fresh fecal microbes’ suspension was prepared and immediately transplanted into the terminal ileum and the right colon by colonoscopy. The endoscopic image during fecal microbes’ infusion is shown in Figure 1B.
After FMT, food intake was limited to 500 g semi-liquid in the first 24 hours and then normal diet was followed. One week after FMT, the patient was assessed by gastrointestinal symptoms. We found that the frequency and severity of abdominal pain significantly reduced. The stool frequency decreased to 2 to 3 times daily and the bloody stool was also significantly improved. At one month after FMT; reviewed colonoscopy revealed significant improvement in colonic mucosal lesions (Fig. 1C). During the colonoscopy, the patient received the second FMT as planned using the same donor (Fig. 1D). Three months after the second FMT, the patient had no diarrhea and any blood in stool. He underwent colonoscopy again and the damaged colon mucosa had reached full healing (Fig. 1E, F). During the 9-month follow-up, his condition did not recur without any treatment. No adverse event was observed during the FMT and follow-up.
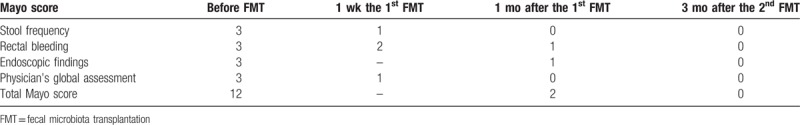
To evaluate the disease activity and efficacy of FMT, Mayo scores were calculated before and after FMT. The score that ranges from 0 to 12 point was based on the total of stool frequency, rectal bleeding, endoscopic findings, and a physician's global assessment. The total Mayo score and sub-item score were listed in Table 1. As shown, clinical and endoscopic remission was induced and maintained by FMT. The clinical response of this patient to FMT was considered effective.
Table 1.
The Mayo scores before and after FMT.
3. Discussion
The concept of FMT for treatment of human intestinal diseases was firstly recorded in China at least since the 4th century.[16] FMT has become an effective strategy for the treatment of UC in recent years. Zhang et al[9] investigated the therapeutic role of step-up FMT for steroid-dependent UC. After step-up FMT, more than half of the recruited patients obtained clinical improvement, suggesting that patients with steroid-dependent UC may benefit from FMT. In our study, we first reported a UC patient with allergy to 5-ASA benefited from FMT after failure of steroid therapy. In our report, the patient was allergic to 5-ASA and short-term steroid therapy was ineffective. In this case, we performed the FMT strategy. The gastrointestinal symptoms were immediately controlled and the patient obtained clinical remission during the 9 months’ follow-up without using any drug.
Increasing studies have focused on the therapeutic effect of FMT in UC; however, the effectiveness was reported differently. The following points should be concerned for the explanation of these variable results: the donor source, fecal microbiota purification methods and fecal microbiota status, disease activity of UC, and permitted concomitant therapies. These possible influencing factors might be different for each case. FMT re-established the intestinal microbiome and showed benefit for UC patients. The mechanism has been still unstated, although it has been hypothesized that healthy donor microbiota may restore homeostasis to the aberrant microbiome and host immune response.[3] Healthy donors are usually recruited from family members; however, there is a lack of evidence to suggest that kinship or non-kinship donor present different therapeutic effects. In this report, a 13-year-old healthy non-kinship boy was selected as the donor after strict screening. Unfortunately, we could not analyze the fecal microbiota composition because the container that holds the stool samples broke during the preservation. In our opinion, the donor selection is important for achieving good therapeutic effect and the fecal microbiota purification methods are crucial for low adverse events rate after FMT by our recent study (new article is being prepared). In this case report, FMT was considered effective based on the improvement of gastrointestinal symptoms and mucosal healing by colonoscopy after FMT, although there is lack of fecal microbiota composition analyses. Another factor that might affect the therapeutic effect is the time spent in the purification of fecal microbiota. Reducing the exposure time of the fecal microbiota to the air is important for preserving its function. In our laboratory, the time was about 1 hour, significantly shorter than the 6 to 12 hours reported by other studies.[17] This was important for obtaining success of FMT for the UC patient.
5-ASA administration is the maintenance treatment of UC. Although the drug is mostly well tolerated, a few patients are allergic to 5-ASA. The limited therapeutic choice for these patients is long-term use with immunosuppressants. However, several patients in China are unwilling to use immunosuppressants. Hence, the quest for an effective and safe approach for these patients is important. To the best of our knowledge, there is no report on using FMT for UC with allergy to 5-ASA in the publicly available literature. We performed FMT for this refractory UC patient and succeeded. This patient lives well without any treatment during the 9 months’ follow-up.
In conclusion, FMT has been considered as an effective treatment for refractory UC through remodeling of intestinal microbiota.
Author contributions
Investigation: Hong-Gang Wang, Shi-Peng Liu, Jing-Fang Zhou, Peng Shen, Shang-Nong Wu.
Methodology: Tian-Heng Ma, Wei Yan, Xiao-Zhong Yang.
Project administration: Yun-Tao Shi, Xiao-Zhong Yang, Shang-Nong Wu.
Writing – original draft: Hong-Gang Wang, Shi-Peng Liu.
Writing – review & editing: Shang-Nong Wu.
Footnotes
Abbreviations: 5-ASA = 5-aminosalicylic acid, FMT = fecal microbiota transplantation, UC = ulcerative colitis.
H-GW and S-PL contributed equally to this work.
The authors report no conflicts of interest.
Ethics statement: This study was reviewed and approved by Huai’an First People's Hospital, Nanjing Medical University Institutional Review Board. The patient provided written informed consents prior to participation in this study.
References
- [1].Talley NJ, Abreu MT, Achkar JP, et al. American College of Gastroenterology IBD Task Force. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol 2011;106:S2–25. [DOI] [PubMed] [Google Scholar]
- [2].Sheehan D, Shanahan F. The gut microbiota in inflammatory bowel disease. Gastroenterol Clin North Am 2017;46:143–54. [DOI] [PubMed] [Google Scholar]
- [3].Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res 2017;10:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017;14:573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borody TJ, Brandt LJ, Paramsothy S. Therapeutic faecal microbiota transplantation: current status and future developments. Curr Opin Gastroenterol 2014;30:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].He Z, Cui BT, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol 2017;23:3565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989;1:164. [DOI] [PubMed] [Google Scholar]
- [8].Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–9. [DOI] [PubMed] [Google Scholar]
- [9].Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerativecolitis. J Transl Med 2015;13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis 2016;10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218–28. [DOI] [PubMed] [Google Scholar]
- [12].Costello SP, Soo W, Bryant RV, et al. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017;46:213–24. [DOI] [PubMed] [Google Scholar]
- [13].Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 2013;19:2155–65. [DOI] [PubMed] [Google Scholar]
- [14].Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110–8. [DOI] [PubMed] [Google Scholar]
- [15].Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015;30:51–8. [DOI] [PubMed] [Google Scholar]
- [16].Zhang F, Luo W, Shi Y, et al. Should we standardize the 1700-year-old fecal microbiota transplantation? Am J Gastroenterol 2012;107:1755. [DOI] [PubMed] [Google Scholar]
- [17].Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol 2013;29:79–84. [DOI] [PubMed] [Google Scholar]


