Abstract
Pyogenic liver abscess (PLA) are space-occupying lesions in the liver associated with high morbidity and mortality. The aim of this study is to review an Italian hospital experience in epidemiological, clinical patterns, and management of PLA.
We performed a retrospective, descriptive case series at a single center assessing demographic characteristics, presentation patterns, etiological factors, microbiological etiology, and management for patients treated for PLA between 2000 and 2016.
Around 109 patients were identified. The majority of patients presented with fever (73%); right upper abdominal pain in 63.3%, vomiting and nausea in 28.4%. The most common laboratory abnormality among included items was increased C-reactive protein and fibrinogen blood levels, respectively, in 98% and 93.9% of cases. Abdominal ultrasound was the diagnostic investigation in 42.4% of cases; CT scan and MR imaging were performed in 51.1% and 3.3% of cases respectively. We observed blood or pus culture study in 99 cases of which only 53.5% came with positive microbial reports. The most common organism identified was Escherichia coli (26.5%), followed by Streptococcus spp (13.2%). Early antibiotic treatment started on all patients and 66.7% of cases required different approaches, Ultrasound or CT-guided needle aspiration of PLA was performed in 13 patients (11%) and percutaneous abscess drainage was performed on 72 patients (67%).
PLA is a diagnostically challenging problem due to nonspecific presenting characteristics. The microbiological yield identified was a typical European spectrum with a preponderance of Escherichia coli infections. Once recognized, percutaneous drainage and antibiotic treatment are the mainstay of management for PLA.
Keywords: clinical presentation, microbiology, percutaneous drainage, pyogenic liver abscess, risk factors
1. Introduction
Pyogenic liver abscess (PLA), a suppurating infection of the hepatic parenchyma, remains a mortality associated condition and nowadays develops as a complication of biliary tract diseases for about 40% of cases.[1] Recently, the etiologies of PLA have shifted from intra-abdominal infections such as acute appendicitis and trauma to pathologic conditions of the biliary tract; however, up to 55% of patients with PLA have no clear risk factors and these cases are called cryptogenic.[2–4]
The incidence of PLA varies from 8 to 22 patients per 1,000,000 people belonging to a geographical area with substantially higher rates having been reported in Taiwan.[5–8]
Early diagnosis and treatment is a crucial step in the management of these patients, since the presentation may be subtle and not specific (abdominal pain, fever, nausea, and vomiting), so currently constitutes a challenge for physicians: a high index of suspicion is the cornerstone of prevention for misdiagnosis and improvement of prognosis.[9] In recent decades, combined antibiotic therapy and percutaneous drainage have become the first-line treatment in most cases and has greatly improved patients’ prognosis: the mortality rate has dropped from 70% to 6.31%.[10] In terms of causative pathogens, bacteria most frequently associated with PLA are Escherichia coli, Enterobacteriaceae, anaerobes, and other members of the gastrointestinal flora. Over the past 2 decades Klebsiella pneumoniae has been emerging as the predominant pathogen responsible for 50% to 88% of PLA in the Asian population and it has been reported with increasing frequency in South Africa, Europe, and the United States.[7] Because such experiences have not yet been reported in Italy, we reviewed the cases of PLA seen at our institution. The present study is a retrospective analysis of demographic characteristics, etiological factors, presentation patterns, microbiological etiology, and the treatment of PLA cases which were presented in an Italian hospital over a 16-year-period.
2. Materials and methods
2.1. Study population
The study includes 109 consecutive patients with pyogenic liver abscess admitted to the Santa Croce and Carle Hospital of Cuneo from January 2000 to December 2016. These patients were identified by searching the International Classification of Disease code (ICD 9-1997) for the diagnosis of “pyogenic liver abscess” (572.0.0) from the hospital database during that period. The protocol was approved by the Institutional Review Board of Santa Croce and Carle General Hospital, Cuneo, Italy (IRB No. 38132).
The diagnosis of PLA was based upon clinical aspects, imaging studies, and microbiology on blood or aspirate culture results.
Exclusion criteria were: patients with age < 18 years, amoebic liver abscess, hydatid liver abscess, previous episode of PLA, and previous liver transplantation.
Medical records were evaluated by the Hospital Archival records system. Data collected included demographic characteristics, etiopathologic factors, clinical features, laboratory data, number, size and location of lesions, microbiological findings, diagnostic and therapeutic methods, treatment response and mortality.
The presumptive etiology of the liver abscess was a judgement made, at the time of retrospective study by the investigator using a standard protocol. PLA was considered secondary to biliary tract disease in patients with a clinical picture of cholecystitis or cholangitis or a documented biliary duct abnormality. It was considered secondary to hematogenous spread when another source of infection was found. Cases that were judged ambiguous were reviewed by a secondary investigator to confirm the etiology. We defined a cryptogenic abscess as a PLA for which no obvious source of infection was found, despite evaluation by imaging methods and the collection of medical history in immunocompetent patients.
Radiological findings included abdominal ultrasound, computed tomography (CT), and magnetic resonance (MR).
Cultures were isolated for aerobic and anaerobic organisms using the standard diagnostic techniques. If both blood and pus cultures were positive, organisms recovered from the abscess were assumed to be the etiologic.
All patients received antibiotic therapy: the type, duration, and mode of administration were recorded for each case. PLA were treated empirically with antibiotics and, if there was no response to the initial treatment, the antibiotic was changed according to the results of cultures or empirically if cultures were negative. No response to the initial treatment was defined as a patient having persistent signs of sepsis after 5 days of intravenous antibiotics.
Ultrasound-guided percutaneous needle aspiration and drainage were performed in the radiology department.
Cure was defined as the absence of signs and symptoms together with image studies without findings compatible with PLA.
Case fatality was defined as death during hospitalization and related to PLA.
2.2. Statistical analysis
Comparisons of variables between groups were made with standard statistical tests. Survival probability curves were calculated with the Kaplan–Meier method and compared with log-rank test. Multivariate Cox proportional hazards model was used to assess variables independently related to 3-month survival. All statistical analyses were performed using SPSS 20.0 software. Results for continuous variables are expressed as mean ± standard deviation. The significance level for all statistical tests was set at 0.05 two-tailed.
3. Results
3.1. Demographic and clinical features
From January 2000 to December 2016 inclusive, 109 patients were diagnosed with PLA at Santa Croce and Carle General Hospital, of which 62 patients (56.9%) were males and 47 patients (43.1%) were females. The patient's age was ranging from 18 to 92, and mean age was 65.4 (standard deviation, SD: ±14.3).
As shown in Figure 1, the majority of patients with PLA presented with fever, 73%; right upper abdominal pain was reported in 69 cases (63.3%), vomiting and nausea in 31 (28.4%), while asthenia in 29 patients (26.6%), loss of weight in 19 patients (17.4%), and jaundice in 14 cases (12.8%).
Figure 1.
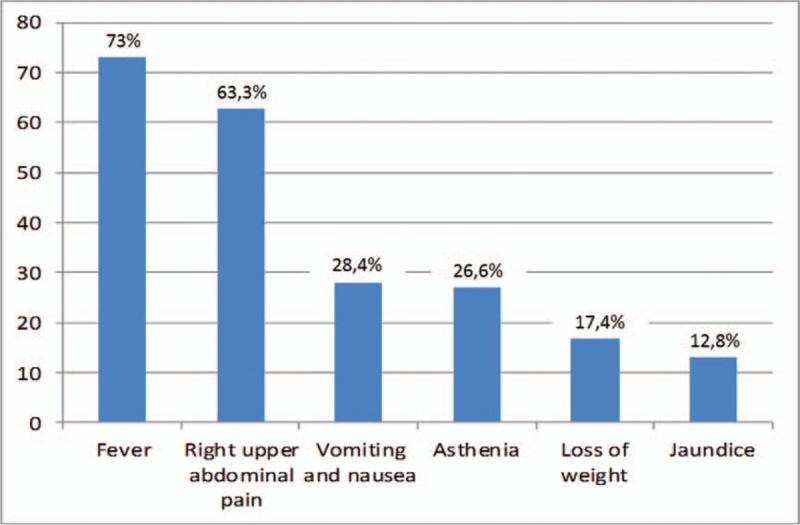
Percentage of patients with various clinical features on presentation.
A total of 25 patients (23%) had diabetes mellitus. Among the diabetic patients, 57% did not have good glycemic control (good glycemic control is categorized as glycated hemoglobin (HbA1c) ≤ 7%).[11]
Charlson comorbility index score (CCIS) was used to determine overall systemic health: with each increased level of CCSI, there was a step-wise increase in the cumulative mortality: score 0 with a 10 year survival rate of 99%, and score 5 with a 10-year survival rate of 34%, respectively.[12] Among the study population, 19 patients (17.4%) had 0–1 CCIS, suggestive of low comorbility, 22 patients (20.2%) had 2–3 CCIS, and 68 cases (62.4%) had high CCIS >3, suggestive of high comorbility and cumulative mortality.
3.2. Laboratory and radiological features
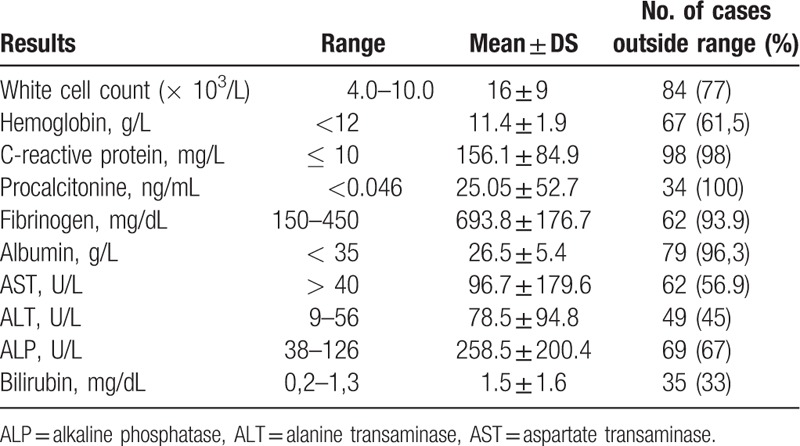
The most common laboratory abnormality among included items was increased C-reactive protein and fibrinogen blood levels, respectively, in 98% and 93.9% of cases (Table 1). These were followed by abnormal total leucocytic count in 77% patients and elevated ALP in 67% of cases. The procalcitonine value was elevated in all patients in which it was tested 34 patients.
Table 1.
Summary of laboratory results at presentation in patients with liver abscess.
Abdominal ultrasound was the diagnostic investigation in 42.4% of cases; CT scan and/or MR imaging were performed if they were further needed for differential diagnosis. Around 51.1% of patients underwent CT scanning and 3.3% needed MR. The mean size of liver abscess was 7 ± 3.5 cm (range, 1.5–15 cm). In this series, 55% of abscesses were single at time of presentation. Two abscesses were found in 18.3% of cases and > 3 lesions in 26.6% of patients. The presence of gas in the abscess cavity was noted in 18.5% of cases. The majority of liver abscesses (65.4%) were found in the right lobe of the liver and about 19.6% were confined to the left lobe. Both lobes were involved in 15% of patients.
3.3. Microbiology and pathogenesis
We observed blood or pus-culture study in 99 cases of which only 53 cases (53.5%) came with positive microbial reports. Culture from aspirate of liver abscess was positive in 25 of 62 patients who underwent percutaneous aspiration of liver abscess (positive rate, 40.3%). The most common organism identified was E coli (26.5%), followed by Streptococcus spp (13.2%) and anaerobics (13.2%). Other organisms were Enterococcus spp (11.3%), Staphylococcus (7.5%) (Fig. 2).
Figure 2.
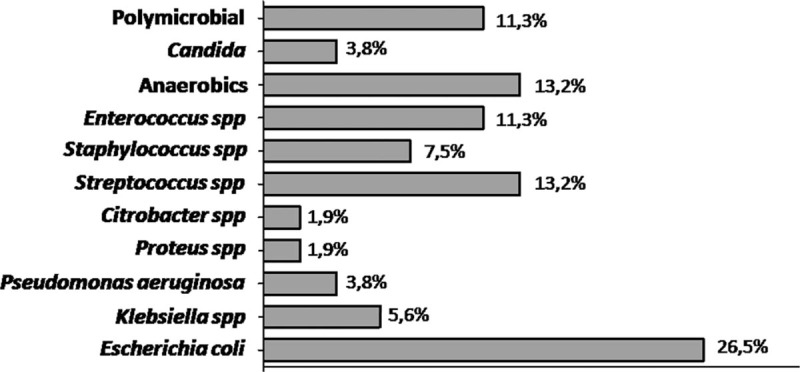
Organisms isolated from all positive cultures.
As shown in Figure 3, of all PLA, in 28 cases (25.7%) the cause was not clear: cryptogenic abscess. Biliary tract diseases, including cholelithiasis, cholecystitis, and malignancies, were identified in 55% of cases (Table 2). In 12% of cases the cause of PLA was assigned to be hematogenous. In 7.3% of PLA the only known risk factor for PLA was diabetes mellitus. Among 28 cryptogenic abscesses, a total of 8 individuals (28.5%) underwent the colonoscopy: nobody was found to have incidental colon cancer or high-grade dysplasia.
Figure 3.
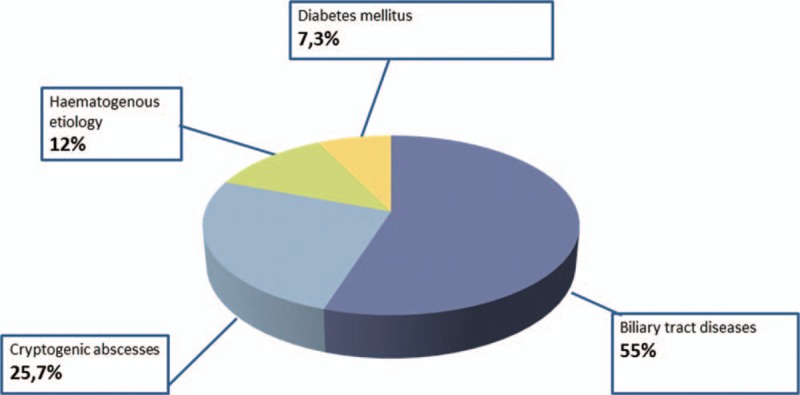
Causes of pyogenic liver abscess.
Table 2.
Detailed biliary tract diseases causes of pyogenic liver abscess. Values are given as number and percentages.

3.4. Management
Intravenous antibiotic therapy started on all patients and 66.7% of cases required intervention in different forms. An empirical antibiotic treatment was started at the onset of the clinical sign of infection and was changed upon the results of antibiotic susceptibility tests. The reason for not performing image-guided aspiration and drainage was due to the small size of the abscess, clinical improvement with antibiotics or advanced malignancy. The range of antibiotic treatment duration was 29.7 days (SD: ±19.8).
Radiological drainage was the most frequent initial intervention: ultrasound or CT-guided needle aspiration of PLA was performed in 13 patients (11%) and percutaneous abscess drainage was performed on 72 patients (67%). Generally, a percutaneous French 7 pigtail catheter was inserted. The removal of the percutaneous drain was based on the patient's clinical and laboratory response. The median of drainage duration was 12.6 ± 14 days. Of all 72 cases that underwent radiological drainage, 7 patients required surgical intervention because of failure of resolution and recurrence of the abscess. Endoscopic retrograde cholangiopancreatography (ERCP) was used to treat liver abscess in 6 patients (5.6%), in which there was a lithiasic or neoplastic biliary obstruction.
The time interval between antibiotic administration and drainage was 8.5 days (SD: ±11.7).
3.5. Complications and outcomes
There were 2 cases of major complication of interventional drainages: right pneumothorax, which resolved without drainage tube, and another patient developed biliary fistulae which resolved after ERCP decompression and nose-biliary drainage. There were no cases of abscess rupture or thoracic empyema. Eleven patients (10.1%) developed pneumonia during hospitalization and 4 cases (3.7%) developed splenic multiple abscesses.
The mean duration of hospitalization was 28 days (SD: ±21). There was no correlation between length of hospital stay and PLA management; (antibiotic treatment alone versus interventional drainage, P=NS).
Eleven patients died during the hospital admission, such death resulting directly from the abscess or from a complication of treatment. The mean age of patients who died was 65.3 ± 9. Seven were males. The mean hospital stay was 24 days (SD: ±12.8).
4. Discussion
In this study, the clinical characteristics, etiology, microbiology, treatment, and mortality of 109 patients with PLA are reviewed and reported.
The average age of PLA patients in our sample was 65.4. This supports other series in demonstrating that the main age of patients who develop PLA has risen: in our study the average presentation was in the seventh decade. Again, a preponderance of males was noted.[13,14]
Presenting symptoms of PLA were multiple and low specific, including fever, right upper abdominal pain, vomiting, nausea and asthenia, while on physical examination jaundice was recognized in 12.8% cases. In association to presenting symptoms, the presence of a raised C-reactive protein, white blood cell and alkaline phosphatase should suggest the possibility of PLA.
Perhaps the low index of suspicion of PLA due to the nonspecific presentation characteristics, the diagnosis could be suggested by the underlying predisposing disease processes. We identified predisposing diseases associated with the abscess formation, hepatic–pancreatic–biliary system problems were present in the majority of cases (55%).[13]
Although it has been suggested that cryptogenic liver abscess is a sign of occult gastrointestinal malignancy,[15,16] few patients with cryptogenic abscess underwent colonoscopy during their indexed hospitalization (28.5%): no colon cancer or polyp with high grade dysplasia was found.
The confirmation of the diagnosis of PLA relies on imaging of the liver: ultrasound alone was the diagnostic investigation in 42.4% of cases; CT scan was performed in 51.1% patients, the reason being the diffuse availability and of course, in case needed further for differential diagnosis. We confirmed the suggestion that PLAs are mostly confined to the right hepatic lobe[9] as the result of the portal vein anatomy, more hepatic mass and the denser network of bile canaliculi in this lobe.
In our study, microbiological yield from blood and abscess culture was 54.2%, lower compared with other studies. A contributory factor to our low microbiological yield may be the early use of antibiotics: as known, a rapid sterilization of blood cultures can occur within a few hours of the first antibiotic dose.[17] There are little formal data regarding the optimum duration of antibiotic therapy, but most units use a regimen of 2 weeks’ parenteral treatment, followed by a more prolonged course (4–6 weeks) of therapy, switching to oral antibiotics when clinical and inflammatory responses allow.[18] In our study, the range of antibiotic treatment duration was 29.7 ± 19.8 days, we also considered long oral antibiotic courses lasted after discharge.
A typical European microbiological spectrum was identified in this study: we observed a preponderance of E coli infections. The other main isolates were Streptococcus spp, Enterococcus spp and anaerobics. In only 2 cases we identified K pneumoniae, the predominantly PLA infection seen in Southeast Asia.[19] The high incident of E coli and Enterococcus spp is probably related to the high incidence of a biliary cause of PLA.[20]
Percutaneous needle aspiration and catheter drainage has been shown to be beneficial in the treatment of PLA in association with antibiotics: this association is the present standard practice. Of our cohort, all patients received antibiotic treatment, 66.7% of patients underwent percutaneous aspiration or drainage, or surgical drainage. Belonging to a meta-analysis, there is a favored opinion for use of percutaneous catheter drainage (PCD) over percutaneous needle aspiration (PNA): PCD facilitates a higher success rate, reduces the time required to achieve clinical relief and supports a 50% reduction in abscess cavity size.[21,22] Image-guided catheter drainage was favored over PNA at our institution. ERCP can be a useful investigation and treatment procedure for patients with PLA: in this series 6.5% of patients underwent ERCP to treat a lithiasic or neoplastic biliary obstruction, which was the cause leading to PLA.[23]
The most frequent complications were pulmonary: pneumonia was seen in 11.9% of patients.
The in-hospital mortality rate was 10.1%. Nowadays, better imaging, more effective use of antibiotics and abscess percutaneous drainage improved AEP mortality rate from 40% to 10% to 25%.[24,25] Previous studies reflected that septicaemia and underlying malignancy were strong indicators of in-hospital mortality during the period from 1993 to 2008 in one UK center.[15] In another European study, from 1992 to 2005, mortality associated with PLA was high, 19%, and the main risk factors were the development of sepsis and/or septic shock.[9]
The overall mean hospital stay was 24 days, which was also compatible with previous reports.[26,27]
This is a retrospective study; there are few reports in recent years about PLA diagnosis and management in Europe. Our experience may provide valuable information to clinicians who encounter this uncommon condition.
The present study has some important limitations: it is a retrospective study and some relevant information may not have been documented in the patient's medical chart, strong conclusions cannot be made due to the small sample size and the results represent the experience of a single-center and may not be generalized in reference to other areas with different epidemiological or clinical settings.
5. Conclusion
The study attempts to show our first-hand real, original experiences in this field and to provide useful information. In practice, clinicians should maintain a high index of suspicion for PLA in patients who present with the risk factors; in particular, hepatic-pancreatic-biliary system pathologies, and clinical scenarios of fever, right abdominal pain, increased levels of C-reactive protein and white blood cell count. Prompt diagnosis of liver lesion and administration of antibiotics and percutaneous drainage can conduce to successful treatment.
Author contributions
Conceptualization: Cristina Serraino, Luigi Maria Fenoglio.
Data curation: Cristina Serraino, Christian Bracco, Gianluca Rinaldi.
Formal analysis: Chiara Elia, Remo Melchio.
Methodology: Remo Melchio.
Supervision: Fulvio Pomero, Alberto Silvestri, Luigi Maria Fenoglio.
Writing – original draft: Cristina Serraino, Chiara Elia.
Footnotes
Abbreviations: CCIS = Charlson comorbility index score, CT = computed tomography, ERCP = endoscopic retrograde cholangiopancreatography, MR = magnetic resonance, PCD = percutaneous catheter drainage, PLA = pyogenic liver abscess, PNA = percutaneous needle aspiration, SD = standard deviation.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004;39:1654–9. [DOI] [PubMed] [Google Scholar]
- [2].Malik AA, Bari SU, Rouf KA, et al. Pyogenic liver abscess: changing patterns in approach. World J Gastrointest Surg 2010;2:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuo SH, Lee YT, Li CR, et al. Mortality in emergency department sepsis score as a prognostic indicator in patients with pyogenic liver abscess. Am J Emerg Med 2013;31:916–21. [DOI] [PubMed] [Google Scholar]
- [4].Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 1996;223:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alvarez Pérez JA, Gonzàlez JJ, Baldonedo RF, et al. Clinical course, treatment and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg 2001;181:177–86. [DOI] [PubMed] [Google Scholar]
- [6].Mohsen AH, Green ST, Read RC, et al. Liver abscess in adults: ten years experience in a UK centre. QJM 2002;95:797–802. [DOI] [PubMed] [Google Scholar]
- [7].Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008;14:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu Y, Wang JY, Jiang W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: etiology, diagnosis, treatment. Gastroenterol Res Pract 2013;2013:258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ruiz-Hernàndez JJ, Leòn-Mazorra M, Conde-Martel, et al. Pyogenic liver abscesses: mortality-related factors. Eur J Gastroenterol Hepatol 2007;19:853–8. [DOI] [PubMed] [Google Scholar]
- [10].Su YJ, Lai YC, Lin YC, et al. Treatment and prognosis of pyogenic liver abscess. Int J Emerg Med 2010;3:381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang HH, Tsai SH, Yu CY, et al. The association of haemoglobin A1c levels with the clinical and CT characteristics of Klebsiella pneumonia liver abscess in patients with diabetes mellitus. Eur Radiol 2014;24:980–9. [DOI] [PubMed] [Google Scholar]
- [12].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbility in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [13].Bosanko NC, Chauhan A, Brookes M, et al. Presentations of pyogenic liver abscess in one UK center over a 15-year period. J R Coll Physicians Edinb 2011;41:13–7. [DOI] [PubMed] [Google Scholar]
- [14].Heneghan HM, Healy NA, Martin ST, et al. Modern management of pyogenic hepatic abscess: a case series and review of the literature. BMC Res Notes 2011;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lai HC, Lin HC. Cryptogenic pyogenic liver abscess as a sign of colorectal cancer: a population-based 5-year follow-up study. Liver Int 2010;30:1387–93. [DOI] [PubMed] [Google Scholar]
- [16].Qu K, Liu C, Wang ZX, et al. Pyogenic liver abscesses associated with nonmetastatic colorectal cancers: an increasing problem in eastern Asia. World J Gastroenterol 2012;18:2948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of sepsis and severe sepsis. Crit Care Med 2008;36:296–327. [DOI] [PubMed] [Google Scholar]
- [18].Sharara AI, Rockey DC. Pyogenic liver abscess. Curr Treat Options Gastroenterol 2002;5:437–42. [DOI] [PubMed] [Google Scholar]
- [19].Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol 2010;16:2458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhu X, Wang S, Jacob R, et al. A 10-year retrospective analysis of clinical profiles, laboratory characteristics and management of pyogenic liver abscesses in a Chinese Hospital. Gut Liver 2011;5:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cai YL, Xiong XZ, Lu J, et al. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: a systematic review and meta-analysis. HPB (Oxford) 2015;17:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mangukiya DO, Darshan JR, Kanani VK, et al. A prospective series case study of pyogenic liver abscess: recent trends in etiology and management. Indian J Surg 2012;74:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dull JS, Topa L, Balgha V, et al. Non-surgical treatment of biliary liver abscesses: efficacy of endoscopic drainage and local antibiotic lavage with nasobiliary catheter. Gatrointest Endosc 2000;51:55–9. [DOI] [PubMed] [Google Scholar]
- [24].Verlenden WL, Frey CF. Management of liver abscess. Am J Surg 1980;140:53–9. [DOI] [PubMed] [Google Scholar]
- [25].Chu K, Fan S, Lai EC, et al. Pyogenic liver abscess: an audit of experience over the past decade. Arch Surg 1996;131:148–225. [DOI] [PubMed] [Google Scholar]
- [26].Pang TC, Fung T, Samra J, et al. Pyogenic liver abscess: an audit of 10 years’ experience. World J Gastroenterol 2011;17:1622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu L, Chen W, Lu X, et al. Pyogenic liver abscess: a retrospective study of 105 cases in an emergency department from East China. J Emerg Med 2017;52:409–16. [DOI] [PubMed] [Google Scholar]


