Supplemental Digital Content is available in the text
Keywords: adverse events, CO2 exhalation, electronic cigarettes, smoking cessation, smoking reduction
Abstract
Background:
Electronic cigarettes (e-cigarettes) are a prevalent smoking cessation aid worldwide; however, a consensus regarding their efficacy and safety has yet to be reached.
Methods:
We conducted a systematic review of the literature from related studies written in English or Chinese and published between January 1, 2003, and July 30, 2017. Eligible studies reporting the number of smokers who reduced or quit smoking and suffered from adverse events after e-cigarette use were selected according to predefined criteria; pertinent data were then extracted for a meta-analysis.
Results:
Our search produced 198 articles; of these publications, 14 including 35,665 participants were analyzed. The pooled efficacy rate of e-cigarettes ranged from 48.3% to 58.7% for smoking reduction and from 13.2% to 22.9% for smoking cessation. The pooled rate of adverse events associated with e-cigarettes ranged from 49.1% to 51.6% based on 11 studies including 16,406 participants. The most prevalent adverse events were mouth or throat irritation, anxiety, depressed mood, nausea, and insomnia. No significant differences in overall CO2 exhalation (eCO) levels were observed after e-cigarette use according to the data from 5 studies.
Conclusion:
Our findings suggest that e-cigarettes are moderately effective with regard to smoking reduction and smoking cessation. eCO levels are unreliable for evaluating the efficacy of e-cigarettes. E-cigarette related adverse events frequently occur, especially due to high-dose nicotine-containing cartridges.
1. Introduction
Cigarettes smoking, which is a serious public health issue, is a substantial economic and social burden worldwide.[1] Nicotine smoking is recognized to be an independent risk factor for various diseases, such as lung cancer, cardiovascular disease, stroke, and chronic lung disease.[2] In United States, smoking-attributable deaths are estimated to be as high as 200,000 for men and 180,000 for women yearly.[3] In European Union, approximately 700,000 people die prematurely each year because of cigarettes smoking[4]; in addition, an estimated 443,000 people die prematurely from exposure to second-hand smoking each year,[5] making tobacco use the single largest preventable cause of diseases, disabilities, and deaths.
Various tobacco replacements have been developed to reduce the hazard caused by smoking nicotine cigarettes.[6] The most popular program is nicotine replacement therapy (NRT), which is a strategy to deliver pharmaceutical grade nicotine in the form of patch, gum, lozenge, sublingual tablet, inhalator, or spray.[7] Although NRT is a short-term, safe, and effective smoking substitute, fewer than 20% of the users have reported successfully quitting smoking after 12 months.[8] In addition, NRT use is accompanied by considerable complaints on unpleasant adverse events, or difficulty in using NRTs.[9] Recently, e-cigarettes have been promoted as an alternative to smoking owing to its potential in helping quit.[10] Despite the wide variations in design, contents, and operational features, the majority of e-cigarettes mimic cigarettes behaviors and vaporize the mist from a nicotine-containing liquid after activating the heating element.[11] Consequently, the smokers inhale the mist produced, which is behaviorally similar to the cigarette. Besides that, e-cigarettes have been generally regarded as more cost-effective, amenable to use in smoking-restricted environments, and more socially acceptable than other cigarettes alternatives.[12] In addition, proponents maintain that e-cigarettes are a safer smoking cessation aid because of less tobacco toxicants and chemical exposure to the users.[13]
Despite the recent prevalence of e-cigarettes, their efficiency in cutting down smoking and quitting smoking accompanied by their potential health risk particularly with respect to their long-term use have not yet reached a consensus.[14] A 6-month prospective study indicated the use of e-cigarettes substantially cut down cigarettes use without causing significant adverse events in smokers not intending to quit.[15] A prospective 12-month randomized controlled trial (RCT) suggested that the use of e-cigarettes with or without nicotine would decrease cigarettes consumption and elicited enduring tobacco abstinence without causing significant adverse events in smokers not intending to quit.[16] Another RCT involving 657 smokers revealed that e-cigarettes were modestly effective in helping users quit, which was similar to the efficiency of nicotine patches in 6-month quit rates.[17] Whereas, a recent online survey on 3627 US smokers showed that no increased quitters were observed after 1-year consumption of e-cigarettes compared with other substitutes,[18] which is consistent to a previous longitudinal international study demonstrating that e-cigarettes users did not quit more frequently than nonusers.[19] Meanwhile, the debates on the safety have been raised as a primary consideration of e-cigarettes. Previous studies showed that e-cigarettes contained very low levels of tobacco-specific nitrosamines and much lesser toxin than tobacco cigarettes.[20] However, the US FDA has warned against e-cigarette use and argued that e-cigarettes be classified as drug-delivery devices similar to nicotine inhalers. Several countries such as Canada, Australia, Brazil, and Panama have taken a more aggressive stance, banning e-cigarettes because of safety concerns.[21] In addition, a web-based survey on 179 Polish considered that e-cigarettes were a source of second-hand exposure to nicotine but not to combustion toxicants.[22] Under this circumstance, no consistent conclusions have been drawn concerning the efficiency and adverse effects of e-cigarettes.
This study aims to assess the efficiency of e-cigarettes on smoking reduction and smoking cessation together with their adverse events using meta-analysis that scientifically summarized all of the existing studies. For better evaluation, factors that have potential influences, such as smoker age, cigarettes, and e-cigarettes utilization, were also considered in this systematic review.
2. Methods
2.1. Search strategy
A literature search was conducted in online databases, including PubMed, EMBASE, web of science, Google scholar, the Chinese Medical Citation Index (CMCI/CMCC integrated version), and the CENTRAL database of the Cochrane Library. The search strategy was as follows: “electronic cigarette (s),” “e-cigarette (s),” “e-cig (s),” “smoking alternatives,” “electronic cigarettes vapor,” “e-cigarettes liquid,” “e-cig composition,” “e-cig chemicals,” “e-cig chemical composition,” “electronic cigarettes gas,” “electronic cigars,” “electronic nicotine delivery device,” and “electronic nicotine delivery systems.” The language of the papers was restricted to English and Chinese. The search covered the literature published from January 2003 to July 2017.
2.2. Selection criteria
The studies that met all of the following four criteria were enrolled: Smoking reduction and smoking cessation attributed to e-cigarettes were reported. The smokers had a consumption history of both cigarettes and e-cigarettes. The chemical constituent of the e-cigarettes was reported. A quantitative assessment of the efficiency of e-cigarettes was presented by calculating the accurate number of smoking reduction, smoking cessation, and quit failures.
2.3. Exclusion criteria
The studies that met any of the following 4 criteria were excluded: The participants who had diseases reported in studies; The description of the baseline conditions of participants and e-cigarette ingredients is unclear; The endpoint indicators of the efficacy about smoking reduction, smoking cessation, and adverse events were absent; Any duplicated publications, reviews, and systematic reviews.
2.4. Definition
Smoking reduction is defined as a minimum 50% cutting down of daily tobacco consumption from baseline to 1-year follow up.[23,24] Whereas, the definition of smoking cessation ranges from single point prevalence to sustained abstinence (multiple point prevalence with self-report of no slips or relapses),[25] which is determined according to the definitions in the original studies.
2.5. Selection criteria and date extraction
The data were independently extracted by 2 reviewers (Xing Liu and Wan Lu) and all the data should include the following characteristics: a description of the study population (age, gender, sample size, and groups), study details (first author, year of publication, country of publication, study designs, and endpoint indicators), and pooled data (rates of smoking reduction, smoking cessation, adverse events, and measured eCO levels). Among them, the pooled data of smoking reduction, smoking cessation, and adverse events were extracted from the RCTs, observational studies, and online surveys, respectively. In addition, the pooled data of measured eCO level was extracted from both RCTs and online surveys. Considering the difference in study design that 3 groups (e-cigarettes, patches, and placebo) existed in RCTs and only e-cigarettes group existed in both observational studies and surveys, only data in the e-cigarettes group were extracted from RCTs for better comparison. When a discrepancy in the data is existing, a third researcher was consulted to reach a final consensus after discussion.
2.6. Quality assessment
The non-RCTs were evaluated using a Newcastle–Ottawa Scale for quality assessing (Cross-Sectional/Prevalence Study Quality).[26] The RCTs were evaluated using a CONSORT 2010 statement.[27] Blinded quality assessments of the included literature were performed by 2 researchers, and a third researcher was consulted for the final grading if discrepancy was presented.
2.7. Statistical analysis
We anticipated heterogeneity between studies due to different study designs, methods of analysis, different using time of e-cigarettes, e-cigarettes dose, nicotine dose in e-cigarettes, history of cigarettes smoking, and cigarettes dose. We used a random-effects model to account for both within and between study heterogeneity. Heterogeneity was examined using the standard I2 test. The analysis was done using Stata Software (Version 15.0, StataCORP, TX) and Review Manager (RevMan) 5.3 (http://ims.cochrane.org/revman). Statistical significance was taken as 2-sided P < .05.
3. Results
3.1. Search result and methodological quality assessment
A total of 198 publications were eventually retrieved from the search, and no conference materials were included in the systematic review. We identified 75 individual publications after excluding the duplicated materials from the searched electronic library. In addition, we excluded 48 manuscripts that described the chemical constituents or the e-cigarettes structures rather than the actual efficiency of e-cigarettes. Moreover, 13 other publications were ruled out according to the contents of the full text, including 4 studies that failed to publish detailed information, 1 study that provided no data on the exposure dose of e-cigarettes, 1 paper that nearly completely overlapped the content of other included studies, and 7 reports that had significantly different sample sizes between the study groups and control groups. After these exclusions, 14 publications were finally included in the meta-analysis, and the entire selecting procedure is shown in Fig. 1. According to the study designs in Table 1, we categorized these publications into experimental studies (3 RCTs and 7 observational studies) and surveys (4 online surveys). The CONSORT 2010 statement showed these 3 RCT publications have an average score of 8.32, while an average score of 6.4 (6 articles scored 6, 5 articles scored 5) has been obtained for those non-RCT publications using Newcastle–Ottawa Scale (see Table, Supplemental Content 1, which illustrates the result of quality assessment).
Figure 1.
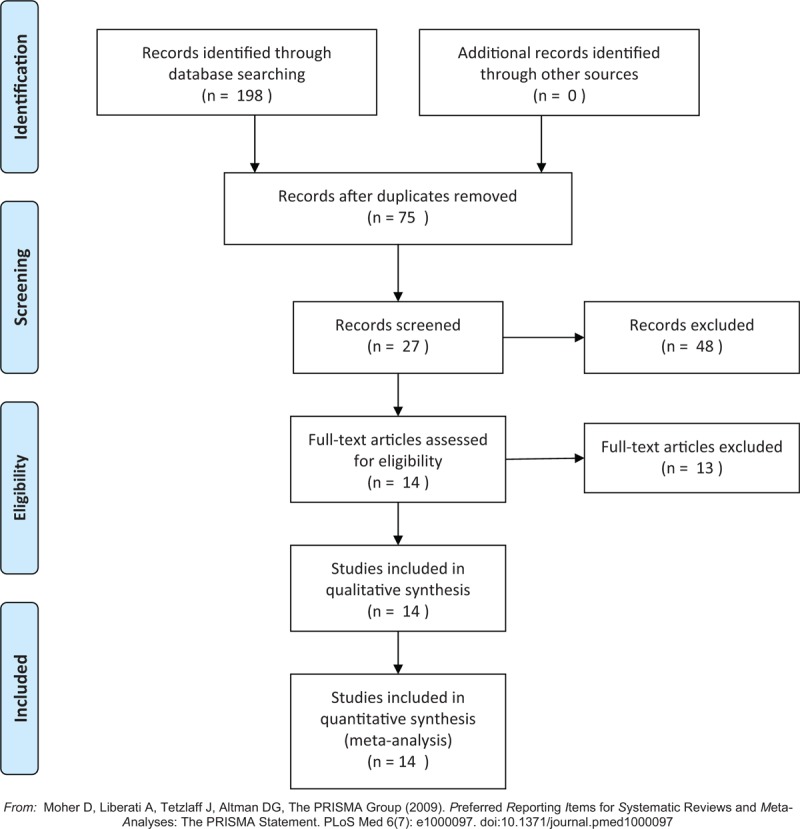
Search results and the selection procedure.
Table 1.
Baseline of the studies included.
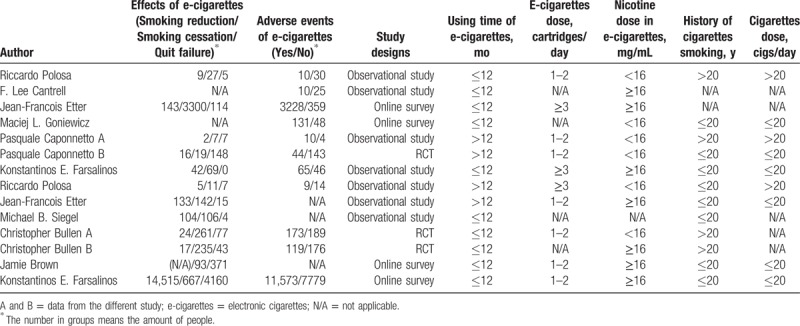
3.2. Study characteristics
Two endpoint indicators, “smoking reduction” and “smoking cessation,” had been promoted to evaluate the efficiency of e-cigarettes as a smoking aid, and a third endpoint indicator “adverse events” was adopted to evaluate the safety of e-cigarettes. We evaluated the baseline levels of these participants and then categorized all of the participants into 6 subgroups according to the following aspects: study designs, using time of e-cigarettes, e-cigarettes dose, nicotine dose in e-cigarettes, history of cigarettes smoking, and cigarettes dose.
3.2.1. Effects of e-cigarettes
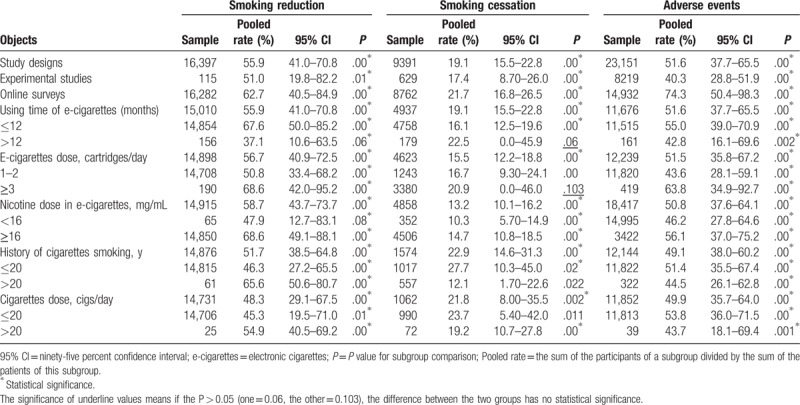
The pooled rates ranged from 48.3% to 58.7% in cutting down smoking subgroups and they ranged from 13.2% to 22.9% in quitting smoking subgroups according to the random effects model (see Figure, Supplemental Content 2, which illustrates the funnel plots and random effects models). In addition, results showed individuals smoked e-cigarettes less than 12 months were easier to cut down their cigarettes use than those who smoked longer than 12 months (pooled rate 67.6% vs 37.1%) (P < .05). Meanwhile, smokers who smoke ≥3 cartridges/day were easier to quit than those whom smoked 1 to 2 cartridges/day (68.6 vs 50.8) (P = .103). Individuals who smoked cigarettes less than 20 years were easier to quit than those who smoked cigarettes over 20 years (27.7% vs 12.1%) (P < 0.05). Similarly, individuals who smoked less than 20 cigarettes/day were easier to quit than those who smoked over 20 cigarettes/day (23.7% vs 19.2%) (P < .05). However, no significant difference was observed in smoking cessation between the individuals who smoked longer or less than 12 months (P = .06) (Table 2).
Table 2.
The outcome of subgroup-based meta-analysis.
3.2.2. Adverse events of e-cigarettes
The dominant adverse events of e-cigarettes were reported as cough, mouth or throat irritation, anxiety, depressed mood, nausea, and insomnia from included 11 studies that involved 16,406 participants (see Table, Supplemental Content 3, which illustrates the baseline of adverse events). Among them, approximately 12.9% claimed to be anxious or nervous, 6.18% complained of having a depressed or sad mood, and 4.57% felt hungry or were concerned about weight gain. Adverse events were also observed in subgroups (ranged from 49.1% to 51.6%) (Table 2). The reported adverse events rate in web-based surveys was significantly higher than that in experimental studies (74.3% vs 40.3%) (P < .05). And participants who smoked e-cigarettes more than 3 cartridges/day had a high incidence of adverse events (pooled rate, 63.8%). Smokers who used e-cigarettes less than 12 months also had a high rate of adverse events (pooled rate, 55.0%).
3.2.3. The eCO levels
Five studies that reported the eCO levels before and after using e-cigarettes have been analyzed.[28–30] Results showed the heterogeneity of forest plots was I2 = 67.2% (Fig. 2), suggesting random effects model being acceptable in assessing the difference of eCO levels. A standardized mean difference of 0.37 [95% confidence interval (95% CI), -0.09 to 0.83] was obtained, suggesting no significant difference could be found on the eCO levels before and after e-cigarettes use. In the sensitivity analysis, standardized mean difference from fixed effects model was 0.23 (95% CI, 0.06–0.41), suggesting that the meta-analysis result was stable because the 95% CI results from 2 effects models were largely overlapped.
Figure 2.
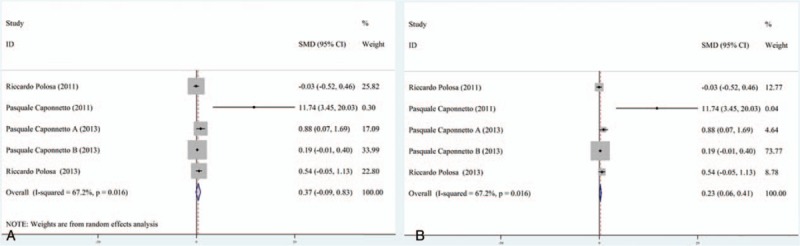
Forest plot of CO2 exhalation variation before and after electronic cigarettes use.
3.3. Publication bias
The funnel plots and forest plots of both the random effects model and the fixed effects model were employed to evaluate the quality of the retrieved data (Supplemental Content 2). Among them, the funnel plots were used to identify and control the publication bias. The results of funnel plots (Supplemental Content 2: F1-F18) were symmetrical, indicating the publication bias did not exist. Because the baseline levels of the included smokers are divergent, random effects model (Supplemental Content 2: R20-R37) was primarily employed to conduct the meta-analysis followed by a confirmation using a fixed effects model (Supplemental Content 2: S39-S56) for sensitivity analysis. The sensitivity analysis (see Table, Supplemental Content 4, which illustrates the outcome of sensitivity analysis) showed that most meta-analysis results of the subgroups were stable.
4. Discussion
Although a series of publications have reported the efficiency of e-cigarettes as an aid for cutting down smoking or quitting smoking, unanimous consent has not been reached on the actual effect of e-cigarettes in real environment, and people are still cautious to their health risk. To the best of our knowledge, this systematic review is the first to address this issue by analyzing all of the published literature that describe both the adverse events and the efficiency of e-cigarettes as a smoking alternative. Although publication bias was observed according to the sensitivity analysis, the included 14 publications that involved 35,665 individuals who smoked both cigarettes and e-cigarettes were the only available sources that could be found by searching the entire publication database until now. As the pilot meta-analysis on the efficiency and reported adverse events of e-cigarettes, this investigation contributes pioneering information to the understanding of the efficiency and adverse events on the ever-increasing use of e-cigarettes.
Our results showed that the e-cigarettes were effective in cutting down smoking (pooled rate, 48.3–58.7%) under a criteria of ≥50% smoking reduction according to the references.[29] In particular, a short-term (≤12 months) use of e-cigarettes would benefit cutting down smoking than a long-term (>12 months) use (pooled rate 67.6% vs 37.1%) (P < .05). This finding indicated that longer-term exposure to e-cigarettes might hamper the efficiency of smoking reduction, which seems to be contrary to the opinion that a higher nicotine dose will facilitate cutting down smoking. This could be explained by the adaption to e-cigarettes after long-term use. In the initial phase of switching to e-cigarettes smoking from cigarettes smoking, higher nicotine dose was desirable because they could relieve anxious and uncomfortable feelings. However, the enjoyable experience would eventually make them addicted to nicotine-contained e-cigarettes after a long-term e-cigarettes practice.[31] This assumption was supported by a recent survey on 39,882 high school students that suggested e-cigarettes use might increase the risk of conventional smoking in adolescence.[32] In this case, a short-term replacement is desirable in the e-cigarettes assisted cutting down smoking.
Meanwhile, e-cigarettes have a pooled rate ranging from 13.2% to 22.9% on quitting smoking according to the definitions of smoking cessation in the original studies.[17] The definition of smoking cessation ranges from single point prevalence to sustained abstinence (multiple point prevalence with self-report of no slips or relapses).[25] One of these definitions is a minimum 80% reduction of daily tobacco consumption.[15] But some researchers suggested that a 30-day time frame for abstinence would be more appropriate,[33] or a 7-day prevalence abstinence could be used for validation of smoking cessation.[34] Considering each included publication has its own definition of smoking cessation, the definition from the original literature was employed in this meta-analysis to reduce the system errors.
The history of cigarettes smoking is a key factor affecting the efficiency of e-cigarettes in helping people quit. It was observed that shorter cigarettes using time (≤20 years) benefited smoking cessation than longer using time (>20 years) (pooled rate, 27.7% vs 12.1%), suggesting that shorter cigarettes using time would lead to less addiction.[35,36] However, we noted that users who smoked cigarettes ≤20 years were more difficult to cut down smoking than those who smoked cigarettes >20 years (pooled rate, 46.3% vs 65.6%), which seems contrary to the conclusion above. It is reasonable when considering the psychological status of those smokers. Individuals with a cigarette history over 20 years have much stronger desire to cut down their smoking than those with a shorter cigarette history because the formers are suffering more smoking-related diseases. Unfortunately, most of the attempts failed because the long histories of addiction make them extremely difficult to quit.
The results showed e-cigarettes containing more nicotine (≥16 mg/mL or ≥3 cartridges/day) would benefit cutting down smoking and quitting than those of less nicotine (<16 mg/mL or <3 cartridges/day), which has been supported by the previous publications showing that high levels of nicotine are needed to achieve smoking reduction or cessation and low-nicotine e-cigarettes were rarely used.[37]
This systematic review shows that the high occurrence rate of adverse events ranges from 49.1% to 51.6% and most of these events appear to wane spontaneously with time.[38] Mouth irritation, throat irritation, and cough that were frequently reported by participants might be caused by hyperventilation, which was associated with longer puffing time with e-cigarettes.[15] Alternatively, physical symptoms such as anxious and nervous, as well as other adverse events such as insomnia and headache, depressed mood, and sadness might be due to nicotine overuse. However, it should be noted that the reported adverse events might not be completely attributed to e-cigarettes use because the control group is generally missing in both observational studies and surveys. Although the potential health risks of long-term use of e-cigarettes are not fully addressed, the indoor air pollution from e-cigarettes has been reported in several investigations. It was reported that liquid particles of less than 2.5 micrometer diameter (PM2.5) were emitted when using e-cigarettes.[39,40] In addition, the potential initiation (gateway) efficiency of e-cigarettes has been reported in several internet-based surveys, showing that approximately 20% of the participants admitted that their initial cigarettes smoking is during the period of e-cigarettes use.[41,42] The occurrence rate of adverse events with e-cigarettes ≥3 cartridges/day (63.8%) was significantly higher than that of 1 to 2 cartridges/day (43.6%); it is most likely because higher e-cigarettes exposure dose would increase the risk of more toxic nicotine intake, which in turn will increase the risk of adverse events. As a result, the 6th session of the Conference of the Parties to the WHO Framework Convention on Tobacco Control (FCTC) in October 2014 acknowledged the need for e-cigarettes regulations, until sufficient data were provided on their efficiency and the safety.[43]
Notably, this study found that the variation of eCO level before and after e-cigarettes use was not remarkable (SMD 0.37 vs 0.23), suggesting eCO level is not a sensitive criterion to assess the efficiency of e-cigarettes. This finding seems to be controversial to the existing study that eCO level was universally biomarker in assessing exposure to cigarettes smoking.[15] Actually, it is understandable if we know the necessity of eCO as a toxic gas normally generated during cigarettes combustion. No eCO could be measured in the case of e-cigarettes because no combustion existed when e-cigarettes are smoked. Thus, the vast majority of the detected eCO may be owing to the natural physical breath procedure. It is reasonably regarded that eCO is not a reliable parameter in evaluating the efficiency of e-cigarettes, thus newer biomarkers are desired.
Relapse is frequently reported on participants who have stopped using e-cigarettes for some time. A recent RCT study revealed that 30.0% participants (197 of 656) relapsed on the 50th day after stopping using e-cigarettes, and 47.3% participants (311 of 656) relapsed on the 100th day. In addition, the relapsed number increased to 431 participants (65.6%) on the 150th day and 443 participants (67.4%) on the 200th day.[17] A prospective 12-month pilot study showed that participants began to relapse at the 8th week, and the relapse rate reached 71.3% after 52 weeks.[16] In addition, 1 online survey that recruited 1006 UK adult e-cigarettes users showed that a staggering 84% of e-cigarettes users continue to smoke both cigarettes and e-cigarettes.[44] According to another web-based survey on 222 first-time e-cigarettes buyers, 56.7% of ex-smokers continued to use e-cigarettes.[45] With these data in mind, it could be inferred that the relapse rate after stopping using e-cigarettes for 1 year is considerably high because most reported relapse rates were higher than 60%. Whereas, lower relapse rates are also reported in a longitudinal study showing that only 6% participants (15 of 250) relapsed, and that 8% participants (20 of 250) relapsed to occasional smoking after 1 month of stopping e-cigarettes, and 15 participants (6%) relapsed overall, and 13 participants (5%) relapsed to occasional smoking even 1 year later.[46] The lower relapse rate might be attributed to a small sample size. These results revealed that the high relapse rate of e-cigarettes after a long-term duration might be an important factor in evaluating the efficiency of e-cigarettes.
There are several limitations in this study. First, the control group is generally missing in the observational studies and surveys especially in the section of healthy risk so that adverse events could only be analyzed using single factor analysis, increasing the likelihood of bias. Second, the majority of the surveys are internet-based, so it cannot be excluded that more enthusiastic users are disproportionately participated; thus, the overall rate of satisfaction to the e-cigarettes may be overestimated. Third, the definition of the relapse is not consistent throughout the research because each included study had its own standard for relapse. Fourth, instability is found among the 6 subgroups according to the sensitivity analysis, which is probably because either the included publications are of fair quality, or the sample size is too small. In this case, the findings of this research maybe not generalizable to the general population, and should be viewed as suggestive, rather than definitive.
5. Conclusion
E-cigarettes are the promising smoking substitute for cutting down smoking and quitting smoking by potentially diminishing the attractiveness or temptation of nicotine cigarettes smoking. Considering the adverse events and potential air pollution of e-cigarettes smoking, areas where e-cigarettes were permitted should be rationally regulated until additional studies with more rigorous study designs are warranted. eCO level is unsuitable in evaluating the efficiency of e-cigarettes and more reliable biomarkers for assessing and reducing adverse events are desired.
Author contributions
Conceptualization: Weizhong Lu.
Data curation: Weizhong Lu, Wan Lu, Sheng Liao, Zhongrong Zhang.
Formal analysis: Weizhong Lu, Wan Lu, Zhongliang Deng.
Funding acquisition: Weizhong Lu, Wan Lu, Sheng Liao, Zhongrong Zhang.
Investigation: Xing Liu, Yun Liu.
Methodology: Zhongliang Deng, Zhongrong Zhang, Yun Liu.
Project administration: Xing Liu, Sheng Liao.
Resources: Xing Liu, Sheng Liao, Zhongrong Zhang, Yun Liu.
Software: Zhongliang Deng.
Supervision: Xing Liu, Zhongliang Deng.
Validation: Yun Liu.
Supplementary Material
Footnotes
Abbreviations: E-cigarettes = electronic cigarettes, eCO = CO2 exhalation, NRT = nicotine replacement therapy.
XL and WL were cofirst authors.
XL is the co-cultured postdoctor of Chongqing Medical University and Chongqing Traditional Chinese Medicine Hospital.
The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Öberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377:139–46. [DOI] [PubMed] [Google Scholar]
- [2].Pirie K, Peto R, Reeves GK, et al. Million Women Study Collaborators. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rostron B. Smoking-attributable mortality by cause in the United States: revising the CDC's data and estimates. Nicotine Tob Res 2012;15:238–46. [DOI] [PubMed] [Google Scholar]
- [4].Oxford University Press, Peto R, Lopez AD, Boreham J, et al. Mortality from smoking in developed countries 1950–2000. Indirect estimates from national statistics 1994. 1996. [Google Scholar]
- [5].Glassman TJ, Reindl DM, Whewell AT. Strategies for implementing a tobacco-free campus policy. J Am Coll Health 2011;59:764–8. [DOI] [PubMed] [Google Scholar]
- [6].Overland S, Hetland J, Aaro LE. Relative harm of snus and cigarettes: what do Norwegian adolescents say? Tob Control 2008;17:422–5. [DOI] [PubMed] [Google Scholar]
- [7].Sutherland G. Current approaches to the management of smoking cessation. Drugs 2012;62:53–61. [DOI] [PubMed] [Google Scholar]
- [8].Lancaster T, Silagy C, Fowler G. Training health professionals in smoking cessation. Cochrane Database Syst Rev 2000. CD000214. [DOI] [PubMed] [Google Scholar]
- [9].Bullen C, Howe C, Lin RB, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction 2010;105:1474–83. [DOI] [PubMed] [Google Scholar]
- [10].Caponnetto P, Campagna D, Papale G, et al. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med 2012;6:63–74. [DOI] [PubMed] [Google Scholar]
- [11].Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res 2013;15:1787–91. [DOI] [PubMed] [Google Scholar]
- [12].Odum LE, O’Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? J Pharm Pract 2012;25:611–4. [DOI] [PubMed] [Google Scholar]
- [13].Lindsay JC. Technical Review and Analysis of FDA Report: “Evaluation of E-cigarettes”. Houston, TX: Exponent Health Sciences; 2009. [Google Scholar]
- [14].Bialous SA, Sarma L. Electronic cigarettes and smoking cessation: a quandary? Lancet 2014;383:407–8. [DOI] [PubMed] [Google Scholar]
- [15].Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 2013;8:e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382:1629–37. [DOI] [PubMed] [Google Scholar]
- [18].Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med 2014;62:14–9. [DOI] [PubMed] [Google Scholar]
- [19].Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med 2013;44:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Pol 2011;32:16–31. [DOI] [PubMed] [Google Scholar]
- [21].Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med 2010;153:607–9. [DOI] [PubMed] [Google Scholar]
- [22].Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev 2013;32:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hughes JR, Solomon LJ, Naud S, et al. Natural history of attempts to stop smoking. Nicotine Tob Res 2014;16:1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction 2010;105:1176–89. [DOI] [PubMed] [Google Scholar]
- [25].Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Db Syst Rev 2008. CD001188. [DOI] [PubMed] [Google Scholar]
- [26].Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol 2013;66:982–93. [DOI] [PubMed] [Google Scholar]
- [27].Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726–32. [DOI] [PubMed] [Google Scholar]
- [28].Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med 2014;9:537–46. [DOI] [PubMed] [Google Scholar]
- [29].Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health 2013;10:446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Caponnetto P, Polosa R, Russo C, et al. Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. J Med Case Rep 2011;5:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McKie L, Laurier E, Taylor RJ, et al. Eliciting the smoker's agenda: implications for policy and practice. Soc Sci Med 2003;56:83–94. [DOI] [PubMed] [Google Scholar]
- [32].Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among US adolescents: a cross-sectional study. JAMA Pediatr 2014;168:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mermelstein R, Colby SM, Patten C, et al. Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine Tob Res 2002;4:395–403. [DOI] [PubMed] [Google Scholar]
- [34].Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediat Adol Med 2003;157:295–302. [DOI] [PubMed] [Google Scholar]
- [35].Hughes JR, Adams EH, Franzon MA, et al. A prospective study of off-label use of, abuse of, and dependence on nicotine inhaler. Tob Control 2005;14:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 2012;107:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Farsalinos KE, Romagna G, Tsiapras D, et al. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abus 2013;7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 2011;106:2017–28. [DOI] [PubMed] [Google Scholar]
- [39].McAuley TR, Hopke PK, Zhao J, et al. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol 2012;24:850–7. [DOI] [PubMed] [Google Scholar]
- [40].Cheng T. Chemical evaluation of electronic cigarettes. Tob Control 2014;23:ii11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Choi K, Forster J. Awareness, perceptions and use of snus among young adults from the upper Midwest region of the USA. Tob Control 2013;22:412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pearson JL, Richardson A, Niaura RS, et al. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health 2012;102:1758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yach D, Bettcher D. Globalisation of tobacco industry influence and new global responses. Tob Control 2000;9:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramo DE, Young-Wolff KC, Prochaska JJ. Prevalence and correlates of electronic-cigarette use in young adults: findings from three studies over five years. Addict Behav 2015;41:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med 2011;40:472–5. [DOI] [PubMed] [Google Scholar]
- [46].Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav 2014;39:491–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


