Abstract
Objective
We examined imaging surrogates of white matter microstructural abnormalities which may precede white matter lesions (WML) and represent a relevant marker of cerebrovascular injury in adults in midlife.
Methods
In 698 community-dwelling adults (mean age 50 years ±3.5 SD) from the Coronary Artery Risk Development in Young Adults (CARDIA) Brain MRI sub-study, WML were identified on structural MR and fractional anisotropy (FA), representing WM microstructural integrity, was derived using Diffusion Tensor Imaging. FA and WML maps were overlaid on a parcellated T1-template, based on an expert-delineated brain atlas, which included 42 WM tract ROIs. Analyses occurred in stages: 1) WML were quantified for the different tracts (i.e., frequency, volume, volume relative to tract size); 2) the interdependence of FA in normal appearing WM (NAWM) and WML was examined across tracts; 3) associations of NAWM FA and hypertension status were assessed controlling for WML volume. In the latter analysis, both overall hypertension (i.e. hypertension vs. normotension and prehypertension vs. normotension) and hypertension categorized by antihypertensive treatment status (yes/no) and blood pressure control (e.g., diastolic <90 mmHg, systolic <140 mmHg), were assessed.
Results
WML were widely distributed across different WM tracts, however, WML volume was small. Mean NAWM FA was lower in participants with vs. participants without WML in given tracts. Hypertension was significantly associated with lower mean NAWM FA globally across tracts, both before and after adjustment for WML volume. Moreover, the magnitude of this association differed by treatment status and the level of control of the hypertension.
Conclusions
In middle-aged adults, NAWM FA could represent a relevant marker of cerebrovascular injury when WML are minimally present.
Keywords: Cerebrovascular disease, Diffusion tensor imaging, Hypertension
Highlights
-
•
Relations of white matter lesions (WML), fractional anisotropy in normal white matter tissue (NAWM FA), and hypertension status were examined for multiple WM tracts.
-
•
WML were widespread but overall WML burden was low.
-
•
Lower NAWM FA was associated with hypertension after accounting for WML.
-
•
NAWM FA could represent an early surrogate marker for vascular-related WM changes.
1. Introduction
Changes in white matter microstructure, specifically reduced white matter integrity, may represent an important development in WM pathophysiology and its sequelae in adults in midlife. In contrast to white matter signal that is apparent on structural MR imaging (MRI) and represents white matter lesions (WML) or WM atrophy, reduced WM integrity cannot be observed using structural MR sequences, but instead is observed using alternative imaging techniques - e.g., diffusion tensor imaging (DTI). Lower WM integrity may, but not necessarily, precede the development of WML and WM atrophy which are more common manifestations of WM disease in later life (de Leeuw et al., 2001; Debette and Markus, 2010; Nordahl et al., 2006; Pugh and Lipsitz, 2002). Moreover, compared to WML, which markedly increase in late life, reduced WM integrity may be relatively more prevalent, and more strongly related to midlife vascular risk factors such as hypertension. Consequently, measures reflective of reduced WM integrity could represent a more sensitive marker of vascular-related brain change in adults in midlife when WML may be relatively sparse or absent.
Assessment of WM microstructure in the absence, or near absence of WML may be informative given that such measures have been associated with important brain outcomes. Studies have observed associations between lower fractional anisotropy (FA) - a measure of white matter integrity, reduced brain connectivity, and cognitive decline (Fjell et al., 2011; Greicius et al., 2009; Lockhart et al., 2012; Turken et al., 2008; Weiler et al., 2014). Decline in brain connectivity and cognition may in part reflect reduced integrity and disruption of signaling pathways in WM tracts, which connect spatially separated regions of the brain to form networks (Damoiseaux and Greicius, 2009; Greicius et al., 2009; Soriano-Raya et al., 2014; van den Heuvel et al., 2008; Weiler et al., 2014). Examination of WM in these tracts could aid our understanding of their potential structural contributions to brain-related changes in functional and behavioral characteristics.
Different reports point to WM microstructural abnormalities related to vascular risk factors in otherwise healthy individuals. For example, in a cohort of adults aged 19–63 years, age and systolic blood pressure were found to be associated with measures of reduced WM integrity based on DTI, but not WML, which may not have been sufficiently prevalent in the cohort (Maillard et al., 2012). Similarly, others have found associations between WM integrity and arterial blood pressure variation in otherwise healthy subjects without evidence of cerebrovascular disease (McEvoy et al., 2015; Salat et al., 2012) or atrophy (Davatzikos and Resnick, 2002; Fjell et al., 2008).
Other studies that include subjects with WML have found lower WM integrity in otherwise normal appearing WM tissue (NAWM). In a study of older adults (mean age 67), lower FA was observed in NAWM 3.5 years prior to development of lesions in those regions (de Groot et al., 2013). Additionally, reports suggest the occurrence of penumbra, or perilesional abnormalities, based on DTI, in the NAWM tissue surrounding lesions (Maillard et al., 2011; Maniega et al., 2015; Nasrallah et al. (unpublished results); Pelletier et al., 2015). Together, these studies suggest that WML may only represent the “tip of the iceberg” – a reference with regard to a wider distribution of pathophysiologic alterations in WM prior to the development of WML.
Few studies, however, have assessed the joint relationships of WM microstructural integrity, WML, and hypertension, nor have such studies examined these relationships in individual WM tracts. In the current study, we characterize FA and WML using multimodal MRI data from a large community-based cohort of middle-aged adults, and apply a region-of-interest (ROI) analysis to evaluate the relationships indicated above in a large representation of WM tracts. First, we examine the interdependence of NAWM FA and WML in different tracts. Second, we assess the association of FA in NAWM and hypertension. And lastly, we examine whether the potential association of the latter was independent of WML. Application of these steps addresses our hypothesis that FA in NAWM represents vascular-related processes that will be associated with hypertension independently of WML, which may be minimally present.
2. Methods
2.1. Study participants
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is a bi-racial sample of community-dwelling adults selected randomly within strata of race, sex, age and education (n = 5115). At the initial exam (1985–86), participants were between 18 and 30 years of age and were examined every 2–5 years thereafter (Friedman et al., 1988). At the Year 25 exam, a sub-sample was invited to participate in the CARDIA Brain MRI sub-study with a goal of recruiting approximately equal numbers of volunteers within each race-sex group. The sub-study recruited participants from 3 of the 4 CARDIA field sites. All participants were eligible for inclusion in the sub-study. Exclusion criteria included contraindication to MRI or a body size too large for the MRI scanner. MRI scans were acquired for a total of 719 subjects.
All participants provided written informed consent at each CARDIA exam, and institutional review boards (IRB) from each field center approved the study. A separate written consent was obtained for the CARDIA Brain sub-study. The IRB for Intramural Research at the National Institute on Aging (NIA) reviewed and approved the current study.
2.2. MRI acquisition and processing
Multi-modal MRI scans including structural and diffusion weighted imaging were obtained for patients using 3-Tesla MR scanners. Structural scans had voxel size = 1 × 1 × 1 mm3, and DTI scans had voxel size = 2.2 × 2.2 × 2.2 mm3. Details of the scanners, scan types and protocols, training of MRI technologists at the different sites, implementation of study protocols, and quality assurance of scanner stability and performance are provided elsewhere (Launer et al., 2015). All scans were reviewed by a radiologist for incidental but clinically relevant findings.
Post-scan image processing was performed by the Section of Biomedical Image Analysis (SBIA), Department of Radiology, University of Pennsylvania, as previously described (Launer et al., 2015). The protocol included an automated processing pipeline and quality control procedures applied at initial, intermediate, and final processing steps that included both visual inspection and automated identification of outliers based on the distributions of study variables.
Of the 719 participants with MRI scans, 709 passed quality control for initial structural MRI. DTI data were excluded for separate quality control issues (i.e. pipeline processing (n = 6), missing images (n = 1), image quality (n = 3)). One individual was excluded who had DTI measures that were consistently greater than the group average (>3 SD). After these exclusions, 698 subjects were available for further analysis.
2.3. MRI measures
Structural MRI scans were preprocessed by removal of extra-cranial material on the T1-weighted image (Doshi et al., 2013), followed by bias correction and tissue segmentation into gray matter, white matter, and cerebrospinal fluid, excluding the cerebellum (Zhang et al., 2001). A multimodal white matter lesion segmentation technique (WMLS) (Lao et al., 2008; Zacharaki et al., 2008) was applied using T1, FLAIR and T2 images to segment WML. WMLS is a supervised learning method that trains on lesions manually delineated by an expert radiologist. The lesion segmentation involves data preprocessing via histogram standardization and co-registration, feature extraction, training a voxelwise discriminative model, voxelwise label assignment and false-positive elimination.
DTI measures were computed using previously described techniques (Le Bihan et al., 2001). Specifically, fractional anisotropy (FA), i.e. the degree of anisotropy of the diffusion at a voxel, was calculated from diffusion tensor images at each brain voxel. FA maps were subsequently aligned to the T1 image of the subject using FLIRT (FMRIB's Linear Image Registration Tool, also part of FSL (Jenkinson et al., 2002)).
Automated segmentation of WM tract regions of interest (ROIs) was performed on T1 images, through deformable atlas registration using the deformable registration tool DRAMSS (Ou et al., 2011) and the JHU-MNI-ss atlas, which is often called the “Eve Atlas” (Oishi et al., 2009). The current analysis included values computed in 42 WM tract ROIs that were delineated on the atlas image. Mean FA was calculated separately within the normal and the abnormal (i.e., WML) regions of each target ROI (See Supplemental Table 1 for list of ROIs).
2.4. Hypertension status
Resting blood pressure was recorded using an Omron HEM907XL oscillometer (HRM USA Inc., Warminster PA USA) while participants were sitting. Medications were recorded by the interviewer, or were reported by the participant if not available. Normotension was defined based on systolic blood pressure (in mmHg) (SBP) <120 and diastolic blood pressure (in mmHg) (DBP) < 80, and not taking antihypertensives. Prehypertension was defined based on SBP 120–139, or DBP 80–89, and not taking antihypertensives. Hypertension was defined based on SBP ≥ 140, or DBP ≥ 90, or taking antihypertensives (Chobanian et al., 2003). Additionally, hypertensive subjects were classified as having: 1) hypertension based on SBP ≥ 140 or DBP ≥ 90, but not taking antihypertensives; 2) controlled hypertension, based on taking antihypertensives and SBP < 120 and DBP < 80; 3) controlled hypertension, based on taking antihypertensives and SBP 120–139 or DBP 80–89; and 4) uncontrolled hypertension, based on taking antihypertensives and SBP ≥ 140 or DBP ≥ 90.
2.5. Covariates
Covariates included age at the 25-year visit, sex, race (black/white), education (<16 years/≥16 years), and smoking status (0 = never, 1 = former, 2 = current). Dyslipidemia was defined based on the following: in men, if triglycerides ≥150 mg/dL or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL; in women, if triglycerides ≥150 mg/dL or HDL-C < 50 mg/dL; or if participants took medications for cholesterol (Miller et al., 2011). Diabetes was defined based on one or more of the following criteria: fasting glucose ≥126 mg/dL, where the duration of the fast was ≥480 min; 2-h glucose oral glucose tolerance test (OGTT) ≥ 200 mg/dL; glycosylated hemoglobin (HbA1c) ≥ 6.5%; or diabetes medication use (American Diabetes, 2011). For subjects who were missing either the OGTT or the HbA1c measure, diabetes status was defined as absent given the values of the remaining variables (e.g., fasting glucose <126 mg/dL). Of these, diabetes status was set to missing for two subjects for whom medication use was not known.
2.6. Statistical analysis
Initially, we examined the distribution of WML with respect to each of the 42 WM tract ROIs including: 1) lesion frequency (i.e. presence of lesions in different tracts regardless of size); 2) absolute lesion volume (cm3); and 3) % WML, a relative measure of lesion volume/total tract volume. Lesion frequency was plotted for the different tracts. For assessing lesion volume and % WML, values were ranked in participants from smallest to highest (including 0 values in the distributions), for each ROI, and percentile rankings were determined.
For the different WM tracts, FA was examined and was normally distributed. For each WM tract in the left and right hemispheres, we assessed differences in mean FA in NAWM in subjects with lesions in these tracts compared to subjects without lesions in these same tracts. In the subjects with lesions in given tracts, we examined mean FA in NAWM compared with FA in the lesions themselves. Next, we examined mean FA in NAWM by hypertension status - i.e., prehypertension vs. normotension and hypertension vs. normotension, first for all subjects, then for all subjects after removing tracts that included any lesions. For each group comparison above, differences in mean FA were computed for each tract and these differences were summed across all 42 tracts for each subject and used to assess an overall group difference in mean FA (i.e., two-tailed t-test, alpha = 0.05). Given standard errors (SE) varied for different tracts (e.g., fewer representative N for some tracts with lesions), inverse-variance weights were applied to estimate mean differences with greater precision.
Next, we examined the association of FA in NAWM and hypertensive status with multivariable models. We pooled available tract data for each subject (n = 42), and conducted a repeated measures regression which accounted for within-subject correlation. Models included terms for prehypertension (present/absent) and hypertension (present/absent). Normotension represented the reference category. Details of these models are provided in the Appendix A.
Lastly, we conducted a post-hoc analysis which was similar to the preceding analysis of association of FA in NAWM and hypertension status. In this analysis, hypertension (present/absent) was replaced with different categories of hypertension (i.e., treated and controlled to normotensive levels, treated and controlled to prehypertensive levels, treated and uncontrolled, untreated) in the models. Normotension represented the reference category. Subjects with prehypertension were excluded from the analysis.
Analyses were performed using SAS version 9.3 and R version 3.0.1.
3. Results
Of the 698 men and women in the study (mean age 50 ± 3.5), 48% were male, 40% were black, and 49% completed college (Table 1). Mean total brain volume was 1105 ± 122 cm3. Median WML volume (range) in the sample was 0.13 cm3 (0.00–21.24) for the whole brain. Prevalence of vascular risk factors was roughly as follows: prehypertension 26%; hypertension 29%; diabetes 9%; and dyslipidemia 37%. Among participants who were classified as hypertensive (n = 200): 45 (22.5%) were not taking antihypertensives; 70 (35%) were taking antihypertensives and had controlled hypertension at normotensive levels; 56 (28%) were taking antihypertensives and had controlled hypertension at prehypertensive levels; and 29 (14.5%) were taking antihypertensives and had uncontrolled hypertension.
Table 1.
Study characteristics.
| N | 698 |
| Age, years | 50 (3.5)a, b |
|
42–56 |
| Gender: Male | 47.6 |
| Race: Black | 39.7 |
| College education | 49.1 |
| Smoking status | |
|
23.6 |
|
15.6 |
| Hypertension status | |
|
26.0 |
|
28.9 |
| Systolic BP, mmHg | 118 (15) |
|
85–206 |
| Diastolic BP, mmHg | 74 (11) |
|
43–118 |
| Diabetes | 9.3 |
| Dyslipidemia | 36.6 |
| TBV, cm3 | 1105 (122) |
|
724–1510 |
| WM volume, cm3 | 492 (69) |
|
298–760 |
| Total WML volume, cm3 | 0.13 [0.06,0.32]c |
|
0.00–21.24 |
Abbreviations: BP, blood pressure; TBV, total brain volume excluding the cerebellum; WML, white matter lesions.
Mean(SD) or %.
0–9 Missing values for different covariates.
50th[25th,75th] percentiles.
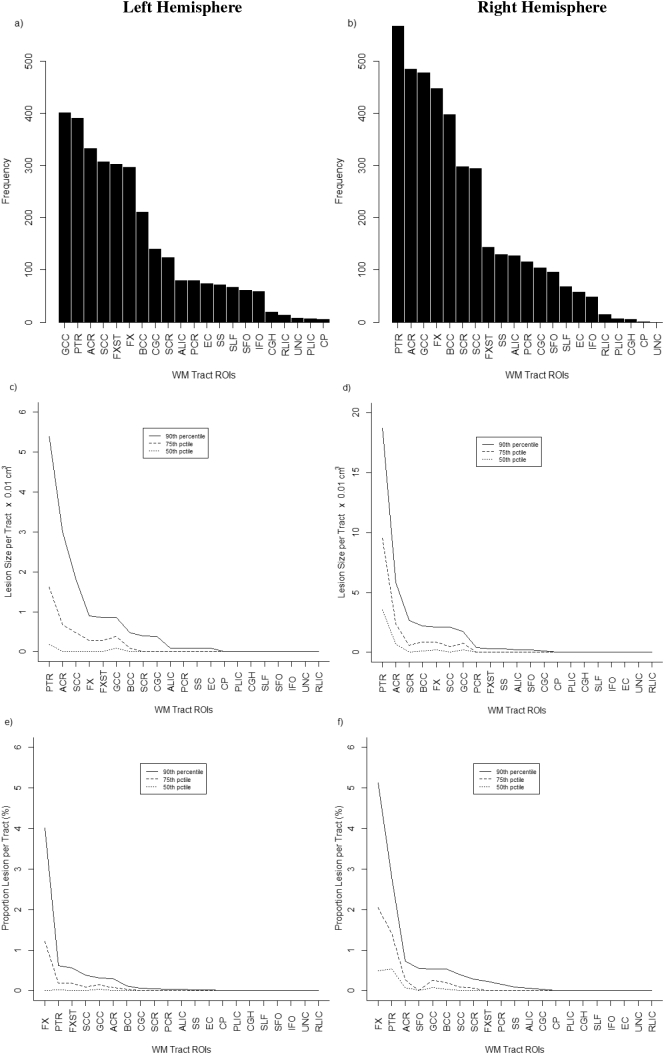
Characteristics of WML in different WM tracts are presented in Fig. 1. Lesion frequency varied from tract to tract and differed to some extent for left vs. right hemispheres (Fig. 1, top row). For some tracts, lesions were common and occurred in over half of participants. For example, lesions occurred most frequently in the genu of the corpus callosum (n = 401 subjects) on the left hemisphere and the posterior thalmic radiation (n = 567 subjects) on the right (See Supplemental Table 2 for detailed list of specific tracts affected). Lesion volume varied also across tracts (Fig. 1, middle row). For example, the largest lesions occurred in a few specific tracts (e.g., posterior thalmic radiation, anterior corona radiata) for both the left and right hemispheres in a relatively smaller number of participants (i.e., 90 percentile ranking). In most tracts, however, lesion volume was relatively small and approached zero for almost all subjects. Plots of % WML could be interpreted similarly (Fig. 1, bottom row): with exception of a few tracts (i.e., fornix (left and right hemisphere), posterior thalmic radiation (right hemisphere)), % WML was <1% in most subjects (i.e., 90 percentile ranking).
Fig. 1.
Distribution of WML for different WM tracts in left hemisphere (plots a, c, e) and right hemisphere (plots b, d, f) based on frequency, absolute lesion volume, and relative lesion volume (as % of total tract volume). A full description of WM tract ROIs is provided in Supplemental Table 1. Supplemental Table 2 includes numeric results of ordered ROIs that correspond with the plots where tracts with highest to lowest values of each metric (e.g. WML volume) are shown.
Different group comparisons of DTI-based measures are summarized for the 42 WM tract ROIs (Table 2) (detailed individual tract data with the different measures are presented in Supplemental Tables 3–5). For subjects without lesions compared to subjects with lesions in the same tracts (Table 2, row 1), analyses indicated a slightly higher mean FA in NAWM of subjects without lesions in given tracts (0.004, 95% CI: 0.002, 0.006). By contrast, in subjects with lesions in given tracts, mean FA was expectedly higher in the NAWM portion of the tracts than FA measured in the WML (0.101, 95% CI: 0.059, 0.143) (Table 2, row 2).
Table 2.
Unadjusted mean differences in FA summarized across white matter tract ROIs for different group comparisonsa.
| Comparisons | Group 1 Mean (SD)d |
Group 2 Mean (SD)d |
Mean difference Δ (95% CI)e |
P-valuef |
|---|---|---|---|---|
| NAWM (No lesion group “Group 1”) vs. NAWM (lesion group “Group 2”)b |
0.380 (0.038) | 0.375 (0.040) | 0.004 (0.002, 0.006) | <0.001 |
| NAWM (Lesion group “Group 1”) vs. WML (lesion group “Group 2”)b |
0.375 (0.040) | 0.292 (0.110) | 0.101 (0.059, 0.143) | <0.001 |
| NAWM (Normotensive “Group 1”) vs. NAWM (Prehypertensive “Group 2”) (all subjects)c |
0.382 (0.038) | 0.382 (0.037) | 0.000 (−0.001, 0.001) | 0.514 |
| NAWM (Normotensive “Group 1”) vs. NAWM (Hypertensive “Group 2”) (all subjects)c |
0.382 (0.038) | 0.376 (0.041) | 0.005 (0.004, 0.007) | <0.001 |
| NAWM (Normotensive “Group 1”) vs. NAWM (Prehypertensive “Group 2”) (subjects without lesions in tracts)c |
0.382 (0.039) | 0.383 (0.036) | −0.001 (−0.002, 0.001) | 0.059 |
| NAWM (Normotensive “Group 1”) vs. NAWM (Hypertensive “Group 2”) (subjects without lesions in tracts)c |
0.382 (0.039) | 0.378 (0.040) | 0.004 (0.003, 0.005) | <0.001 |
FA, fractional anisotropy; NAWM, normal appearing white matter; WML, white matter lesions.
“No lesion group” represents subjects without lesions in given tracts and “lesion group” represents subjects with lesions in these same tracts. Mean differences were assessed at level of each tract and summarized for the 42 tracts. “All subjects” represents all subjects in the study with all tracts included. “Subjects without lesions in tracts” represents all subjects in the study with tracts containing lesions excluded.
See Supplemental Tables 3–5 for mean differences for individual tract data.
Comparison based on 39 tracts (excluded 2 tracts which had no lesions and 1 tract where a lesion occurred for 1 subject only).
Comparison based on 42 tracts.
Group number reflects order in which group appear in “Comparisons” column. Mean and SD calculations were summarized for WM Tract ROIs by: , X2,…, XN)/N and SD() = Sqrt()2/N-1), where Xi represents mean FA for a given tract.
Mean difference (Δ) and estimation of 95% confidence interval takes into account differences in SE of different tracts (See Supplemental Tables 3–5). Weights were calculated from inverse SEs and applied to each tract. Confidence intervals were adjusted for multiple testing using False Discovery Rate.
P-values based on t-test of mean difference and adjusted for multiple testing using False Discovery Rate.
Mean differences in FA in NAWM were assessed by hypertension status as well. First, in all subjects, regardless of whether WML in tracts were present, mean FA was significantly lower overall in NAWM (−0.005, 95% CI: −0.007, −0.004) in hypertensives compared with normotensives (Table 2, row 4). Similarly, in a restricted sample where any tract containing a lesion was excluded from the analysis, mean FA was significantly lower in NAWM in those with hypertension vs. normotension (−0.004, 95% CI: −0.005, −0.003) (Table 2, row 6). Conversely, mean differences of FA based on comparisons between prehypertensives vs. normotensives were almost 0 (Table 2, rows 3,5).
Models that examined the association of mean FA, prehypertension, and hypertension had a similar pattern as the group comparisons above (Table 3). In an unrestricted analysis of all subjects, mean FA in NAWM was lower in hypertensives vs. normotensives in unadjusted (−0.006, 95% CI: −0.010,−0.003) and adjusted analyses (−0.005, 95% CI: −0.009, −0.001). Moreover, these results persisted after adjusting for % WML as an additional covariate. When the analysis excluded tracts with lesions, mean FA remained lower in hypertensives vs. normotensives in both unadjusted (−0.006, 95% CI: −0.010, −0.002) and adjusted analyses (−0.005, 95% CI: −0.009, 0.000). No associations with FA were observed for prehypertensives vs. normotensives.
Table 3.
Adjusted associations of FA in NAWM, prehypertension and hypertension summarized across white matter tract ROIs.
| All subject tractsa |
All subject tracts without lesionsb |
|||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P-value | Coefficient | 95% CI | P-value | |
| Model 1 | ||||||
| Interceptc | 0.382 | 0.379, 0.385 | <0.001 | 0.375 | 0.372, 0.378 | <0.001 |
| Prehypertensiond | 0.001 | −0.003, 0.004 | 0.720 | 0.002 | −0.002, 0.006 | 0.423 |
| Hypertensiond | −0.006 | −0.010, −0.003 | 0.001 | −0.006 | −0.010, −0.002 | 0.004 |
| Model 2 | ||||||
| Intercept | 0.379 | 0.374, 0.383 | <0.001 | 0.371 | 0.366, 0.376 | <0.001 |
| Prehypertension | 0.000 | −0.003, 0.004 | 0.823 | 0.001 | −0.004, 0.005 | 0.781 |
| Hypertension | −0.005 | −0.009, −0.001 | 0.016 | −0.005 | −0.009, 0.000 | 0.037 |
| Model 3 | ||||||
| Intercept | 0.379 | 0.375, 0.383 | 0.001 | NA | NA | NA |
| Prehypertension | 0.001 | −0.003, 0.004 | 0.745 | NA | NA | NA |
| Hypertension | −0.005 | −0.009, −0.001 | 0.023 | NA | NA | NA |
| % WMLe | −0.003 | −0.004, −0.002 | 0.001 | NA | NA | NA |
Model coefficients and 95% CI based on repeated measures regression (See Methods for details).
Model 1: Adjustment for study center only.
Model 2: Adjustment for age, sex, race, education, smoking status, diabetes, dyslipidemia, and study center.
Model 3: Model 2 + adjustment for %WML (i.e. relative % lesion in each tract).
Results are summarized over white matter tracts in 698 subjects each with 42 white matter tracts (n = 29,316).
Results are summarized over white matter tracts in 698 subjects with tracts that exclude lesions (n = 22,384).
Intercept represents the mean FA associated with reference values of the variables in the different models.
Coefficients represents the difference in mean FA associated with prehypertension and hypertension, respectively, compared to normotension.
Coefficient represents the difference in mean FA associated with a + 1% difference in %WML.
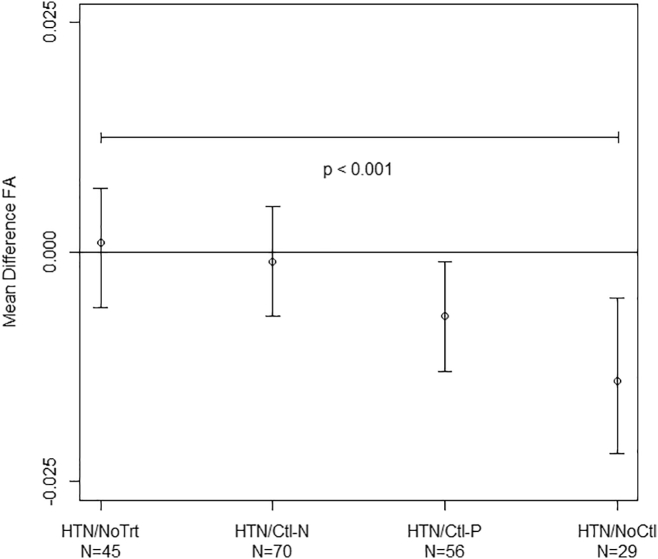
Our post-hoc examination of FA in NAWM and categories of hypertension indicated group differences in both unadjusted and adjusted analyses (Fig. 2) (test for trend p < 0.01). Among participants who were hypertensive but not taking antihypertensive medication, as well as those whose hypertension was controlled at normotensive levels through antihypertensives, there was essentially no difference in mean FA compared with participants who were normotensive. By comparison, mean FA was lower in subjects on antihypertensive medication whose hypertension was controlled at prehypertensive levels, as well as lower in subjects on antihypertensives but whose blood pressure remained high (i.e., uncontrolled).
Fig. 2.
Adjusted mean differences in NAWM FA (95% CI) for different hypertensive groups (No Trt = not treated; Ctl-N = treated and controlled at normotensive levels; Ctl-P = treated and controlled at prehypertensive levels; NoCtl = treated and uncontrolled) relative to normotensive subjects across WM tracts. A test for linear trend was found to be significant between the different groups. A similar relationship was observed between the different groups after restricting the analysis to WM tracts without lesions.
4. Discussion
As reported previously (Launer et al., 2015, Nasrallah et al. (unpublished results)), we observed relatively low WML burden in this cohort of middle-aged adults. Although WML were widely present across different WM tracts, overall WML in the brain and the percentage of each tract occupied by WML (i.e. %WML) in the study group was small, for example, compared to WML burden observed in older adults (de Leeuw et al., 2001; Söderlund et al., 2003). We observed lower FA in NAWM overall for participants that had lesions in different tracts compared to participants that did not have lesions in those same tracts. Mean FA in NAWM was associated with hypertension both in the whole sample as well as the sample that excluded tracts with lesions. This association differed depending on the clinical characteristics (i.e., subgroups) of the hypertensive participants. Given low WML burden, DTI-based measures, like FA, may represent a sensitive marker of vascular-related WM abnormalities that could have implications for assessment of brain health in midlife.
The observed findings with FA in NAWM could represent a pathophysiologic process that is a direct result of hypertension on the NAWM as well as changes in NAWM induced by WML. Lower FA in NAWM might be expected in particular where WML occur nearby (e.g., penumbra) (Maillard et al., 2011; Nasrallah et al. (unpublished results); Pelletier et al., 2015). NAWM FA was slightly lower in subjects with lesions in their tracts compared with subjects without lesions in their tracts, and our results showed an association between higher % WML and lower mean FA in NAWM. We found that hypertension was still associated with lower FA after accounting for %WML, which would suggest that NAWM FA is not solely a marker of WML. It is possible that lower FA in NAWM in this group could be part of the same pathway that leads to WML as previous studies suggest (de Groot et al., 2013). Follow-up data will be necessary to clarify the potential bi-directional relationships between WML and DTI-based measures in NAWM.
We observed large variation in the presence of lesions spatially across different WM tracts. For example, over half of participants had lesions in representative tracts (i.e., genu of the corpus callosum, posterior thalmic radiation, and the anterior corona radiata) in the left hemisphere, with an even greater proportion of participants with lesions in these same tracts in the right hemisphere. Conversely, less than ten participants had lesions that occurred in three representative tracts (i.e., uncinate fasciculus, cerebral peduncle, and the posterior limb of the internal capsule) in both left and right hemispheres. By comparison, our analysis did not indicate differences in the association of NAWM FA and prehypertension/hypertension between the different tracts. It is possible that this latter result is a reflection of the low WML volume that was found for most tracts compared to wide-varying differences observed between tracts in lesion frequency. Hypertension-related regional alterations in WM microstructure have been identified for some studies, but results are inconsistent as other studies suggest these effects are widespread (Kennedy and Raz, 2009; Salat et al., 2012; McEvoy et al., 2015).
Previous studies have reported interdependencies of WM microstructure in NAWM and WML for the whole brain (Maniega et al., 2015; Nasrallah et al. (unpublished results); Pelletier et al., 2015). The current work examined these relationships for individual WM tracts. The observed associations between lower FA in NAWM and hypertension globally across different WM tracts could have implications for understanding brain function at multiple levels (e.g., network functionality, cognition), which is dependent on the integrity of WM tracts (Damoiseaux and Greicius, 2009; Fjell et al., 2011; Greicius et al., 2009; Weiler et al., 2014). Moreover, the characterization of WM tracts in the current study (e.g., affected tracts, lesion volume, DTI measures) could be informative with respect to future work that examines early structural markers in relationship to brain changes and pathophysiologic pathways in brain disease.
While our results showed significant differences with hypertension, little or no differences were observed for prehypertension. Salat et al. investigated associations of normotension to moderate-severe levels of hypertension, with respect to FA, in a healthy but relatively older sample aged 43–87 years (Salat et al., 2012). Although the findings from the study suggested significant associations between FA and inter-individual variation in blood pressure levels that extended into normotensive and medically controlled hypertensive individuals, the results may have been related to the underlying distribution of age of the sample. McEvoy et al. reported significant associations between high blood pressure and lower FA in a middle-aged cohort of men, even among individuals whose blood pressure was medically controlled; however, associations were among subjects who experienced a longer duration of hypertension >5.6 years (McEvoy et al., 2015). It is possible that the results observed for prehypertension in the current study reflect the relatively young age of the cohort who, consequently, may not have experienced prehypertension for the duration necessary to observe differences in their brain measures.
While FA in NAWM was associated with hypertension overall, this result varied depending on the different clinical characteristics of hypertension observed in the sample. Participants taking antihypertensives, particularly those whose blood pressure remained high (i.e., uncontrolled hypertension), were observed to have the lowest mean FA when compared with normotensives. It is possible that these individuals may not be adhering to their treatment, may not be receiving adequate treatment, or may be phenotypically disposed to high blood pressure. Interestingly, participants who met the clinical threshold for hypertension, but who were not on antihypertensives, had mean FA comparable to normotensives. These individuals may have experienced hypertension for a shorter duration than their counterparts and have not yet received treatment. Participants whose hypertension was controlled at normotensive levels with antihypertensive medication were observed to have FA levels more favorable (i.e., not significantly different from normotensive subjects) than their counterparts on antihypertensives whose hypertension was controlled at prehypertensive levels (i.e., mean FA was significantly lower than mean FA in normotensives).
Previously, Liu et al. examined the relationships of the same hypertensive subgroups described above with respect to cardiovascular outcomes (e.g., coronary heart disease) based on the larger cohort of CARDIA participants, which included subjects who did not participate in the CARDIA Brain sub-study. In that study, individuals whose blood pressure was well-controlled (i.e., SBP <120 and DBP < 80) were observed to have better outcomes than those whose blood pressure was controlled at prehypertensive levels. Also, participants taking antihypertensives whose hypertension remained uncontrolled had worse health outcomes compared to any of the other groups (Liu et al., 2015). The results in the current study underscore the likely heterogeneity in samples of individuals with clinically manifest hypertension and the implications of this heterogeneity for early brain markers like FA.
The current study has some limitations to consider when interpreting the findings. We examined WML and FA in NAWM in the same tracts and made an assumption that these were independent of WML in other tracts. However, WML may have effects on DTI measures in NAWM in adjoining tracts that are potentially as large as those WML in the same tract. Therefore, the result that suggested hypertension was independently associated with lower FA after accounting for %WML may have been explained in part by WML in a nearby tract. Additionally, our results persisted after we excluded tracts with lesions as part of our analysis, however, it possible that we may not have removed the detectable influence of WML of an adjoining tract even after we excluded the adjoining tract containing the WML from the analysis.
Our study measured FA in NAWM of any given tract as one measure. However, previous studies have shown that DTI measures in NAWM differ depending on their proximity to WML (i.e., penumbra) (Maillard et al., 2011; Nasrallah et al. (unpublished results)). Differences in FA closer to lesions compared to FA measured in NAWM of tract as a whole could have important implications for future work that compares structural integrity of WM with functional measures; therefore, future studies would need to account for both FA and lesions, if present. Similarly, categories of blood pressure control or no control are based on blood pressure readings taken on one occasion, which should be considered when one interprets differences in NAWM FA with respect to these different categories.
It is possible that FLAIR and DTI maps may have been misaligned for some ROIs. Contrary to expectations, mean FA was higher in WML compared to NAWM for the genu of the corpus callosum in all subjects. A closer examination of the data showed that these results were confined to the WML-NAWM comparison in this region only, where the contribution of voxel space of the WML was small and therefore vulnerable to misalignment. We would not expect the study findings to be affected given that the analyses involved comparisons of larger voxels spaces (i.e., NAWM of tracts) and, therefore, would be less vulnerable to potential misalignment of imaging maps.
Non-vascular factors, for example, inflammation, atrophy and genetic factors, may have contributed to the findings. Also, other processes may have affected the findings other than those investigated (e.g., lower FA in NAWM due to Wallerian degeneration); however, given the age and relative healthiness of the study group, the influence of these factors on the results are likely to be minimal.
The study has a number of important strengths. This is one of the first studies to characterize the distribution of WML and relate these to DTI-based measures at the level of WM tracts in a community-based sample of middle-aged adults. Detailed tract parcellation, based on the EVE atlas, allowed for assessment of WML location and their magnitude, and thus could be a useful tool for understanding the early advancement of vascular-related brain injury starting in midlife. Moreover, multi-modal imaging allowed for evaluation of interdependencies as well as potential independent processes between WML and markers of microstructural injury based on DTI. Analytically, analyses were stratified by tract – i.e., mean comparisons of measures were within tracts, which allowed for more direct comparisons than comparisons across the brain as a whole. DTI measures between different tracts may vary (e.g., fiber density, myelination differences) which could have led to potential confounding in an analysis based on the whole brain (de Groot et al., 2013). Our analytic strategy allowed for comparisons to be made within each tract with respect to the effect of interest (e.g., hypertension status), which was later combined and summarized for the different tracts.
5. Conclusions
In sum, in a population-based study of middle-aged adults with low WML burden, we observed hypertension-related differences in FA in NAWM. Given NAWM constitutes the bulk of WM in these individuals, FA could represent an early surrogate marker for vascular-related WM changes, which, in turn, could have important implications for brain health.
Acknowledgements
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.032.
Appendix A. Details of association models of NAWM FA and hypertension status
Initially, we examined the associations of interest without respect to WM tract using the following model:
| (1) |
where β0 represents the mean FA in normotensive subjects; β1 represents the mean difference in FA in prehypertensive compared with normotensive subjects; and β2 represents the mean difference in FA in hypertensive compared with normotensive subjects. The model was adjusted for covariates including: age, sex, race, study center, education, smoking status, diabetes, and dyslipidemia. % WML, measured for each tract, was subsequently added as an additional covariate to examine its relationship with FA in NAWM. Although the distribution % WML was skewed, it was left untransformed given that it was a covariate, not a dependent variable, in the analysis.
We examined whether the associations of interest between FA in NAWM and hypertensive status varied by tract, in order to test the assumption of a global effect of hypertension. We selected a reference tract (i.e., uncinate fasciulus in right hemisphere) and applied the following model:
| (2) |
Coefficients based on this model represent the following: β0 is equal to the mean FA in the reference tract in subjects who are normotensive; β1 represents the mean difference in FA in the reference tract for subjects with prehypertension compared to normotensive subjects; β2 represents the mean difference in FA in the reference tract for subjects with hypertension compared to normotensive subjects; β3j represents the mean differences in FA for tract j and the reference tract in normotensive subjects; β4j represents mean differences in FA for tract j and the reference tract, in subjects with prehypertension compared to normotensive subjects; and β5j represents mean differences in mean FA for each tract j and the reference tract, in subjects with hypertension compared with normotensive subjects. False discovery rate (FDR) was applied to account for multiple testing. Given that no significant tests were associated with coefficients β4j and β5j in model (2), based on FDR, results are reported only for model (1).
Appendix B. Supplementary data
Supplementary tables
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl. 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr., Jones D.W., Materson B.J., Oparil S., Wright J.T., Jr., Roccella E.J., Joint National Committee on Prevention, D.E., Treatment of High Blood Pressure. National Heart, L., Blood, I, National High Blood Pressure Education Program Coordinating, C Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Greicius M.D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Davatzikos C., Resnick S.M. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cereb. Cortex. 2002;12:767–771. doi: 10.1093/cercor/12.7.767. [DOI] [PubMed] [Google Scholar]
- de Groot M., Verhaaren B.F., de Boer R., Klein S., Hofman A., van der Lugt A., Ikram M.A., Niessen W.J., Vernooij M.W. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- de Leeuw F.-E., de Groot J.C., Achten M., Oudkerk M., Ramos L., Heijboer R., Hofman A., Jolles J., van Gijn J., Breteler M. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi J., Erus G., Ou Y., Gaonkar B., Davatzikos C. Multi-atlas skull-stripping. Acad. Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Greve D.N., Fischl B., Benner T., van der Kouwe A.J., Salat D., Bjornerud A., Due-Tonnessen P., Walhovd K.B. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. NeuroImage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Amlien I.K., Walhovd K.B. Reduced white matter integrity is related to cognitive instability. J. Neurosci. 2011;31:18060–18072. doi: 10.1523/JNEUROSCI.4735-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman G.D., Cutter G.R., Donahue R.P., Hughes G.H., Hulley S.B., Jacobs D.R., Jr., Liu K., Savage P.J. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kennedy K., Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Z., Shen D., Liu D., Jawad A.F., Melhem E.R., Launer L.J., Bryan R.N., Davatzikos C. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad. Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer L.J., Lewis C.E., Schreiner P.J., Sidney S., Battapady H., Jacobs D.R., Lim K.O., D'Esposito M., Zhang Q., Reis J., Davatzikos C., Bryan R.N. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N., Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Liu K., Colangelo L.A., Daviglus M.L., Goff D.C., Pletcher M., Schreiner P.J., Sibley C.T., Burke G.L., Post W.S., Michos E.D., Lloyd-Jones D.M. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA) J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S.N., Mayda A.B., Roach A.E., Fletcher E., Carmichael O., Maillard P., Schwarz C.G., Yonelinas A.P., Ranganath C., Decarli C. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front. Hum. Neurosci. 2012;6:56. doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Fletcher E., Harvey D., Carmichael O., Reed B., Mungas D., DeCarli C. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Seshadri S., Beiser A., Himali J.J., Au R., Fletcher E., Carmichael O., Wolf P.A., DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniega S.M., Valdes Hernandez M.C., Clayden J.D., Royle N.A., Murray C., Morris Z., Aribisala B.S., Gow A.J., Starr J.M., Bastin M.E., Deary I.J., Wardlaw J.M. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol. Aging. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy L.K., Fennema-Notestine C., Eyler L.T., Franz C.E., Hagler D.J., Jr., Lyons M.J., Panizzon M.S., Rinker D.A., Dale A.M., Kremen W.S. Hypertension-related alterations in white matter microstructure detectable in middle age. Hypertension. 2015;66:317–323. doi: 10.1161/HYPERTENSIONAHA.115.05336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N., Goldberg A.C., Howard W.J., Jacobson M.S., Kris-Etherton P.M., Lennie T.A., Levi M., Mazzone T., Pennathur S., American Heart Association Clinical Lipidology, T., Prevention Committee of the Council on Nutrition, P.A., Metabolism, Council on Arteriosclerosis, T., Vascular, B., Council on Cardiovascular, N., Council on the Kidney in Cardiovascular, D Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Nordahl C.W., Ranganath C., Yonelinas A.P., Decarli C., Fletcher E., Jagust W.J. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J. Cogn. Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Jiang H., Li X., Akhter K., Zhang J., Hsu J.T., Miller M.I., van Zijl P.C.M., Albert M., Lyketsos C.G., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Mazziotta J., Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46:486. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y., Sotiras A., Paragios N., Davatzikos C. DRAMMS: deformable registration via attribute matching and mutual-saliency weighting. Med. Image Anal. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier A., Periot O., Dilharreguy B., Hiba B., Bordessoules M., Chanraud S., Peres K., Amieva H., Dartigues J.F., Allard M., Catheline G. Age-related modifications of diffusion tensor imaging parameters and white matter hyperintensities as inter-dependent processes. Front. Aging Neurosci. 2015;7:255. doi: 10.3389/fnagi.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.G., Lipsitz L.A. The microvascular frontal-subcortical syndrome of aging. Neurobiol. Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Salat D.H., Williams V.J., Leritz E.C., Schnyer D.M., Rudolph J.L., Lipsitz L.A., McGlinchey R.E., Milberg W.P. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. NeuroImage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Nyberg L., Adolfsson R., Nilsson L.G., Launer L. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39:1093–1105. doi: 10.1016/s0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Soriano-Raya J.J., Miralbell J., Lopez-Cancio E., Bargallo N., Arenillas J.F., Barrios M., Caceres C., Toran P., Alzamora M., Davalos A., Mataro M. Tract-specific fractional anisotropy predicts cognitive outcome in a community sample of middle-aged participants with white matter lesions. J. Cereb. Blood Flow Metab. 2014;34:861–869. doi: 10.1038/jcbfm.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A., Whitfield-Gabrieli S., Bammer R., Baldo J.V., Dronkers N.F., Gabrieli J.D. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M., Mandl R., Luigies J., Pol H.H. Microstructural organization of the cingulum tract and the level of default-mode connectivity. J. Neurosci. 2008;28:10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler M., de Campos B.M., Nogueira M.H., Pereira Damasceno B., Cendes F., Balthazar M.L. Structural connectivity of the default mode network and cognition in Alzheimers disease. Psychiatry Res. 2014;223:15–22. doi: 10.1016/j.pscychresns.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Zacharaki E.I., Kanterakis S., Bryan R.N., Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med. Image Comput. Comput. Assist. Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables