Abstract
BACKGROUND
Infants and young children are particularly susceptible to viral encephalitis, but the mechanisms are unknown. We determined the age-dependent contribution of innate and adaptive immune functions to reovirus-induced encephalitis in mice.
METHODS
Newborn wild-type mice, 2 to 20 days of age, were inoculated with reovirus or diluent and monitored for mortality, weight gain, and viral load. Four and 15-day-old IFNAR−/− and RAG2−/− mice were inoculated with reovirus and similarly monitored.
RESULTS
Weight gain was impaired in mice inoculated with reovirus at 8 days of age or less. Clinical signs of encephalitis were detected in mice inoculated at 10 days of age or less. Mortality decreased when mice were inoculated after 6 days of age. Survival was ≤ 15% in WT, RAG2−/−, and IFNAR−/− mice inoculated at 4 days of age. All WT mice, 92% of RAG2−/− mice, and only 48% of IFNAR−/− mice survived following inoculation at 15 days of age.
CONCLUSIONS
Susceptibility of mice to reovirus-induced disease decreases between 6 and 8 days of age. Enhanced reovirus virulence in IFNAR−/− mice relative to WT and RAG2−/− mice inoculated at 15 days of age suggests that maturation of the innate immune system contributes to age-related mortality following reovirus infection.
INTRODUCTION
Viral encephalitis is a major cause of morbidity and mortality worldwide. Neurotropic viruses continue to emerge and re-merge due to changes in viral virulence, expanded distribution of viral vectors, fluctuations in population immunity, and increased travel associated with globalization (1). The young are particularly susceptible to poor outcomes of viral encephalitis such as developmental delays, learning disabilities, epilepsy, and death. Neurotropic viruses replicate more efficiently and display enhanced apoptosis capacity in immature versus mature neurons through mechanisms that are incompletely understood (2).
Reovirus serves as a highly tractable experimental system in which to study neurotropic virus-host interactions. Following peroral inoculation of newborn mice, reovirus infects the intestine and disseminates systemically to the mesenteric lymph nodes, liver, spleen, lungs, heart, and brain. Serotype 1 reovirus strains disseminate exclusively via hematogenous routes, whereas serotype 3 strains disseminate via hematogenous and neural routes (3, 4). Upon entry into the brain, serotype 3 strains infect neurons located in the frontal and parietal cortex, CA1 to CA4 regions of the hippocampus, the cingulate gyrus, the thalamus, and Purkinje neurons in the cerebellum (5, 6). Reovirus infection of neurons causes apoptosis and triggers inflammation, resulting in a lethal meningoencephalitis (3, 7, 8, 9).
The susceptibility of mice to reovirus infection and death is age-dependent. Infection of young mice with serotype 3 reovirus leads to a lethal encephalitis and death, whereas signs of clinical disease are absent following infection of older mice (10, 11). One study suggests that the age-susceptibility of mice to reovirus neurovirulence is due to the inhibition of viral replication by neuronal cell intrinsic factors (12). However, the precise mechanism is unknown.
The type I interferon (IFN) pathway is a major component of the innate immune system. Following production and secretion, IFNs act in an autocrine and paracrine fashion to induce an antiviral state in infected and neighboring cells, promote a balanced natural killer cell response with controlled anti-inflammatory activities, and enhance antigen presentation to activate the adaptive immune system (13). Mice deficient in signal transducer and activator of transcription-1 (STAT-1), a key component of IFN signaling, display increased mortality and higher viral loads in the brain following intracranial inoculation with reovirus at 2 days of age, suggesting that IFN signaling reduces reovirus replication and virulence (14).
Strategies to prevent and treat viral encephalitis are limited in part due to a lack of knowledge of the cellular factors and molecular mechanisms that contribute to viral virulence. We hypothesized that the susceptibility to reovirus disease is an effect of age-dependent control of the virus by maturing components of the immune system. To test this hypothesis, we defined the age at which mice lose susceptibility to reovirus-induced disease, determined the relationship between viral replication in vital organs and disease severity, and delineated age-dependent contributions from innate and adaptive immune functions. Since age restriction is a shared determinant of disease severity in many neurotropic virus infections, it is possible that mechanisms underlying reovirus age restriction will be applicable to other viral infections.
METHODS
Cell Lines and Viruses
Murine L929 cells were maintained in Eagle’s minimal essential medium (SMEM); (Lonza; Walkersville, MD) with 5% fetal bovine serum (Gibco; Gaitersburg, MD), 2 mM L-glutamine (Invitrogen; Carlsbad, CA), 100 U/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), and 25 ng/ml amphotericin B (Sigma-Aldrich; St. Louis, MO). Reovirus strain type 3 Dearing (T3D) is a laboratory stock recovered by plasmid-based reverse genetics (15). Reovirus was amplified in L929 cells and purified by Vertrel-XF (Dupont; Wilmington, DE) extraction, followed by CsCl-gradient centrifugation (16). Viral plaque-forming unit (PFU) titers were determined by plaque assay using L929 cells (17).
Mouse Strains
C57BL/6J (WT) mice were obtained from The Jackson Laboratory. C57BL/6 IFNAR−/− mice were provided by John Williams (Vanderbilt University School of Medicine; Nashville, TN), and C57BL/6 RAG2−/− mice were provided by Danyvid Olivares-Villagomez (Vanderbilt University School of Medicine; Nashville, TN).
Infection of Mice
Mice were weighed and inoculated intracranially (18) with purified reovirus T3D in PBS at 100 PFU/g. For analysis of virulence, mice were monitored for weight gain and symptoms of disease for 20 days post-inoculation (d p.i.) and euthanized when moribund. For analysis of viral replication, organs were harvested into 1 ml of PBS, and homogenized using a TissueLyser (Qiagen; Hilden, Germany). Viral titers were determined by plaque assay. Animal husbandry and experimental procedures were performed in accordance with Public Health Service policy and approved by the Vanderbilt University School of Medicine Institutional Animal Care and Use Committee.
Histology
Mice were inoculated intracranially with reovirus T3D at 100 PFU/g. Brains were resected and divided sagittally. Left brain hemispheres were processed for plaque assay. Right brain hemispheres were fixed in 10% formalin and embedded in paraffin. Consecutive 6 μm sections were stained with hematoxylin and eosin (H&E) or processed for immunohistochemical detection of reovirus antigen or the cleaved (active) form of caspase-3 (19).
Immunoblotting
Brain homogenates were diluted 2-fold in RIPA lysis buffer (Sigma-Aldrich) containing Complete Protease Inhibitor Cocktail (Roche; Basel, Switzerland). Protein extract (50 μg) was resolved by electrophoresis in 10% Tris-glycine gels (Bio-Rad; Hercules, CA) and transferred to Immun-Blot PVDF membranes (Bio-Rad). Membranes were blocked for at least 1 h in Odyssey blocking buffer (LI-COR; Lincoln, NE) and incubated with an anti-actin antibody (1:500) (Santa Cruz Biotechnologies; Dallas, TX) and a cleaved (active) caspase-3 antibody (1:1000) (Cell Signaling Technologies; Danvers, MA) in Odyssey blocking buffer overnight. Membranes were washed and incubated with secondary antibodies IRDye 680CW-conjugated donkey anti-goat (1:2,000) and IRDye 800CW-conjugated goat anti-rabbit (1:5,000) in Odyssey blocking buffer for 2 h. Membranes were washed three times and scanned using an Odyssey infrared imaging system (LI-COR). Signal intensities of specific bands were quantified using ImageStudio software (LI-COR).
RESULTS
Survival of mice from reovirus infection is age-dependent
Following infection with a neurotropic serotype 3 strain of reovirus, neonatal mice will contract encephalitis, which is often fatal, whereas adult mice do not develop overt signs of disease (10, 11). To determine the age window during which WT mice become refractory to reovirus-induced mortality, mice were inoculated intracranially with 100 PFU/g T3D in PBS or PBS alone (mock) at 2, 4, 6, 8, 10, 15, and 20 d of age and monitored for survival for 20 d. Only 37% of 2 day-old (n = 16), 15% of 4 day-old (n = 20), and 48% of 6 day-old (n = 27) mice survived (Figure 1a). In contrast, 90% of mice inoculated with reovirus at 8 d of age (n = 18) and all mice inoculated at 10, 15, and 20 d of age (n ≥ 15) survived. Mice that became ill exhibited clinical signs of encephalitis including lethargy, seizures, ataxia, and paralysis. All PBS-inoculated mice survived. These data indicate that reovirus-induced mortality is reduced in mice inoculated at or after 8 d of age.
Figure 1. Survival and weight gain in mice following reovirus inoculation at various ages.
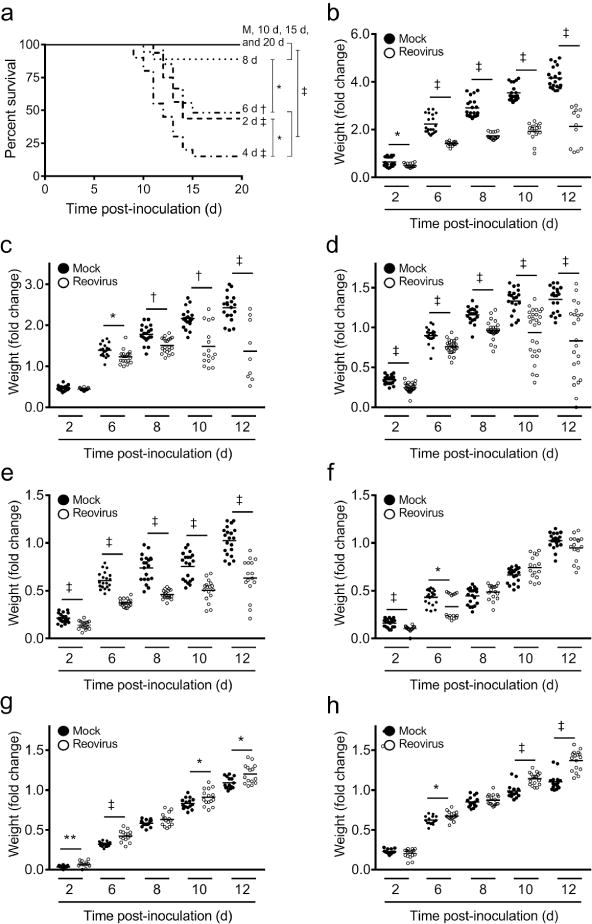
WT mice were inoculated intracranially with reovirus T3D at 100 PFU/g at 2, 4, 6, 8, 10, 15, or 20 d of age or with PBS alone (mock; M) at 2 d of age. (a) Mice (n ≥ 15 per experimental group) were monitored for morbidity for 20 d and euthanized when moribund. Statistical analyses compared each curve to mock, to the next age of inoculation (e.g., 2 d versus 4 d; 4 d versus 6 d; etc.), and between susceptible and non-susceptible age groups (e.g., 2–6 d versus 8–20 d). *, P < 0.05; †, P < 0.005; ‡, P < 0.0001 as determined by Log-rank test. WT mice were inoculated intracranially with reovirus T3D at 100 PFU/g at (b) 2, (c) 4, (d) 6, (e) 8, (f) 10, (g) 15, or (h) 20 d of age and monitored for weight gain for 20 d. Data are represented as fold-change normalized to weight on the day of inoculation compared with age-matched, mock controls at 2, 6, 8, 10, and 12 d p.i. *, P < 0.05; **, P < 0.01; †, P < 0.005; ‡, P < 0.0001 as determined by Mann-Whitney U test.
Reovirus-induced disease is age-dependent
We monitored weight gain of reovirus inoculated mice as a quantitative surrogate measure of reovirus-induced disease. Mice were inoculated intracranially with 100 PFU/g T3D in PBS at 2, 4, 6, 8, 10, 15, or 20 d of age or PBS alone at 2 d of age and weighed daily for 20 d. The mean weight gain of mice inoculated with reovirus at 2, 4, 6, and 8 d of age was significantly less than that of age-matched controls inoculated with PBS (Figure 1b–e). The mean weight gain of mice inoculated with reovirus at 10 d was significantly less than that of age-matched controls at early time points post-inoculation (p.i.), but the differences were reduced at later time points (Figure 1f). Interestingly, the mean weights of mice inoculated with reovirus at 15 and 20 d of age were significantly greater than those of age-matched controls at later time points (Figure 1g, h). These data suggest that the capacity of reovirus to induce disease is diminished in mice inoculated at 10 d of age or older.
Viral loads in the brain and peripheral organs do not strictly correlate with disease severity
Disease severity is often proportional to the level of viral replication in vital organs. To determine whether the age-dependent severity in reovirus disease is a function of viral load, we quantified viral loads in organs at 4, 7, and 10 d p intracranial inoculation with T3D. The average peak viral loads in brains of mice inoculated at 2 and 4 d of age were comparable (Figure 2). In contrast, the average peak viral load in brains of mice inoculated at 6 d of age was lower compared with the average peak viral load in brains of mice inoculated at younger age. Average peak viral load in brains of mice inoculated at 10 and 15 d of age were comparable to average peak viral load in brains of mice inoculated at 6 d of age. We calculated the area under the curve (AUC) for each age of inoculation to test whether the cumulative viral loads in the brain for all three time points differed between mice inoculated at different ages. The AUCs of mice inoculated at 2 d of age (5.5 × 1014) and 4 d of age (5.0 × 1013) were markedly greater than the AUCs of mice inoculated at 6 d of age (4.7 × 107), 10 d of age (4.2 × 107), and 15 d of age (2.2 × 108). Although viral loads in the brains of mice inoculated at 6 d of age were comparable to those in mice inoculated at 10 or 15 d of age, mortality was significantly greater in mice inoculated at 6 d of age compared with mice inoculated at 10 or 15 d of age. These data suggest that viral loads in the brain do not strictly correlate with disease severity.
Figure 2. Viral loads in the brain following reovirus inoculation at various ages.
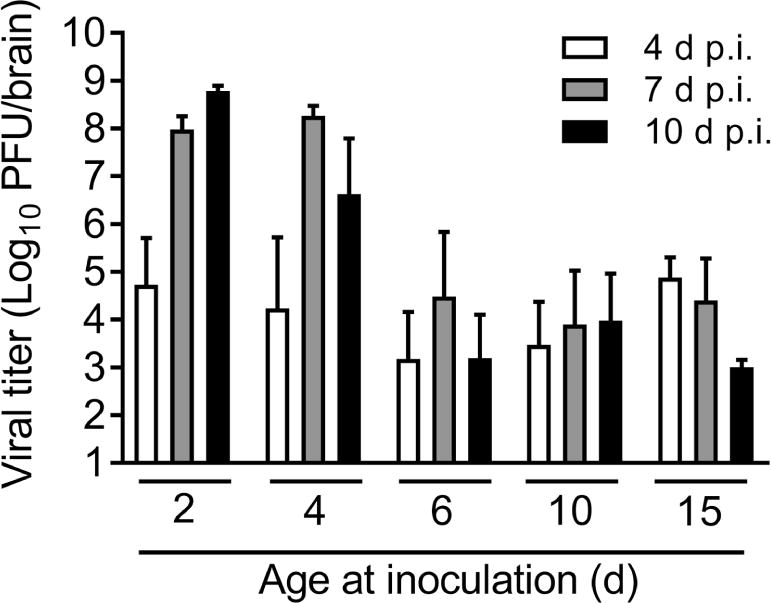
WT mice were inoculated intracranially with reovirus T3D at 100 PFU/g at 2, 4, 6, 10, or 15 d of age. At 4, 7, and 10 d p.i., mice were euthanized, brains were resected, and viral loads were determined by plaque assay. n = 5–7 mice per bar.
To determine whether the capacity to disseminate from the brain to other tissue sites in the host correlates with disease severity and, if so, whether this process is age-dependent, we quantified viral loads in the heart, spleen, liver, and intestine. We found that for each individual mouse, virus was either present in all or none of the peripheral organs assayed. At 4 d p.i., approximately half of the mice inoculated at any age had detectable viral titers in peripheral organs with the exception of mice inoculated at 6 d of age, of which only one-fifth had detectable titers in peripheral organs (Table 1). At 7 d p.i., viral titers were detectable in peripheral organs of the majority of mice inoculated at 2 and 4 d of age compared with less than half the mice inoculated at 6, 10, or 15 d of age. At 10 d p.i., reovirus was detected in the majority of mice inoculated at 2 and 4 d of age, but no reovirus was detected in peripheral organs of mice inoculated at 6, 10, or 15 d of age. These data indicate that the capacity of reovirus to disseminate from the brain to peripheral organs is age-dependent. However, neither viral load in the brain nor the presence of reovirus in peripheral organs alone explain the substantial disease severity observed in mice inoculated at 6 d of age.
Table 1. Viral dissemination to peripheral organs.
WT mice were inoculated intracranially with reovirus T3D at 100 PFU/g at 2, 4, 6, 10, or 15 d of age. Viral loads in the brain, heart, liver, spleen, and intestine were determined by plaque assay at 4, 7, and 10 d p.i. n = 5–7 mice per condition. Numerals represent the number of mice with detectable titer in peripheral organs divided by the total number of mice assayed, and the resulting percentage on mice with detectable titer in peripheral organs in each group.
| Time post-inoculation (d) | ||||
|---|---|---|---|---|
| 4 | 7 | 10 | ||
| Age of inoculation (d) | 2 | 3/6 (50%) |
5/6 (83%) |
6/6 (100%) |
| 4 | 3/6 (50%) |
5/5 (100%) |
5/6 (83%) |
|
| 6 | 1/5 (20%) |
2/5 (40%) |
0/7 (0%) |
|
| 10 | 3/6 (50%) |
2/6 (33%) |
0/6 (0%) |
|
| 15 | 4/6 (67%) |
2/5 (40%) |
0/6 (0%) |
|
Reovirus displays enhanced virulence in IFNAR−/− but not RAG2−/− mice
To test the hypothesis that maturation of innate and adaptive immune responses contributes to age-dependent susceptibility to reovirus disease, we compared reovirus virulence following inoculation of WT, IFNAR−/−, and RAG2−/− mice at different ages. Mice deficient in expression of the interferon α/β receptor (IFNAR) lack an essential component of the antiviral innate immune response, while mice deficient in recombination activating gene 2 (RAG2) lack functional B and T lymphocytes and are incapable of mounting adaptive immune responses. Mice were inoculated intracranially with T3D at 4 or 15 d of age and monitored for survival, weight gain, and clinical signs of neurologic disease such as lethargy, seizures, ataxia, and paralysis (20) for 20 d. The mean survival time for IFNAR−/− mice inoculated with reovirus at 4 d of age was 8 d, whereas the mean survival time for WT and RAG2−/− mice was 12 and 11 d, respectively (Figure 3a). Only 48% of IFNAR−/− mice inoculated with reovirus at 15 d of age survived compared with 92% of RAG2−/− mice and 100% of WT mice inoculated at 15 d of age (Figure 3b). All PBS-inoculated mice survived. A majority of IFNAR−/− mice inoculated at either 4 or 15 d of age displayed reduced weight gain (Figure 3c, d) without neurological signs of illness. RAG2−/− mice inoculated with reovirus displayed reduced weight gain when inoculated at 4 d of age but not when inoculated at 15 d of age, a trend similar to that of WT mice inoculated with reovirus at these ages (Figure 3e, f). These data suggest that the absence of a functional innate but not adaptive immune response prolongs the susceptibility of mice to reovirus-induced disease and raises the possibility that maturation of the IFN response contributes to age-dependent reovirus virulence.
Figure 3. Survival and weight gain of reovirus-infected WT, IFNAR−/−, and RAG2−/− mice.
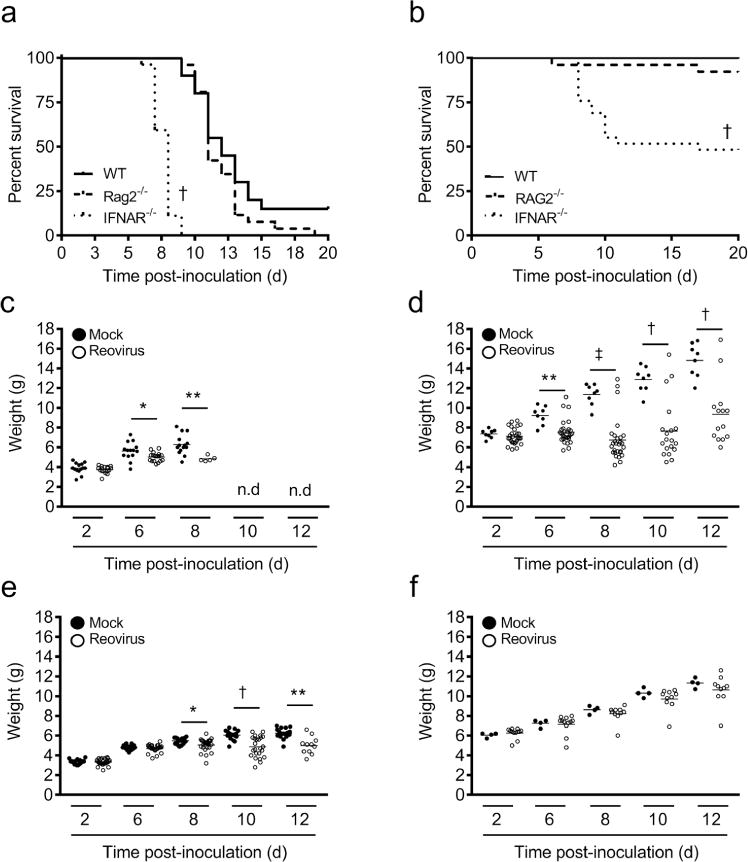
WT, IFNAR−/−, and RAG2−/− mice were inoculated intracranially with reovirus T3D at 100 PFU/g at either 4 or 15 d of age and monitored for morbidity for 20 d. Mice were euthanized when moribund. Survival of mice inoculated at (a) 4 and (b) 15 d of age. N ≥ 23 per experimental group. † P < 0.001 as determined by Log-rank test compared to WT. Weights of IFNAR−/− mice inoculated at (c) 4 or (d) 15 d of age. Weights of RAG2−/− mice inoculated at (e) 4 or (f) 15 d of age. N ≥ 15 per experimental group. *, P < 0.05; **, P < 0.01; †, P < 0.005; and ‡, P < 0.0001 as determined by Mann-Whitney U test.
Viral loads in brains of IFNAR−/− mice are higher than those in WT mice when inoculated at an older age
Based on the increased mortality in IFNAR−/− mice at older ages of inoculation, we hypothesized that reovirus produces higher titers in IFNAR−/− mice when inoculated at ages at which WT mice are no longer susceptible to reovirus disease. To test this hypothesis, WT, IFNAR−/−, and RAG2−/− mice were inoculated intracranially with T3D at the susceptible age of 4 d and the non-susceptible age of 15 d, and viral loads in organs were determined at 4, 7, and 10 d p.i. Viral loads in brains of WT, IFNAR−/−, and RAG2−/− mice inoculated at 4 d of age did not differ significantly on 4 and 7 d p.i. (Figure 4a). However, viral loads in brains of RAG2−/− mice were significantly higher than those in brains of WT mice at 10 d p.i. We were unable to perform statistics comparing viral loads in brains of IFNAR−/− mice at 10 d p.i. due to lack of replicates. However, the viral load in the brain of the single surviving mouse approximated the average load of RAG−/− mice inoculated at that age. At all time points tested, viral loads in brains of IFNAR−/− mice were significantly higher than those in brains of WT mice inoculated at 15 d of age (Figure 4b). No significant differences in viral load were detected between brains of WT and RAG2−/− mice inoculated at 15 d. These data suggest that adaptive immune responses function in the clearance of reovirus from brains of younger mice, while IFN-mediated innate immune responses control viral replication in the brains of older mice. The lack of overwhelmingly significant immune-related differences in viral loads in brains of mice inoculated at 4 d of age suggests that disease severity and mortality at this age of inoculation is not directly related to the modulation of viral replication by immune responses in the brain.
Figure 4. Viral loads in reovirus-infected WT, IFNAR−/−, and RAG2−/− mice.
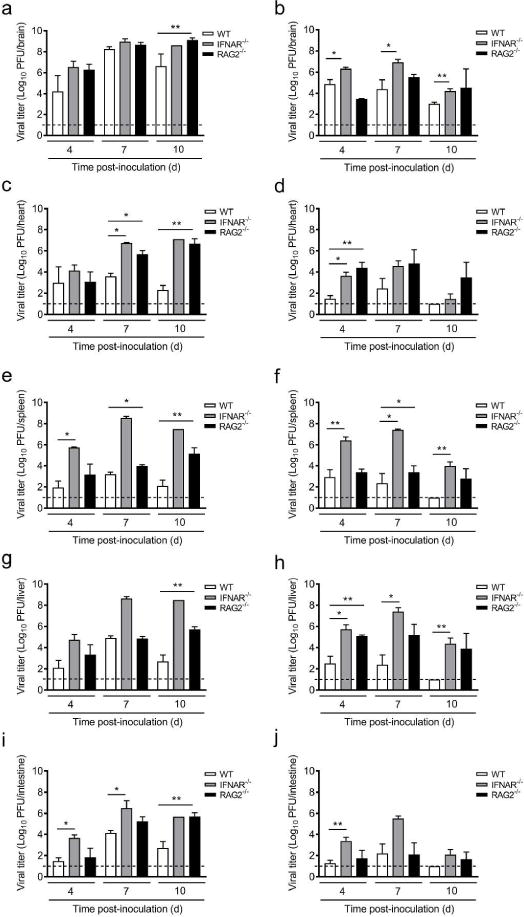
WT, IFNAR−/−, and RAG2−/− mice were inoculated intracranially with reovirus T3D at 100 PFU/g at either 4 or 15 d of age. At 4, 7, and 10 d p.i., mice were euthanized, organs resected, and viral loads were determined. Viral loads in brains of mice inoculated at (a) 4 or (b) 15 d of age, in hearts of mice inoculated at (c) 4 or (d) 15 d of age, in spleens of mice inoculated at (e) 4 or (f) 15 d of age, in livers of mice inoculated at (g) 4 or (h) 15 d of age, and in intestines of mice inoculated at (i) 4 or (j) 15 d of age. N = 2–10 except IFNAR−/− 10 d p.i., for which n = 1 due to low survival rate at that time point. Dotted lines represent limit of detection. *, P < 0.05; **, P < 0.01 as determined by Mann-Whitney U test.
IFN controls viral dissemination to peripheral organ in both younger and older mice
To determine the function of innate and adaptive immune responses in controlling viral dissemination from the site of inoculation to sites of secondary replication, viral loads were quantified in organs of WT, IFNAR−/−, and RAG2−/− mice infected at 4 and 15 d of age (Figure 4 c–j). Contrary to WT (Table 1), reovirus was detected in all peripheral organs in both strains of immune-deficient mice at all time points tested (data not shown). Overall, viral loads in peripheral organs of IFNAR−/− mice were increased compared with those in WT mice (Figure 4c–j). Viral loads in the heart and spleen of RAG2−/− mice inoculated at 4 d of age also were increased compared with those in WT mice at 7 d p.i. and in all organs at 10 d p.i. RAG2−/− mice inoculated at 15 d of age displayed modestly increased viral loads in the heart and liver but not in the spleen and intestine at 4 d p.i., and the differences became smaller at later times (Figure 4d, f, h, and j). Peak viral loads in all organs of IFNAR−/− mice inoculated at 4 d of age coincide with the sharp reduction in survival of IFNAR−/− mice (Figure 3a), whereas differences in weight gain and survival of WT and RAG2−/− mice inoculated at 4 d of age appear at later times post-infection when viral loads in both the brain and peripheral organs of RAG2−/− mice are significantly greater than those in WT mice (Figure 4a, c, e, g, and i). Interestingly, viral loads in IFNAR−/− mice inoculated at 4 d of age increased throughout infection until death, while viral loads in mice inoculated at 15 d of age peaked at 7 d p.i., followed by a decline at 10 d p.i., consistent with the enhanced survival of IFNAR−/− mice inoculated at this age. Together, these results suggest that IFN plays an important role in controlling systemic dissemination and replication at secondary sites in mice of both susceptible and non-susceptible ages at all time points assessed. Adaptive responses function later during infection of mice inoculated at a susceptible age and likely contribute to viral clearance.
Reovirus tropism is unaltered in brains of immune-deficient mice
To determine whether differences in brain pathology link mortality to altered immune responses, the right hemispheres of brains matched as closely as possible for viral load were processed for histology. Consecutive sections were stained with H&E, polyclonal reovirus antiserum to detect viral antigen, or a monoclonal antibody specific for the cleaved (activated) form of caspase-3 to detect cells undergoing apoptosis, the primary mechanism of neuronal cell death following reovirus infection (19). Reovirus antigen was detected in the hippocampus, thalamus, and cortex (Figure 5a) as well as the cerebellum and hindbrain (Figure 5b) of brains resected at 7 d p.i. from mice of all three strains inoculated with reovirus at 4 d of age. Although overall staining intensity varied, no qualitative differences were found in viral tropism. Staining for the activated form of caspase-3 was modest in all sections analyzed and localized with reovirus staining, consistent with the pattern of reovirus-induced tissue injury (8, 19, 21). Histological analysis of brains resected from mice inoculated with reovirus at 15 d of age at 7 d p.i. showed substantially reduced levels of reovirus antigen-positive cells (data not shown). Staining was restricted to small areas within the thalamus and surrounding the lateral ventricles. These areas of the brain also displayed low levels of activated caspase-3 staining (data not shown).
Figure 5. Reovirus tropism in brains of WT, IFNAR−/−, and RAG2−/− mice.
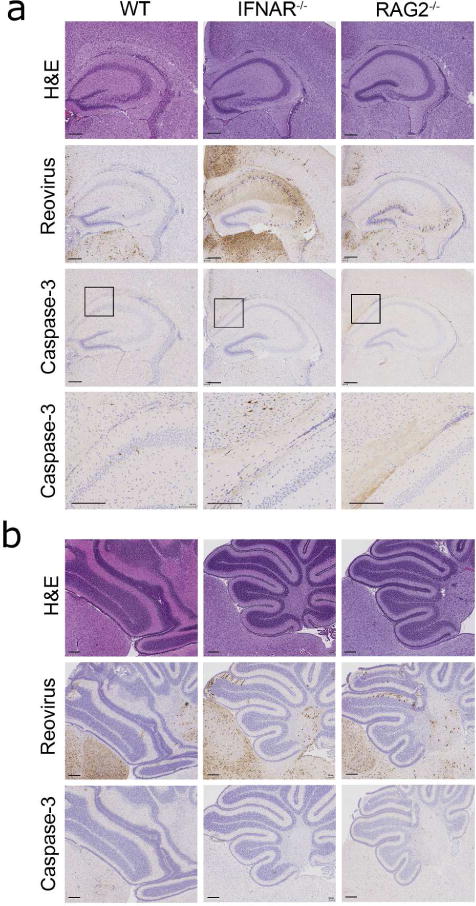
WT, IFNAR−/−, and RAG2−/− mice were inoculated intracranially with reovirus T3D at 100 PFU/g at 4 d of age. At 7 d p.i., brains were removed, the left hemispheres were homogenized for determination of viral titer, and the right hemispheres were processed for immunohistochemistry. Consecutive coronal sections of the brain were stained with H&E, reovirus antiserum, or an anti-cleaved caspase-3 antibody. Representative sections of brains are shown. (a) Hippocampal region stained with H&E or polyclonal reovirus antiserum and higher magnification image of boxed inset stained for cleaved caspase-3 (scale bars, 200 μm). (b) Cerebellum and hind brain. Sections shown are from a WT, IFNAR−/−, and a RAG2−/− mouse with a viral load of 3.9 × 108 PFU/brain, 3.8 × 109 PFU/brain, and 1.2 × 109 PFU/brain, respectively (scale bars, 200 μm).
Apoptosis is unaltered in brains of immune-deficient mice
To determine whether quantitative age- and immune-dependent differences exist in reovirus-induced apoptosis, protein lysates from the brains of three individual mice of each strain resected 7 d p.i. were resolved by SDS PAGE and immunoblotted using an antibody specific for cleaved caspase-3 and an antiserum specific for actin as a loading control. The intensity of the cleaved caspase-3 signal was normalized to the intensity of the actin signal. The overall magnitude of apoptosis in brains of IFNAR−/− and RAG2−/− mice inoculated at 4 d of age (Figure 6a, c) and at 15 d of age (Figure 6b, d) was comparable.
Figure 6. Quantification of apoptosis in brains of WT, IFNAR−/−, and RAG2−/− mice.
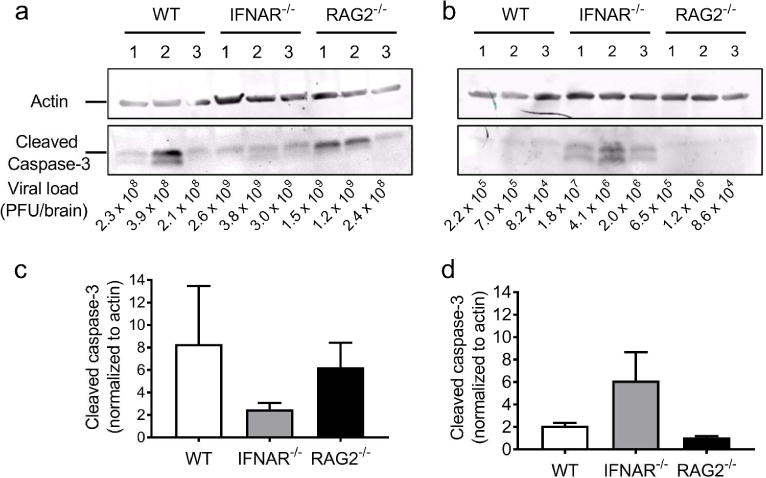
Mice were inoculated intracranially with reovirus T3D at 100 PFU/g at either (a, c) 4 or (b, d) 15 d of age. Protein lysates from brains of three T3D-infected WT, IFNAR−/−, or RAG2−/− mice resected at 7 d p.i. were resolved by SDS-PAGE. (a, b) Lysates immunoblotted using antisera specific for actin (top) and cleaved caspase-3 (bottom). Viral loads are listed for each sample. (c, d) Quantification of the band intensity of cleaved-caspase-3 normalized to actin.
DISCUSSION
In this study, we determined the precise timing of the age restriction to reovirus virulence in C57BL/6J mice based on mortality and determination of weight gain as a surrogate marker for reovirus-induced disease. We found that the age restriction for reovirus mortality and disease lies between 6 and 10 d of age. We were surprised to find that a lower proportion of mice inoculated at 4 d of age survived compared to mice inoculated at 2 d of age. This finding might be attributed to experimental variability caused by a suboptimal nurturing by the dam. Mice inoculated intracranially with serotype 3 reovirus strains likely succumb to encephalitis rather than to disease at other sites of infection, as a reovirus mutant incapable of disseminating systemically is as virulent as WT virus following intracranial inoculation (22). Interestingly, there was a significant increase in weight gain following inoculation with reovirus at 15 and 20 d of age relative to PBS-inoculated controls. It is possible that the virus-mediated increase in weight gain occurs as a consequence of virus-induced damage to the ventromedial hypothalamus, which is associated with increased appetite (23–25). Histological examination of brain tissue from mice infected at additional ages will provide more information about the age-dependent differences in reovirus neural tropism and pathology.
We thought it possible that age-related disease severity might track with virus titers in target tissues. Our results indicate that viral replication occurred even in mice that did not display overt neurological signs of infection. Brains of mice inoculated at 6 d of age harbored viral loads that were similar to those in brains of mice inoculated at non-susceptible ages, yet survival rates and weight gain was comparable to mice inoculated at susceptible ages. Reovirus disseminated systemically in mice inoculated at susceptible ages, whereas systemic dissemination was limited in mice inoculated at non-susceptible ages. Thus, we conclude that viral titers in the brain do not strictly correlate with susceptibility to reovirus-induced disease.
We used immune-deficient mice to investigate whether maturation of innate or adaptive immune responses contributes to the age restriction of reovirus disease. Our results indicate that IFN functions in controlling viral replication in mice of both susceptible and non-susceptible ages, whereas adaptive immune responses are particularly important in controlling replication at later times post-infection in mice of susceptible age. The increased susceptibility of older IFNAR−/− mice to reovirus infection suggests that age-dependent maturation of IFN responses contributes to the age-related virulence of reovirus. Some IFNAR−/− mice had signs of encephalitis, but others died quickly after the onset of illness. A previous study describes intestinal perforation, bacterial sepsis, and acute hepatitis as causes of death in IFNAR−/− mice inoculated with reovirus at 2 d of age (26).
Besides contributing to age-dependent susceptibility to reovirus infection, the type I IFN response functions in the age-dependent susceptibility to infection with herpes simplex virus (HSV-1). Lower basal levels of IFNAR are expressed in the choroid plexus of uninfected newborn mice compared with adults. Concordantly, the adult choroid plexus is less susceptible to infection with HSV-1 relative to the newborn brain. Similar to our findings with reovirus, HSV-1 susceptibility was restored in brains of adult IFNAR−/− mice (27).
Cells of the innate immune system also may contribute directly to the age-dependent susceptibility to reovirus CNS disease. Microglia, the resident macrophage cells in the brain, modulate the immune response to brain infections by secreting inflammatory cytokines such as IL-1α, IL-6, and TNFα (28). Microglia are virtually absent from the mouse hippocampus at birth, but numbers of these cells increase dramatically between 5 and 10 d of age and peak at 15 d of age (29). Microglial activation and pro-inflammatory cytokine production in the brain decrease with age under normal conditions and in response to stimulation with LPS (30, 31), suggesting that the inflammatory response to viral infections of the brain are muted at later points in life. Consistent with this idea, the production of the pro-inflammatory cytokine IL-1α is increased in brains of reovirus-infected newborn mice compared with adults and coincides with nervous tissue injury that precedes encephalitis (32). Our finding that the absence of RAG2 expression does not affect the timing of the age restriction to reovirus disease is consistent with the kinetics of adaptive immune effector maturation, which is initiated after the first month of life (33) and, thus, outside the interval during which reovirus susceptibility diminishes.
Results from this study define an age that mice become refractory to reovirus disease and provide evidence that maturation of innate immune responses contributes to the mechanism of age restriction. These findings suggest that innate immune maturity influences diverse types of neural insults.
Acknowledgments
We thank members of the Dermody lab for helpful discussions and Laurie Silva and Gwen Taylor for critical review of the manuscript, the Vanderbilt Division of Animal Care for animal husbandry, and Kelli Boyd and colleagues from the Vanderbilt Translational Pathology Shared Resource for assistance.
This work was supported by Public Health Service awards T32 HD060554 (A. G. W.), F31 DK108562 (J. J. B.), and R01 AI38296 (T. S. D.). Additional support was provided by the Elizabeth B. Lamb Center for Pediatric Research.
Footnotes
The authors declare no conflicts of interest.
Original research article, basic science.
References
- 1.Griffin DE. Emergence and re-emergence of viral diseases of the central nervous system. Prog Neurobiol. 2010;91:95–101. doi: 10.1016/j.pneurobio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin DE. Viral encephalomyelitis. PLoS Pathog. 2011;7:e1002004. doi: 10.1371/journal.ppat.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyler KL, McPhee DA, Fields BN. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–4. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- 4.Morrison LA, Sidman RL, Fields BN. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci U S A. 1991;88:3852–6. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antar AAR, Konopka JL, Campbell JA, et al. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host & Microbe. 2009;5:59–71. doi: 10.1016/j.chom.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pruijssers AJ, Dermody TS. Reovirus. In: Reiss CS, editor. Neurotropic Viral Infections. 2. New York, NY: Springer Nature; 2016. pp. 337–60. [Google Scholar]
- 7.Weiner HL, Powers ML, Fields BN. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980;141:609–16. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- 8.Danthi P, Coffey CM, Parker JS, Abel TW, Dermody TS. Independent regulation of reovirus membrane penetration and apoptosis by the mu1 phi domain. PLoS Pathog. 2008;4:e1000248. doi: 10.1371/journal.ppat.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danthi P, Pruijssers AJ, Berger AK, Holm GH, Zinkel SS, Dermody TS. Bid regulates the pathogenesis of neurotropic reovirus. PLoS Pathog. 2010;6:e1000980. doi: 10.1371/journal.ppat.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis G, Kilham L, Gonatos N. Reovirus type III encephalitis: observations of virus-cell interactions in neural tissues. I. Light microscopy studies. Laboratory Investigations. 1971;24:91–109. [PubMed] [Google Scholar]
- 11.Raine CS, Fields BN. Ultrastructural features of reovirus type 3 encephalitis. Journal of Neuropathology and Experimental Neurology. 1973;32:19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Tardieu M, Powers ML, Weiner HL. Age dependent susceptibility to Reovirus type 3 encephalitis: role of viral and host factors. Ann Neurol. 1983;13:602–7. doi: 10.1002/ana.410130604. [DOI] [PubMed] [Google Scholar]
- 13.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goody RJ, Beckham JD, Rubtsova K, Tyler KL. JAK–STAT signaling pathways are activated in the brain following reovirus infection. J Neurovirol. 2007;13:373–83. doi: 10.1080/13550280701344983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi T, Antar AA, Boehme KW, et al. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe. 2007;1:147–57. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furlong DB, Nibert ML, Fields BN. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–56. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virgin HW, Dermody TS, Tyler KT. Cellular and humoral immunity to reovirus infection. In: Tyler KT, Oldstone MBA, editors. Curr Top Microbiol Immunol. Berlin: Springer-Verlag; 1998. pp. 147–61. [DOI] [PubMed] [Google Scholar]
- 18.Tyler KL, Bronson RT, Byers KB, Fields B. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985;35:88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Pruijssers AJ, Hengel H, Abel TW, Dermody TS. Apoptosis induction influences reovirus replication and virulence in newborn mice. J Virol. 2013;87:12980–9. doi: 10.1128/JVI.01931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler KL. Pathogenesis of Reovirus Infections of the Central Nervous System. In: Tyler KL, Oldstone MA, editors. Reoviruses II Cytopathogenicity and Pathogenesis. Berlin Heidelberg: Springer Verlag; 1998. pp. 93–124. [DOI] [PubMed] [Google Scholar]
- 21.Richardson-Burns SM, Tyler KL. Regional differences in viral growth and central nervous system injury correlate with apoptosis. J Virol. 2004;78:5466–75. doi: 10.1128/JVI.78.10.5466-5475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehme KW, Guglielmi KM, Dermody TS. Reovirus nonstructural protein sigma1s is required for establishment of viremia and systemic dissemination. Proc Natl Acad Sci U S A. 2009;106:19986–91. doi: 10.1073/pnas.0907412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Vazquez C, Prieto-Gomez B, Dafny N. Interferon modulates central nervous system function. Brain Res. 2012;1442:76–89. doi: 10.1016/j.brainres.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 24.Dafny N, Gillman MA, Lichtigfeld FJ. Cholecystokinin: induced suppression of feeding in fed, fasting and hypothalamic island rats. Brain Res Bull. 1988;21:225–31. doi: 10.1016/0361-9230(88)90235-3. [DOI] [PubMed] [Google Scholar]
- 25.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Dionne KR, Galvin JM, Schittone SA, Clarke P, Tyler KL. Type I interferon signaling limits reoviral tropism within the brain and prevents lethal systemic infection. J Neurovirol. 2011;17:314–26. doi: 10.1007/s13365-011-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox DR, Folmsbee SS, Muller WJ, Longnecker R. The type I interferon response determines differences in choroid plexus susceptibility between newborns and adults in herpes simplex virus encephalitis. mBio. 2016;7:e00437–16. doi: 10.1128/mBio.00437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock RB, Gekker G, Hu S, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–64. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim I, Mlsna LM, Yoon S, et al. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain and behavior. 2015;5:e00403. doi: 10.1002/brb3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrazzano P, Chanana V, Uluc K, et al. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS Neurol Disord Drug Targets. 2013;12:338–49. doi: 10.2174/1871527311312030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inamizu T, Chang MP, Makinodan T. Influence of age on the production and regulation of interleukin-1 in mice. Immunology. 1985;55:447–55. [PMC free article] [PubMed] [Google Scholar]
- 32.Derrien M, Fields BN. Reovirus type 3 clone 9 increases interleukin-1 level in the brain of neonatal, but not adult, mice. Virology. 1999;257:35–44. doi: 10.1006/viro.1999.9611. [DOI] [PubMed] [Google Scholar]
- 33.Landreth KS. Critical windows in development of the rodent immune system. Hum Exp Toxicol. 2002;21:493–8. doi: 10.1191/0960327102ht287oa. [DOI] [PubMed] [Google Scholar]


