Abstract
Chronic pain is a large, unmet public health problem. Recent studies have demonstrated the importance of neuroinflammation in the establishment and maintenance of chronic pain. However, pharmacotherapies that reduce neuroinflammation have not been successfully developed to treat chronic pain thus far. Several preclinical studies have established imidazoline I2 receptor (I2R) agonists as novel candidates for chronic pain therapies, and while some I2R ligands appear to modulate neuroinflammation in certain scenarios, whether they exert anti-neuroinflammatory effects in models of chronic pain is unknown. This study examined the effects of the prototypical I2R agonist 2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI) on hypersensitivity and neuroinflammation induced by chronic constriction injury (CCI), a neuropathic pain model in rats. In CCI rats, twice-daily treatment with 10 mg/kg 2-BFI for seven days consistently increased mechanical and thermal nociception thresholds, reduced GFAP and Iba-1 levels in the dorsal horn of the spinal cord, and reduced levels of TNF-α relative to saline treatment. These results were recapitulated in primary mouse cortical astrocyte cultures. Incubation with 2-BFI attenuated GFAP expression and supernatant TNF-α levels in LPS-stimulated cultures. These results suggest that I2R agonists such as 2-BFI may reduce neuroinflammation which may partially account for their antinociceptive effects.
Keywords: 2-BFI, imidazoline I2 receptor, pain, neuroinflammation, rats, astrocytes, microglia
Graphical Abstract
1. Introduction
Chronic pain is a massive and undertreated healthcare problem despite decades of research. It affects over 100 million people in the United States alone – more than cancer, heart disease, and diabetes combined – carries an economic burden of over $600 billion, and reduces quality of life, second only to bipolar disorder as the leading cause of suicide among medical illnesses [1–3]. This problem is exacerbated by ineffective analgesics currently available and a lack of novel pharmacotherapies introduced to the market within the past 50 years [4, 5].
The important role of neuroinflammation in pain has been increasingly acknowledged over the past several years [for review, see 6], and immunocompetent cells of the central nervous system (CNS) including microglia and astrocytes have become recognized as essential to pain pathophysiology. Under normal conditions, painful stimuli from the periphery are transmitted via primary neurons through the dorsal horn of the spinal cord to numerous brain regions, constituting the ascending pain pathway. The descending pain pathway, comprised of fibers projecting from the brain stem to the spinal cord, can suppress pain transmission by the release of neurotransmitters such as serotonin (5-HT) and norepinephrine (NE). In response to tissue or nerve injury, microglia and astrocytes “activate” by proliferating in number and releasing a range of factors including pro-inflammatory cytokines (e.g., TNF-α, IL-1β) that profoundly affect neurons to promote hyperalgesia and allodynia [7], a relationship demonstrated to be causally related [8]. These pro-inflammatory substances are thought to augment pain signals by a) enhancing pain-transmitting neuron excitability by increasing NMDA and AMPA receptor signaling and calcium permeability, and blocking the uptake of glutamate by astrocytes to increase glutamatergic signaling, while b) diminishing inhibitory transmission by weakening GABA and glycine release by inhibitory interneurons and inhibitory descending projections. However, while this neuroimmune interface appears to be a target for analgesics, traditional glial inhibitors like minocycline have returned low efficacy in clinical trials [9–11]. Therefore, the development of compounds which take advantage of neuroimmune interactions to treat pain is still required.
The imidazoline I2 receptor (I2R) has been established as a promising target for chronic pain therapy. I2R agonists such as 2-BFI are effective in several preclinical chronic pain models as monotherapies but also enhance opioid analgesia in an additive to synergistic manner while decreasing opioid tolerance and physical dependence. Although the clearest role of I2Rs based on the available molecular evidence is allosteric modulation of monoamine oxidase (MAO) A [12] and B [13], the presence of I2Rs on glia and their ability to influence glial activation has also been documented. I2Rs were found in astrocyte but not neuron rat primary cerebral cortical cultures [14], and increased densities of I2Rs were found in human patients with gliomas relative to control and non-glial tumor patients, suggesting that I2Rs may be principally expressed by glial cells [15]. A regulatory role for I2Rs on glial activity was proposed in vitro as the induction of nitric oxide synthase type-2 (NOS-2) in rat primary astrocytes or RAW 264.7 macrophages by lipopolysaccharide (LPS) plus cytokines was attenuated by co-incubation with an I2R ligand [16]. Similar results were found in vivo. In mice with brain and spinal cord injury induced by experimental autoimmune encephalomyelitis (EAE), 2-BFI administration for ten days reduced spinal microglial activation, reduced levels of cytokines such as interferon-γ (IFN- γ) and TNF-α in blood and spinal cord, and improved symptom severity scores [17, 18]. However, despite the causal relationship between chronic pain and neuroinflammation, the effects of I2R agonists on glia in in vivo models of chronic pain have not yet been examined.
This study measured the antinociceptive effects of the I2R agonist 2-BFI in rats with chronic constriction injury (CCI)-induced neuropathic pain over a seven-day treatment period, then examined spinal microglial and astrocytic activation and TNF-α levels to determine if 2-BFI treatment modulated CCI-induced neuroinflammation. In a separate experiment, the effects of 2-BFI on mouse primary cortical astrocyte cultures, stimulated with LPS to mimic neuroinflammation, was examined.
2. Methods
2.1 Subjects
Male (n = 36 rats) Sprague-Dawley rats (Envigo, Indianapolis, IN) 10–12 weeks old and weighing approximately 250 g at experiment onset were individually housed on a 12/12-hour light/dark cycle with behavioral experiments conducted during the light period. All rats had free access to standard rodent chow and water, except during test sessions. Treatment conditions were randomly assigned and group size was determined by previous studies from our laboratory to ensure sufficient statistical power. All animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain [19] and with the 2011 Guide for the Care and Use of Laboratory Animals [20], and all procedures were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York (Buffalo, NY).
2.2 Induction of neuropathic pain
Neuropathic pain was induced by CCI procedure [21, 22]. Briefly, rats were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (15 mg/kg) intraperitoneally (i.p.) prior to surgery. The right sciatic nerve was exposed, and four ligatures (4.0 chromic gut suture, Patterson Veterinary, Devens, MA) were placed around the nerve (approximately 1 mm apart) proximal to the trifurcation. Ligatures were loosely tied such that circulation through the epineural vasculature was uninterrupted. The incisions were closed with surgical clips.
2.3 Mechanical and thermal nociception
Behavioral testing and drug treatment began one day after CCI surgery. Thermal hyperalgesia was measured by the Hargreaves test using equipment and procedures described previously [23]. Briefly, rats (n = 9 per group) were placed in transparent test chambers atop an elevated clear glass platform through which a light beam was projected from a heat source onto the hind paw. This thermal stimulus was applied until the rat withdrew its paw or 20 s had elapsed to determine the paw withdrawal latency (PWL). Measurements were taken in duplicate approximately 1 min apart, and the average was used for statistical analysis. Mechanical hyperalgesia was measured by the von Frey filament test using equipment and procedures as described in detail previously [23]. Briefly, rats were placed in transparent test chambers atop a wire mesh platform through which filaments were applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor to determine the paw withdrawal threshold (PWT), defined as the lowest strength filament that elicited a behavioral response in at least two out of three applications. In all experiments, experimenters were blind to the treatments, and they received extensive training with the von Frey and Hargreaves procedures to ensure accurate judgment of paw withdrawal responses and minimize experimenter bias.
Each day, baseline thermal and mechanical thresholds were measured immediately prior to saline or 10 mg/kg 2-BFI treatment. This dose of 2-BFI was chosen as it produces significant antinociception [23]. Since 2-BFI-induced antinociception reaches a peak effect at 30 min post-injection, thermal thresholds were measured beginning at 25 min post-injection, after which rats were transferred to the mechanical nociception apparatus to habituate before mechanical thresholds were measured beginning at 35 min post-injection. At least 6 h later, rats received either saline or another 10 mg/kg 2-BFI injection, depending on group assignment.
2.4 TNF-α bioassay
The lytic effect of TNF-α on WEHI-13VAR fibroblast cells was used to analyze lumbar spinal cord tissue homogenates for the presence of biologically active TNF-α [24, 25]. Immediately after sacrifice (1 hr following the final drug treatment), spinal cord samples ere harvested on ice, snap frozen, and stored at −30°C until processing, with spinal cords collected from four rats per treatment group, which has been previously demonstrated to provide sufficient statistical power in this assay [25]. Samples were weighed, homogenized in 3 mL RPMI-1640 supplemented with glutamine (2 mM) and a protease inhibitor cocktail (PIC; 2.5 μl/50 mg tissue), then centrifuged at 14,000 × g for 15 min at 4°C. Supernatants were stored at −30°C.
WEHI-13VAR fibroblast cells, a TNF-α-sensitive cell line derived from a mouse fibrosarcoma (ATCC, Manassas, VA), were grown in culture medium containing: RPMI-1640, 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Chicago, IL), and 3 μg/mL gentamicin (Sigma-Aldrich, St. Louis, MO) in T75 flasks at 37°C, 95% relative humidity, 5% CO2. Cells used in the bioassay were cultured to approximately 90% confluency and were always below passage 25 to avoid loss of TNF-α sensitivity. Cells were prepared for the assay by detaching with 0.25% trypsin and 0.02% EDTA (Sigma-Aldrich, St. Louis, MO), adding 10 mL/flask culture medium, then supplementing with 1 μg/mL actinomycin D (Calbiochem, La Jolla, CA) to a concentration of 500,000 cells/mL. 100 μL of cell suspension was added to each well of a flat-bottom 96-well tissue culture plate containing 100 μL of 2-fold serial dilutions of unknown samples, in triplicate, or known concentrations of recombinant rat TNF-α standards (rrTNF, R&D Systems, Minneapolis, MN) in diluting medium, RPMI-1640, 2 mM L-glutamine, 1% heat-inactivated fetal bovine serum, and 15 mM HEPES (Sigma-Aldrich, St. Louis, MO). Following 20 hr incubation at 37°C, 95% RH, 5% CO2, 10 μL of a 1:1 diluted solution of diluting media and the pre-mixed Cell Proliferation Reagent WST-1 (a solution of the tetrazolium salt, WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate and an electron coupling reagent) (Takara Bio USA, Inc., Mountain View, CA) was added to each well. After incubating for 4 hr at 37°C, 95% RH, 5% CO2, the absorbance at 440 and 700 nm was measured using a SpectraMax 190 microplate reader with SoftMax Pro v4.0 acquisition and analysis software (MDS Analytical Technologies, Sunnyvale, CA). A standard curve (0.01 pg/mL – 10,000 pg/mL, reverse sigmoid in shape) of the (OD440 – OD700) vs log[TNF-α] was plotted and the [TNF-α] of each sample was determined from the dilution closest to the inflection point of the standard curve. This assay is based on the specific cytotoxicity of the WEHI-13VAR cells to TNF-α in the presence of actinomycin D, and has a detection limit of approximately 1 pg/mL. WST-1 counting solution was used as a cell viability indicator, which is quantitated spectrophotometrically, so increased TNF-α concentrations result in augmented cell death and thus reduced absorbance at 440 nm. Results are expressed as pg TNF-α/100 mg tissue weight.
2.5 Primary culture of mouse cortical astrocytes
Primary cultures of mouse cortical astrocytes were prepared as described by Amur-Umarjee et al. [26]. Brains from newborn (P1–P2) C57BL/6 mice were dissected under sterile conditions. The cortex was isolated, mechanically dissociated, and plated on poly-D-lysine-coated flasks in Dulbecco’s modified Eagle’s medium and Ham’s F12 (DMEM/F12; 1:1 v/v) (Life Technologies, Carlsbad, CA), containing 100 μg/mL gentamycin and supplemented with 4 mg/mL dextrose anhydrous, 3.75 mg/mL HEPES buffer, 2.4 mg/mL sodium bicarbonate, and 10% FBS. After 24 h the medium was changed and the cells were grown in DMEM/F12 supplemented with insulin (5 μg/mL), human transferrin (50 μg/mL), sodium selenite (30 nM), d-biotin (10 mM), 0.1% bovine serum albumin (BSA), 1% FBS, and 1% horse serum. After 14 days, oligodendrocytes and microglia were removed from the mixed glial culture by the differential shaking and adhesion procedure [27] and the astrocytes were collected from the flasks by trypsinization. The cells were plated on 24-well plates containing coverslips coated with poly-D-lysine (105 cells per well). Cells were grown until confluent in defined culture media G5: DMEM/F12 supplemented with hydrocortisone (10 nM), sodium selenite (30 nM), insulin (5 μg/mL), human transferrin (50 μg/mL) d-biotin (10 ng/mL) bFGF (5 ng/mL), and EGF (10 ng/mL). Cells were treated with or without lipopolysaccharide (LPS; 1 μg/mL) in combination with 0, 1 μM, 10 μM, or 100 μM 2-BFI for 3 d. While no experiments had used 2-BFI in astrocyte cultures previously, similar concentrations were previously used in in vitro experiments with other cell types [28, 29]. Subsequently, cell culture supernatants were collected and snap frozen and stored at −80°C until analyzed. The cells were rinsed in PBS, fixed in 4% buffered paraformaldehyde for 20 min at room temperature (RT), and then stored under PBS at 4°C until analyzed.
2.6 Enzyme-linked immunosorbent assay (ELISA)
The release of TNF-α into the cell culture supernatant was measured using the Mouse TNF-α DuoSet ELISA kit (R&D Systems, Minneapolis, MN). The ELISA procedure was performed according to the manufacturer’s instructions and absorbance was measured at 450 nm with a subtraction of absorbance at 570 nm using a microplate reader (BioRad).
2.7 Immunocytochemistry
1 h following the final drug treatment and behavioral test, rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with ice cold PBS followed by 4% PFA in PBS (pH 7.4). The L4–5 segments of spinal cord were then harvested, post-fixed in the 4% PFA solution overnight at 4°C, and immersed in 30% sucrose in 0.1 M phosphate buffer for 48 h at 4°C for cryoprotection. Spinal cord sections were cut at 20 μm in a cryostat and then processed for immunofluorescence. The sections were blocked with 3% normal donkey serum (NDS; Jackson ImmunoResearch) for 2 h at RT, then incubated at 4°C overnight with gentle rocking with one of the following primary antibodies: Iba-1 antibody (rabbit, 1:500, Wako, Richmond, VA) or GFAP antibody (rabbit, 1:1000, Millipore, Billerica, MA). Following three 15 min rinses in PBS, the sections were incubated for 2 h at RT with gentle rocking with AlexaFluor 488-conjugated secondary antibody (donkey, 1:500, Jackson ImmunoResearch, West Grove, PA), then washed again in PBS. The stained sections were mounted on microscope slides with Vectashield HardSet Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA). Fluorescent images were captured with the 10× objective of an Olympus IX51 microscope and stitched together to create a montage using FIJI software.
Fixed mouse primary cortical astrocytes were stained with and antibody against GFAP and examined by fluorescence microscopy. Briefly, after rinsing in fresh PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 2 min at RT. The cells were then incubated in a blocking solution of 5% normal donkey serum (NDS) in PBS followed by overnight incubation at 4°C with anti-GFAP primary antibody (rabbit, 1:1000, Millipore, Billerica, MA). Cells were then incubated with secondary antibody conjugated with AlexaFluor 488 (1:500, Jackson Immunoresearch) for 2 h at RT. Coverslips were mounted onto microscope slides with Vectashield HardSet Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA). Fluorescent images were captured with the 10× objective of an Olympus IX51 microscope and stitched together to create a montage using FIJI software.
2.8 Data analysis
Data are expressed as mean ± SEM. Nociception data were analyzed by two-way mixed model ANOVA (day × treatment) followed by Bonferroni’s post-test. For the analysis of Iba-1 or GFAP immunoreactivity, the images of the spinal cord dorsal horn were captured, laminae I–IV were outlined, and a numerical value of the average pixel intensity was calculated with ImageJ. The background fluorescence intensity was subtracted within each section, and at least five sections per animal were used in the analysis. For the analysis of GFAP immunoreactivity in mouse cortical astrocytes, the captured pictures had backgrounds subtracted, and then five randomly selected frames were analyzed for the average pixel intensity with ImageJ. All images were analyzed under blinded conditions. Fluorescence intensity and concentration of TNF-α was analyzed with Student’s unpaired two-tailed t-test or one-way ANOVA followed by Bonferroni’s post-test. For all statistical analyses, p < 0.05 was considered statistically significant.
2.9 Drugs
2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI) was synthesized according to standard procedures [30], dissolved in normal saline, and administered i.p. in a volume of 1 mL/kg. The drug purity was confirmed by HPLC (> 98% purity). Depending on group assignment, rats received either two daily injections of saline or two daily injections of 10 mg/kg 2-BFI, with injections separated by at least six hours.
3. Results
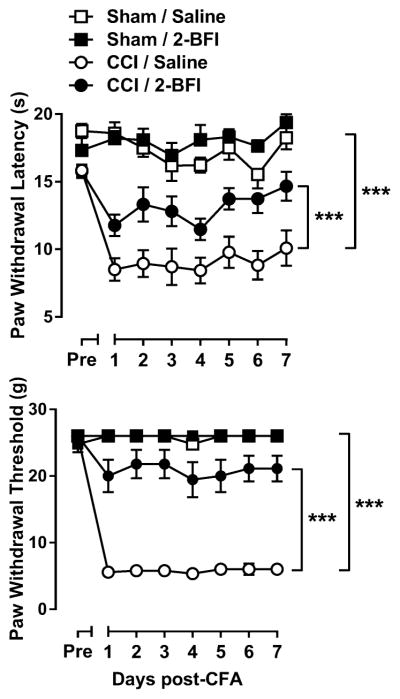
Prior to CCI surgery, the average PWL for all rats was 16.90 ± 0.36 s. According to two-way mixed-model ANOVA, with day as the within-subject factor, CCI surgery significantly affected PWL with significant day × CCI interaction (F(7, 112) = 3.26, p < 0.01), and significant main effects of both day (F(7, 112) = 8.31, p < 0.0001) and CCI surgery (F(1, 112) = 85.66, p < 0.0001). Daily treatment with 10 mg/kg 2-BFI significantly increased PWL in rats with CCI surgery as compared to saline with significant main effects of day (F(7, 112) = 9.38, p < 0.0001) and 2-BFI (F(1, 112) = 17.27, p < 0.001), but no significant day × 2-BFI interaction. 2-BFI treatment did not significantly alter PWL in sham rats (Fig. 1, left). Prior to CCI surgery, the average PWT for all rats was 25.7 ± 0.3 g. According to two-way mixed-model ANOVA, CCI surgery significantly affected PWT with significant day × CCI interaction (F(7, 112) = 139.61, p < 0.0001), and significant main effects of both day (F(7, 112) = 145.37, p < 0.0001) and CCI surgery (F(1, 112) = 946.69, p < 0.0001). Daily treatment with 2-BFI significantly increased PWT in rats with CCI surgery as compared to saline with a significant day × 2-BFI interaction (F(7, 112) = 6.26, p < 0.0001) and significant main effects of day (F(7, 112) = 17.98, p < 0.0001) and 2-BFI (F(1, 112) = 185.04, p < 0.0001). 2-BFI treatment did not significantly affect PWT in sham rats (Fig. 1, right).
Figure 1.
CCI-induced thermal (top) and mechanical (bottom) hyperalgesia and antinociceptive effects of twice-daily 10 mg/kg 2-BFI in rats (n = 9 rats per group). Ordinates: top, paw withdrawal latency (s), bottom, paw withdrawal threshold (g). Abscissas: days after CCI surgery. *** P < 0.0001 main effect of CCI surgery or 2-BFI.
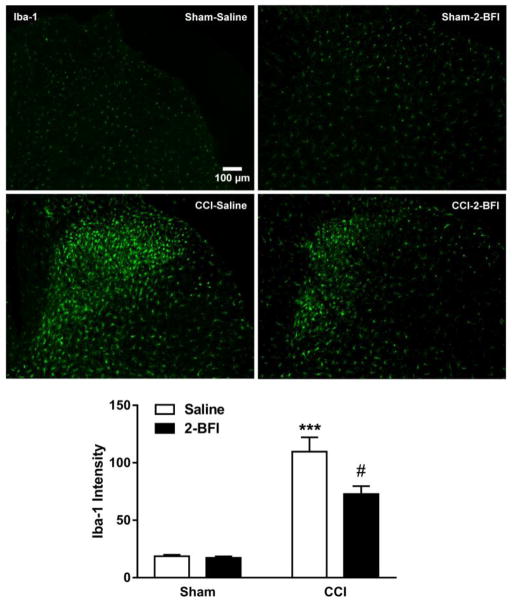
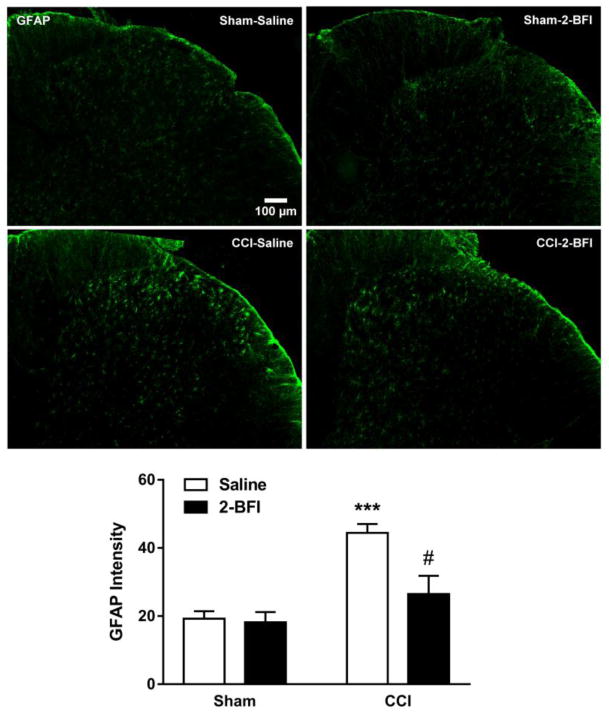
Next, Iba-1 and GFAP expression in the lumbar spinal cord were examined by immunofluorescence staining. Relative to the sham-saline group, Iba-1 average pixel intensity in the CCI-saline group was increased by 5.87-fold, significant according to t-test (t(8) = 7.22, p < 0.0001). Twice-daily treatment with 2-BFI significantly attenuated this increase in Iba-1 expression (t(8) = 2.58), p < 0.05). 2-BFI treatment did not alter Iba-1 expression in sham rats (Fig 2). Similarly, GFAP average pixel intensity in the CCI-saline group was increased by 2.31-fold relative to the sham-saline group, significant according to t-test (t(8) = 7.43, p < 0.0001). Twice-daily treatment with 2-BFI significantly attenuated this increase in GFAP expression (t(8) = 3.03), p < 0.05). 2-BFI treatment did not alter GFAP expression in sham rats (Fig. 3).
Figure 2.
Effects of 2-BFI on microglial activation in the dorsal horn of the lumbar spinal cord seven days following sham or CCI surgery (n = 5 rats per group). Images show representative Iba-1 immunofluorescence in sham-saline (top left), sham-2-BFI (top right), CCI-saline (bottom left), and CCI-2-BFI (bottom right) rats. Graph shows quantification of Iba-1 immunofluorescence (bottom). *** P < 0.0001 compared to sham-saline, # P < 0.05 compared to CCI-saline.
Figure 3.
Effects of 2-BFI on astrocytic activation in the dorsal horn of the lumbar spinal cord seven days following sham or CCI surgery (n = 5 rats per group). Images show representative GFAP immunofluorescence in sham-saline (top left), sham-2-BFI (top right), CCI-saline (bottom left), and CCI-2-BFI (bottom right) rats. Graph shows quantification of GFAP immunofluorescence (bottom). *** P < 0.0001 compared to sham-saline, # P < 0.05 compared to CCI-saline.
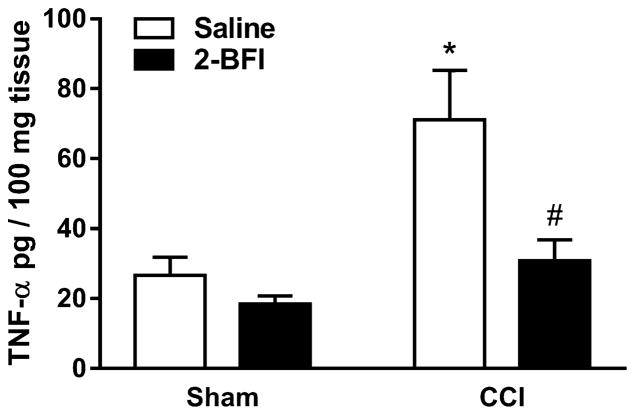
Spinal cord TNF-α levels were then analyzed via WEHI cell-based bioassay. TNF-α was increased by 2.67-fold in spinal cord homogenate from the CCI-saline group as compared to the sham-saline group (t(6) = 2.97, p < 0.05). Twice-daily treatment with 2-BFI significantly attenuated this increase in the CCI-2-BFI group (t(6) = 2.63, p < 0.05). 2-BFI treatment did not significantly alter the TNF-α level in sham rats (Fig. 4).
Figure 4.
Effects of 2-BFI on TNF-α level in the lumbar spinal cord seven days following sham or CCI surgery (n = 4 rats per group). * P < 0.05 compared to sham-saline, # P < 0.05 compared to CCI-saline.
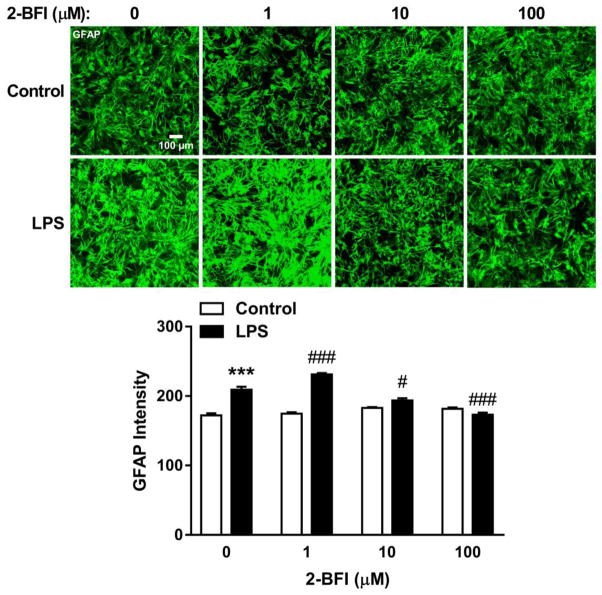
In mouse cortical astrocyte cultures, LPS stimulation significantly increased GFAP staining intensity relative to control cultures (t(8) = 5.99, p < 0.001). 2-BFI produced a significant main effect on GFAP intensity in LPS-stimulated cultures (one-way ANOVA: F(3, 16) = 56.41, p < 0.0001). Bonferroni’s post-test revealed that GFAP intensity in 1 μM 2-BFI-treated, LPS-stimulated cultures was increased as compared to LPS alone (p < 0.01), whereas GFAP intensity was decreased in 10 μM (p < 0.05) and 100 μM (p < 0.001) 2-BFI-treated, LPS-stimulated cultures. No significant effect was observed in cultures treated with 2-BFI alone (Fig 5). Relative to control cultures, stimulation with LPS increased supernatant concentrations of TNF-α by 17.92-fold (t(2) = 25.10, p < 0.01). 2-BFI produced a significant main effect on TNF-α secretion in LPS-stimulated cultures (one-way ANOVA: F(3, 4) = 24.24, p < 0.01). Bonferroni’s post-test revealed that TNF-α concentrations in the 1, 10, and 100 μM 2-BFI LPS-stimulated cultures were significantly reduced as compared to control LPS-stimulated cultures. No significant effect on TNF-α was found in cultures treated with 2-BFI alone (Fig. 6).
Figure 5.
Effects of 2-BFI on GFAP expression in primary mouse cortical astrocyte cultures. Images show representative GFAP immunofluorescence in control and LPS-treated cultures co-incubated with 0–100 μM 2-BFI (top). Graph shows quantification of GFAP immunofluorescence (bottom). *** P < 0.001 compared to control; # P < 0.05, ### P < 0.001 compared to LPS-0 μM 2-BFI.
Figure 6.
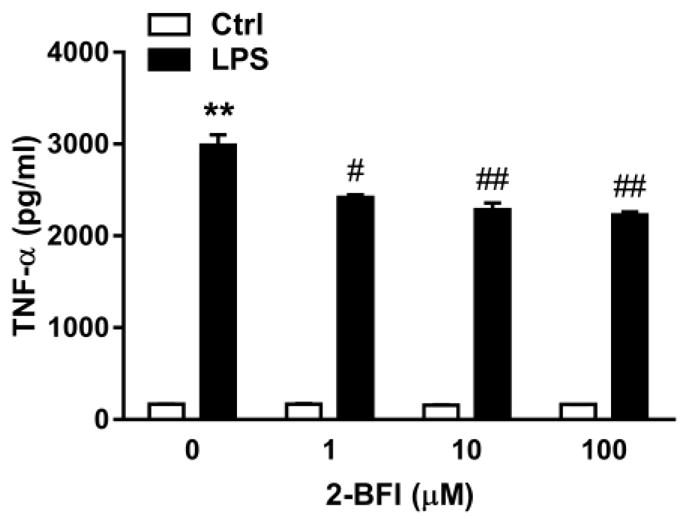
Effects of 2-BFI on supernatant TNF-α concentrations in control and LPS-treated mouse primary cortical astrocyte cultures. ** P < 0.01 compared to control; # P < 0.05, ## P < 0.01 compared to LPS-0 μM 2-BFI.
4. Discussion
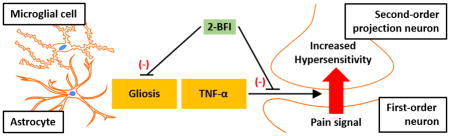
The primary findings of this study were that the I2R agonist 2-BFI produced consistent thermal and mechanical antinociception when administered twice daily over a period of seven days in a model of CCI-induced neuropathic pain. CCI rats treated with 2-BFI displayed significantly attenuated activation of spinal microglia and astrocytes as well as spinal cord levels of TNF-α as compared to saline-treated CCI rats. Likewise, in mouse primary cortical astrocyte cultures stimulated with LPS, 2-BFI significantly attenuated increases in GFAP expression and supernatant TNF-α concentration. These data are the first to suggest that I2R agonists may inhibit neuroinflammation by exerting effects directly on glial populations such as astrocytes, which may in turn contribute to the antinociceptive effects of these compounds.
CCI-induced neuropathic pain is a commonly-used and well-validated rodent model of chronic pain. CCI surgery induces persistent hypersensitivity of the ipsilateral hindpaw to a range of stimuli for over two months after surgery [21, 22], and as such is an appropriate model with which to study possible analgesics. As previously demonstrated, the prototypical I2R agonist 2-BFI produced consistent and acute antinociception over a course of seven days when administered to CCI rats [22], with no apparent development of tolerance despite twice-daily treatment. Studies using other I2R agonists have demonstrated that tolerance to the antinociceptive effects of these compounds develops remarkably slowly as compared to other analgesics such as opioids, and that they also attenuate the development of opioid tolerance and dependence [31, 32]. However, despite years of research, the mechanism of I2R agonists underlying antinociception remains elusive. MAO inhibition is the most often-proposed mechanism for I2Rs, but mechanisms other than increased synaptic monoamine concentrations may be equally if not more important to their antinociceptive effects. Previous studies showed that I2Rs exist on multiple proteins, some of which are yet unidentified, and are expressed either partially [33] or solely [14] by glial cells like astrocytes as opposed to neurons. This is further supported by increased I2R density in human brains with gliomas but not non-glial tumors [15, 34]. Functional relevance of the glial localization of I2Rs was demonstrated in vitro as an I2R ligand attenuated the induction of NOS-2, an inflammatory mediator, in stimulated rat primary astrocytes or RAW 264.7 macrophages [16]. In vivo, 2-BFI attenuated microglial activation, reduced levels of pro-inflammatory cytokines including TNF-α in blood and spinal cord, and improved symptom severity scores in EAE mice [17, 18]. These previous data suggest that I2R activation can attenuate glial reactivity and neuroinflammation in certain models. Given the bidirectional relationship between neuroinflammation and chronic pain, it was feasible that anti-neuroinflammatory action contributes to the antinociceptive effects of I2R agonists in chronic pain models. However, this possibility has not yet been tested.
We first examined whether twice-daily treatment with 2-BFI for 7 days altered glial activation in the lumbar spinal cord, a CNS region previously demonstrated to be important for hind paw pain hypersensitivity in rodents [8]. Relative to healthy control rats, CCI induced a significant increase in Iba-1 and GFAP expression, similar to results reported previously [35–38]. 2-BFI treatment did not alter the Iba-1 or GFAP expression in healthy rats, but significantly attenuated the increases in staining for both glial markers in CCI rats. While microglial and astrocytic activation were only partially attenuated to intermediate levels between CCI and control rats, this level of inhibition has been previously shown to be behaviorally relevant in producing antinociception [39]. Indeed, 10 mg/kg 2-BFI is not a maximally analgesic dose, thus it is possible that higher doses may have led to greater inhibition. Further studies are needed to discern the dose-dependence relationship of this effect. CCI also induced a significant increase in spinal TNF-α protein levels, a phenomenon that has also been described in rodent chronic pain models [7, 40, 41], and was significantly attenuated by 2-BFI treatment. These data suggest that repeated treatment with 2-BFI suppresses both gliosis as well as pro-inflammatory cytokine secretion which may underlie, at least in part, the antinociceptive effects of 2-BFI.
Due to interactions between cell types, it is difficult to determine the direct action of a pharmacological agent on a particular cell type in vivo. For instance, neurons alter GFAP levels in astrocytes [42] and release glutamate which induces astrocytic expression of the glutamate transporters GLAST and GLT-1, and induces Ca2+ oscillations in astrocytes [43, 44]. Thus, I2R-mediated attenuation of glial reactivity could have been secondary to a direct effect on neurons. To further elucidate this possibility, we performed in vitro experiments using mouse primary astrocyte cultures. Although mouse and rat astrocytes exhibit differences in morphology, differentiation, and functionality [45, 46], they display similar reactivity to stimuli such as LPS, which mimics in vivo neuroinflammation [45, 47, 48]. Similar to these and other previous reports [39], three-day incubation with LPS induced a significant increase in GFAP expression in the astrocyte cultures. 2-BFI alone did not alter GFAP expression, but co-incubation of 2-BFI in LPS-stimulated cultures dose-dependently attenuated the increase in GFAP. Parallel analysis of astrocyte culture supernatants revealed that 2-BFI also attenuated LPS-induced TNF-α secretion. While these results cannot completely rule out the possibility that I2R activation on other cell types may contribute to the inhibitory effect on neuroinflammation, they do indicate astrocytes as one direct site of action.
Although previous literature had suggested a role of I2Rs in modulating glial reactivity, a lack of molecular information on I2Rs makes the mechanistic basis of the effects reported in the current study difficult to propose. Interestingly, several antidepressant monoaminergic drugs, including reversible MAO inhibitors, have been demonstrated to produce antinociception in vivo [49–52] as well as anti-neuroinflammatory effects in vivo and in LPS-stimulated glial cultures [53–57]; since I2Rs modulate MAO activity, monoaminergic mechanisms may explain the present results. While the mechanisms underlying the anti-neuroinflammatory effects of antidepressant drugs are also unclear, their suppression of glial activation and cytokine release may be a consequence of binding to 5-HT and NE transporters which are expressed by astrocytes [58, 59], or to monoaminergic receptors; microglia and astrocytes express several adrenergic receptor subtypes, and astrocytes additionally express several serotonin receptor subtypes [60, 61]. Yet again, direct activation of these receptors also reduces cytokine release [62, 63] so it is difficult to discern whether the driving factor to reduce neuroinflammation is increased monoamine concentrations or a mechanistically distinct direct effect on glia by antidepressants. I2R activation on currently unknown proteins could also be involved in these effects. Whatever the mechanistic explanation may be, it seems clear that antidepressant drugs have anti-neuroinflammatory effects which are at least partially exerted directly on glia to produce antinociception, and the data from the current study suggest that the same may be true for I2R agonists. While spinal infiltrating macrophages as opposed to glia were shown to mediate chronic pain in female mice [37], I2R agonists may also suppress inflammatory activation of these cells as reported previously in vitro to account for the efficacy of these drugs in female animals [16, 23]. Should the anti-neuroinflamamatory effects of I2R agonists be further established, they may be found to account for some other beneficial I2R-associated effects such as reduced tolerance to opioids [31], as opioid tolerance may result from increased neuroinflammation following repeated opioid exposure [64, 65]. However, further information regarding the distribution and molecular roles of I2Rs is ultimately needed to explain their mechanisms of action on glia.
In summary, this is the first study to report that repeated treatment with an I2R agonist reduces glial reactivity and pro-inflammatory cytokine secretion in a rodent model of chronic pain. Similar results were found in an in vitro model of neuroinflammation in cultured mouse astrocytes, suggesting a primary site of I2R action on astrocytes. These findings provide evidence that I2R agonists reduce neuroinflammation, which may underlie their antinociceptive effects.
Acknowledgments
Funding
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806) and by a grant from the National Natural Science Foundation of China (81373390). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 2-BFI
2-(2-benzofuranyl)-2-imidazoline
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- CCI
chronic constriction injury
- EAE
experimental autoimmune encephalomyelitis
- ELISA
enzyme-linked immunosorbent assay
- GABA
γ-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- Iba-1
ionized calcium-binding adapter molecule 1
- IL-1β
interleukin 1β
- LPS
lipopolysaccharide
- MAO
monoamine oxidase
- NMDA
N-methyl-D-aspartate
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- TNF-α
tumor necrosis factor α
Footnotes
Disclosure
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIH. Pain in America. 2013. [Google Scholar]
- 2.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depression and anxiety. 2009;26(10):888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 3.Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Progress in neurobiology. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesthesia and analgesia. 2010;110(3):780–9. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- 5.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. The Lancet. 2011;377(9784):2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 6.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nature reviews. Immunology. 2014;14(4):217–31. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20(2):467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 9.Martinez V, Szekely B, Lemarie J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau de Loubresse C, Chauvin M, Bouhassira D, Fletcher D. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain. 2013;154(8):1197–203. doi: 10.1016/j.pain.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Sumitani M, Ueda H, Hozumi J, Inoue R, Kogure T, Yamada Y, Kogure T. Minocycline Does Not Decrease Intensity of Neuropathic Pain Intensity, But Does Improve Its Affective Dimension. Journal of pain & palliative care pharmacotherapy. 2016;30(1):31–5. doi: 10.3109/15360288.2014.1003674. [DOI] [PubMed] [Google Scholar]
- 11.Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, Heylen R, Roubos E, Vissers K. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 2015;122(2):399–406. doi: 10.1097/ALN.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 12.Jones TZ, Giurato L, Guccione S, Ramsay RR. Interactions of imidazoline ligands with the active site of purified monoamine oxidase A. The FEBS journal. 2007;274(6):1567–75. doi: 10.1111/j.1742-4658.2007.05704.x. [DOI] [PubMed] [Google Scholar]
- 13.McDonald GR, Olivieri A, Ramsay RR, Holt A. On the formation and nature of the imidazoline I2 binding site on human monoamine oxidase-B. Pharmacological research. 2010;62(6):475–88. doi: 10.1016/j.phrs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Regunathan S, Feinstein DL, Reis DJ. Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J Neurosci Res. 1993;34(6):681–8. doi: 10.1002/jnr.490340611. [DOI] [PubMed] [Google Scholar]
- 15.Callado LF, Martin-Gomez JI, Ruiz J, Garibi JM, Meana JJ. Imidazoline I(2) receptor density increases with the malignancy of human gliomas. Journal of neurology, neurosurgery, and psychiatry. 2004;75(5):785–7. doi: 10.1136/jnnp.2003.020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinstein DL, Reis DJ, Regunathan S. Inhibition of astroglial nitric oxide synthase type 2 expression by idazoxan. Molecular pharmacology. 1999;55(2):304–8. doi: 10.1124/mol.55.2.304. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Zhang ZX, Liu YF, Xu HQ, Hou ST, Zheng RY. 2-BFI ameliorates EAE-induced mouse spinal cord damage: effective therapeutic time window and possible mechanisms. Brain research. 2012;1483:13–9. doi: 10.1016/j.brainres.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YB, Xia NG, Zhang YT, Wang XS, Liang SS, Yin WY, Xu HQ, Hou ST, Zheng RY. Brain protection conferred by long-term administration of 2-(2-benzofuranyl)-2-imidazoline against experimental autoimmune encephalomyelitis. Neurochemical research. 2015;40(3):572–8. doi: 10.1007/s11064-014-1502-0. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 20.Guide for the Care and Use of Laboratory Animals. National Academy of Sciences; Washington DC: 2011. [Google Scholar]
- 21.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 22.Li J-X, Thorn DA, Qiu Y, Peng B-W, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Brit J Pharmacol. 2014;171(6):1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemian JN, Obeng S, Zhang Y, Zhang Y, Li JX. Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the mu-Opioid Receptor. The Journal of pharmacology and experimental therapeutics. 2016;357(3):509–19. doi: 10.1124/jpet.116.232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khabar KS, Siddiqui S, Armstrong JA. WEHI-13VAR: a stable and sensitive variant of WEHI 164 clone 13 fibrosarcoma for tumor necrosis factor bioassay. Immunology letters. 1995;46(1–2):107–10. doi: 10.1016/0165-2478(95)00026-2. [DOI] [PubMed] [Google Scholar]
- 25.Ignatowski TA, Spengler RN. Tumor necrosis factor-alpha: presynaptic sensitivity is modified after antidepressant drug administration. Brain research. 1994;665(2):293–9. doi: 10.1016/0006-8993(94)91350-1. [DOI] [PubMed] [Google Scholar]
- 26.Amur-Umarjee S, Phan T, Campagnoni AT. Myelin basic protein mRNA translocation in oligodendrocytes is inhibited by astrocytes in vitro. J Neurosci Res. 1993;36(1):99–110. doi: 10.1002/jnr.490360111. [DOI] [PubMed] [Google Scholar]
- 27.Suzumura A, Bhat S, Eccleston PA, Lisak RP, Silberberg DH. The isolation and long-term culture of oligodendrocytes from newborn mouse brain. Brain research. 1984;324(2):379–83. doi: 10.1016/0006-8993(84)90054-4. [DOI] [PubMed] [Google Scholar]
- 28.Han Z, Yang JL, Jiang SX, Hou ST, Zheng RY. Fast non-competitive and reversible inhibition of NMDA-activated currents by 2-BFI confers neuroprotection. PloS one. 2013;8(5):e64894. doi: 10.1371/journal.pone.0064894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugedo L, Pineda J, Ruiz-Ortega JA, Martin-Ruiz R. Stimulation of locus coeruleus neurons by non-I1/I2-type imidazoline receptors: an in vivo and in vitro electrophysiological study. Br J Pharmacol. 1998;125(8):1685–94. doi: 10.1038/sj.bjp.0702255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63(6):1474–1480. [Google Scholar]
- 31.Thorn DA, Zhang Y, Li JX. Tolerance and cross-tolerance to the antinociceptive effects of oxycodone and the imidazoline I2 receptor agonist phenyzoline in adult male rats. Psychopharmacology. 2017;234(12):1871–1880. doi: 10.1007/s00213-017-4599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorn DA, Zhang Y, Li JX. Effects of the imidazoline I receptor agonist 2-BFI on the development of tolerance and behavioral/physical dependence to morphine in rats. Br J Pharmacol. 2016;173(8):1363–72. doi: 10.1111/bph.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller B, Garcia-Sevilla JA. Immunodetection and subcellular distribution of imidazoline receptor proteins with three antibodies in mouse and human brains: Effects of treatments with I1- and I2-imidazoline drugs. Journal of psychopharmacology (Oxford, England) 2015;29(9):996–1012. doi: 10.1177/0269881115586936. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Gomez JI, Ruiz J, Callado LF, Garibi JM, Aguinaco L, Barturen F, Javier Meana J. Increased density of I2-imidazoline receptors in human glioblastomas. Neuroreport. 1996;7(8):1393–6. doi: 10.1097/00001756-199605310-00013. [DOI] [PubMed] [Google Scholar]
- 35.Popiolek-Barczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM, Pilat D, Mika J. Parthenolide Relieves Pain and Promotes M2 Microglia/Macrophage Polarization in Rat Model of Neuropathy. Neural plasticity. 2015;2015:676473. doi: 10.1155/2015/676473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz J, Abele A, Marian C, Haussler A, Herbert TA, Woolf CJ, Tegeder I. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain. 2008;138(1):130–42. doi: 10.1016/j.pain.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience. 2015;18(8):1081–3. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain research. 1991;565(1):1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- 39.Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Scientific reports. 2015;5:10278. doi: 10.1038/srep10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA. Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain. 2015;156(7):1320–33. doi: 10.1097/j.pain.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bianchi M, Martucci C, Ferrario P, Franchi S, Sacerdote P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugs. Anesthesia and analgesia. 2007;104(4):949–54. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- 42.Kremsky I, Morgan TE, Hou X, Li L, Finch CE. Age-changes in gene expression in primary mixed glia cultures from young vs. old rat cerebral cortex are modified by interactions with neurons. Brain, behavior, and immunity. 2012;26(5):797–802. doi: 10.1016/j.bbi.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, Pietrini G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. Journal of neurochemistry. 2000;75(3):1076–84. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- 44.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(20):7817–30. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarassishin L, Suh HS, Lee SC. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. 2014;62(6):999–1013. doi: 10.1002/glia.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puschmann TB, Dixon KJ, Turnley AM. Species differences in reactivity of mouse and rat astrocytes in vitro. Neuro-Signals. 2010;18(3):152–63. doi: 10.1159/000321494. [DOI] [PubMed] [Google Scholar]
- 47.Cheli VT, Santiago Gonzalez DA, Smith J, Spreuer V, Murphy GG, Paez PM. L-type voltage-operated calcium channels contribute to astrocyte activation In vitro. Glia. 2016;64(8):1396–415. doi: 10.1002/glia.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souza DG, Bellaver B, Souza DO, Quincozes-Santos A. Characterization of adult rat astrocyte cultures. PloS one. 2013;8(3):e60282. doi: 10.1371/journal.pone.0060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. The Journal of pharmacology and experimental therapeutics. 2004;311(2):576–84. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka N, Kiuchi Y, Yokogawa F, Masuda Y, Oguchi K, Hosoyamada A. Antinociceptive efficacy of antidepressants: assessment of five antidepressants and four monoamine receptors in rats. Journal of anesthesia. 2001;15(3):154–8. doi: 10.1007/s005400170018. [DOI] [PubMed] [Google Scholar]
- 51.Mika J, Zychowska M, Makuch W, Rojewska E, Przewlocka B. Neuronal and immunological basis of action of antidepressants in chronic pain - clinical and experimental studies. Pharmacological reports: PR. 2013;65(6):1611–21. doi: 10.1016/s1734-1140(13)71522-6. [DOI] [PubMed] [Google Scholar]
- 52.Villarinho JG, de Pinheiro KV, de Pinheiro FV, Oliveira SM, Machado P, Martins MA, Bonacorso HG, Zanatta N, Fachinetto R, Ferreira J. The antinociceptive effect of reversible monoamine oxidase-A inhibitors in a mouse neuropathic pain model. Progress in neuro-psychopharmacology & biological psychiatry. 2013;44:136–42. doi: 10.1016/j.pnpbp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Vollmar P, Haghikia A, Dermietzel R, Faustmann PM. Venlafaxine exhibits an anti-inflammatory effect in an inflammatory co-culture model. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008;11(1):111–7. doi: 10.1017/S1461145707007729. [DOI] [PubMed] [Google Scholar]
- 54.Obuchowicz E, Kowalski J, Labuzek K, Krysiak R, Pendzich J, Herman ZS. Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2006;9(1):27–35. doi: 10.1017/S146114570500547X. [DOI] [PubMed] [Google Scholar]
- 55.Bielecka AM, Paul-Samojedny M, Obuchowicz E. Moclobemide exerts anti-inflammatory effect in lipopolysaccharide-activated primary mixed glial cell culture. Naunyn-Schmiedeberg’s archives of pharmacology. 2010;382(5–6):409–17. doi: 10.1007/s00210-010-0535-4. [DOI] [PubMed] [Google Scholar]
- 56.Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain, behavior, and immunity. 2012;26(3):469–79. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Zychowska M, Rojewska E, Makuch W, Przewlocka B, Mika J. The influence of microglia activation on the efficacy of amitriptyline, doxepin, milnacipran, venlafaxine and fluoxetine in a rat model of neuropathic pain. European Journal of Pharmacology. 2015;749(Supplement C):115–123. doi: 10.1016/j.ejphar.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. Journal of neurochemistry. 2003;84(1):136–44. doi: 10.1046/j.1471-4159.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- 59.Inazu M, Takeda H, Ikoshi H, Sugisawa M, Uchida Y, Matsumiya T. Pharmacological characterization and visualization of the glial serotonin transporter. Neurochemistry international. 2001;39(1):39–49. doi: 10.1016/s0197-0186(01)00010-9. [DOI] [PubMed] [Google Scholar]
- 60.Hertz L, Schousboe I, Hertz L, Schousboe A. Receptor expression in primary cultures of neurons or astrocytes. Progress in neuro-psychopharmacology & biological psychiatry. 1984;8(4–6):521–7. doi: 10.1016/0278-5846(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 61.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends in neurosciences. 2007;30(10):527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Idzko M, Panther E, Stratz C, Muller T, Bayer H, Zissel G, Durk T, Sorichter S, Di Virgilio F, Geissler M, Fiebich B, Herouy Y, Elsner P, Norgauer J, Ferrari D. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172(10):6011–9. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 63.Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43(6):1026–34. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 64.Horvath RJ, Romero-Sandoval EA, De Leo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150(3):401–13. doi: 10.1016/j.pain.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(22):9980–9. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]