Abstract
The mammalian heart is the first organ to form and is critical for embryonic survival and development. With an occurrence of 1%, congenital heart defects (CHDs) are also the most common birth defects in humans, and major cause of childhood morbidity and mortality [1, 2]. Understanding how the heart forms will not only help to determine the etiology and to design diagnostic and therapeutic approaches for CHDs, but may also provide insight into regenerative medicine to repair injured adult hearts. Mammalian heart development requires precise orchestration of growth, differentiation and morphogenesis to remodel a simple linear heart tube into an intricate, four-chambered heart with properly connected pulmonary artery and aorta, a structural basis for establishing the pulmonary and systemic circulation. Here we will review the recent advance in our understanding of how the planar cell polarity pathway, a highly conserved morphogenetic engine in vertebrates, regulates polarized morphogenetic processes to contribute to both the arterial and venous pole development of the heart.
Brief overview of early mammalian heart development
The heart arises from cardiac progenitor cells situated along the anterior lateral plate mesoderm. In the mouse, they are amongst the earliest mesodermal progenitors to exit the primitive streak during gastrulation, and traverse anterior-laterally to reach their final position in the splanchnic mesoderm below the neural headfolds. By embryonic day (E) 7.5, they form the cardiac crescent, a “crescent shaped epithelium” located cranially and cranio-laterally at the ventral part of the embryo [3].
Lineage studies showed that at this early stage, the cardiac progenitors are arranged into two juxtaposed cohorts, known as the First Heart Field (FHF) and the Second Heart Field (SHF). Together, they generate most of the cardiac structures and cell types. The FHF and SHF originate from a contiguous population of mesodermal progenitors, but differ in the timing at which their contribute to the heart. The FHF reflects the first wave of mesodermal cells that undergo myocardial differentiation in the crescent, and coalesce at the midline to form a linear, beating heart tube by E8.25. This initial heart tube will eventually give rise to the left ventricle and a portion of the atria. Conversely, the SHF cells, positioned dorso-medially to the FHF, remain as rapidly proliferating progenitors in the pharyngeal and splanchnic mesoderm (SpM). The SHF cells undergo gradual differentiation, and then deploy to the heart tube to form the right ventricle and the outflow tract (OFT) at the arterial pole, and the atria and dorsal mesenchymal protrusion (DMP) at the venous pole [4–11]. The two-heart field concept of cardiogenesis provides the crucial foundation for our current understanding of heart development.
The OFT is initially a single conduit linking the right ventricle and aortic sac, and is septated later to give rise to the aorta and pulmonary artery that connect with the left and right ventricles, respectively. From E8.5 to E9.5 in the mouse, the OFT undergoes rapid elongation, leading to rightward looping of the heart. As a result, the OFT also acquires a characteristic rightward curvature. Sufficient elongation of the OFT is critical for appropriate cardiac looping to re-position the OFT above the interventricular septum, between the left and the right ventricles. Subsequently, the OFT is invaded by cardiac neural crest (CNC) cells that arise from the dorsal neural tube, and migrate through the pharyngeal arches to reach the OFT. The CNC cells, along with the endocardium in the OFT, form the OFT cushion that spirals around to give rise to the aorticopulmonary septum (APS). The formation of the APS converts the single OFT vessel into the ascending aorta and pulmonary artery. The proper alignment of the OFT with the ventricles at early stage is important to ensure that upon septation, the aorta and pulmonary artery can be connected properly with the left and right ventricles to establish systemic and pulmonary circulatory systems, respectively.
Given the complexity of the morphogenetic processes involved in OFT formation, it is not surprising that conotruncal anomalies are the most common CHDs in humans. OFT defects can manifest in various forms. Mis-alignment of the OFT can lead to double outlet right ventricle (DORV), overriding aorta or transposition of the great arteries, whereas OFT septation defect can cause persistent truncus arteriosus (PTA)/common arterial trunk (CAT). Identifying the developmental mechanisms of OFT formation in model organisms is the critical first step to define the etiology and to develop early detection, prevention and therapies for these common, devastating CHD in humans.
Planar cell polarity signaling: a conserved morphogenetic engine in vertebrates
Planar cell polarity (PCP) refers to the cellular polarity on the plane of epithelium, perpendicular to the apico-basal polarity of the cell [12–16]. First discovered in Drosophila, PCP can manifest in various forms in different tissue, such as uniformly oriented trichomes on the fly wing and bristles on the abdomen, and coordinated rotation and chirality of the ommatidia in the fly eye. In mammals, PCP can also be observed as coordinated orientation of nodal cilia in the node, stereocilia in the inner ear, and hair follicles on the skin [17–21].
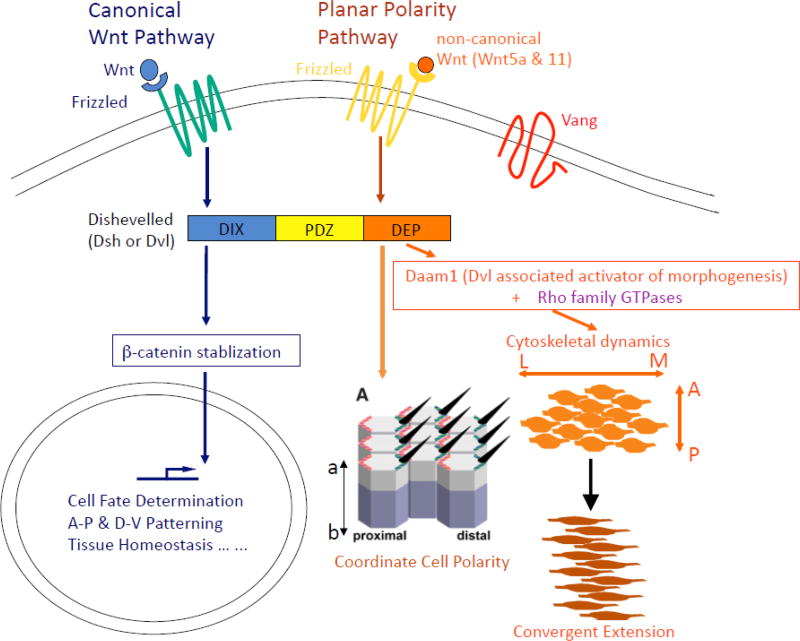
Genetic studies in flies have identified a group of six core proteins that are required for proper PCP establishment in all tissues. These include Dishevelled (Dsh/Dvl), Frizzled (Fz), Flamingo (Fmi), Diego (Dgo), Van Gogh (Vang) and Prickle (Pk). Dsh and Fz are also well-known members of the canonical Wnt pathway, where binding of the secreted ligand Wnt to the Fz receptor and co-receptors Arrow/Lrp5/6 triggers Dsh to stablize the β-catenin protein to regulate gene transcription [22–25]. However, in the PCP pathway, Dsh and Fz interact with PCP-specific core proteins, and act in a β-catenin independent manner to coordinate cell polarity (Figure 1). As such, the PCP pathway has also been referred to as a branch of the β-catenin independent non-canonical Wnt signaling pathway.
Figure 1. The canonical Wnt and the planar cell polarity pathway.
Dishevelled (Dsh/Dvl) and Frizzled (Fz) and important components of both the canonical Wnt and the PCP pathway. In the Wnt pathway, binding of Wnt to Fz and it co-receptors Arrow/Lrp5/6 promotes Dsh/Dvl to stablize β-catenin, which then enters the nucleus to regulate gene transcription. In the PCP pathway, however, Dsh/Dvl and Fz interact with PCP-specific core proteins, such as Van Gogh (Vang), and act in a β-catenin independent manner to coordinate cell polarity or regulate directional cell behavior during convergent extension (CE) tissue morphogenesis.
Later studies in Xenopus and zebrafish indicate that the PCP pathway also regulates a polarized tissue morphogenesis process known as convergent extension (CE) [26–29]. CE was first described in gastrulating Xenopus embryos as simultaneous lengthening of the antero-posterior (A-P) axis and narrowing of the medio-lateral (M-L) axis of the mesodermal tissues. Further studies in Xenopus explants indicated that the force for CE is generated through medio-laterally oriented cell intercalation [16, 28]. More recent studies in zebrafish revealed that CE can also occur through directional migration and oriented cell division [26, 27, 30]. Interestingly, vertebrate orthologs of the fly core PCP genes are all required for CE, suggesting that a common ancestral mechanism may be evolved to coordinate both cell polarity among static epithelial cells and dynamic, polarized cell behavior in mesodermal cells undergoing CE [13, 16, 27, 28, 31–34]. In mice, disruption of PCP signaling can abolish various CE-like morphogenesis processes in the neural plate, limb buds, cochlea tube, stomach and intestine [18, 35–48].
There are, however, apparent distinctions of PCP signaling in epithelial PCP vs. CE. During CE, PCP signaling acts through an effector Daam1 (Dishevelled-associated activator of morphogenesis 1) [49, 50] and small GTPases Rho/Rac/Cdc42 to modulate cytoskeletal organization and dynamics [51, 52]. In epithelial PCP, however, Daam1 is clearly not required [53]. Therefore, it appears that the distinct output of PCP signaling in these two processes may involve different downstream effectors. Secondly, PCP signaling activation during CE requires non-canonical Wnts, such as Wnt5a and Wnt11, in frogs and zebrafish [26, 54, 55], but there are conflicting evidences regarding the requirement of Wnt ligands for epithelial PCP [56, 57]. Finally, a hallmark of epithelial PCP is asymmetric plasma membrane localization of core PCP proteins. This has led to a feedback competition model to explain how intracellular antagonism between Vang/Pk and Fz/Dsh, coupled with intercellular interaction between Vang and Fz, results in segregation of Vang/Pk and Fz/Dsh complexes on opposing cell cortices to coordinate and propagate cell polarity in epithelium [56, 58–60]. During CE, however, asymmetric distribution of PCP proteins has not been consistently observed [32]. And while Vang is thought to antagonize Fz/Dsh in epithelial PCP, prior genetic studies suggested that its vertebrate ortholog Vangl2 (Vang like 2) interacts in a synergistic manner with Wnt5a/Fz/Dvl during CE [37, 40, 45, 61].
Using mouse genetics and Xenopus models, Seo et. al. have recently re-investigate the mode of functional interaction between Vangl2 and Dvl, and found that Vangl2 exerts dual positive and negative regulation on Dvl during CE [62]. Biochemical and imaging studies showed that Vangl2 binds to Dvl to cell-autonomously promote efficient Dvl plasma membrane recruitment, which is required for PCP activation. Simultaneously, Vangl2 inhibits Dvl from interacting with its downstream effector Daam1, and functionally suppresses Dvl→Daam1 cascade during CE. Based on these findings, a novel model was proposed to explain how direct interaction between Vangl2 and Dvl may act as a key bi-functional switch that underlies the central logic of PCP. In this parsimonious model, interaction with Vangl2 recruits Dvl to the plasma membrane but maintains Dvl in an inactive state. At the same time, Vangl2 also helps to spatially enrich and poise Dvl at the plasma membrane for efficient activation, likely by Fz upon the presence of non-canonical Wnt ligands. In support of this model, Wnt11 is able to release Dvl binding with Vangl2 [62]. Future studies on how other PCP components may modulate Vangl2-Dvl interaction affinity and dynamics will help to establish a conceptual framework for the molecular mechanism of PCP signaling during CE.
PCP signaling in mammalian OFT development
Prior mouse genetic studies have revealed that the PCP pathway is critical for OFT formation in mammals. Loss of the presumptive PCP ligands Wnt5a and Wnt11 results in OFT malformation primarily in the form of PTA [63], and DORV or TGA [64, 65], respectively. Similarly, mutations of core PCP genes, including Fz1, 2, 7; Dvl1, 2, 3; and Vangl2 cause various OFT defects ranging from DORV, TGA to PTA [37, 61, 66–69].
We and others have been using the mouse Dvl mutants to understand how PCP signaling may contribute to mammalian OFT development. Of the 3 Dvl orthologs in mammals, loss of Dvl1 does not cause any heart defect, while loss of Dvl2 and Dvl3 cause OFT defects with ~50% and 100% penetrance, respectively [37, 66]. In Dvl1−/−; Dvl2−/− double mutants, the penetrance of OFT defects is increased to 100% [66, 67]. Conversely, over-expressing Dvl1 or Dvl2 using BAC (bacterial artificial chromosome) transgenes in Dvl3−/− mutant was able to rescue the OFT defects [37]. Therefore, it appears that for OFT formation, the three Dvl genes function largely in a redundant fashion, and that a threshold level of Dvl proteins is required. The different penetrance of OFT defects in each Dvl single mutant may be due to different expression level/pattern of each Dvl gene during OFT formation.
Given that Dsh/Dvl can function in both the canonical Wnt and the PCP pathways, a structure-function analysis was performed to determine which pathway disruption contributes to the fully penetrant OFT defects in Dvl1−/−; Dvl2−/− double mutant. It is known that the N-terminal DIX domain of Dvl is required only for Wnt, whereas the C-terminal DEP (Dishevelled, Egl-10 and Pleckstrin) domain is required only for PCP signaling [31, 70, 71]. Furthermore, a point mutation identified in flies, known as dsh1, results in a K to M substitution at aa446 in the DEP domain and specifically abolishes PCP signaling but leaves Wnt signaling intact [72, 73]. By testing deletion mutants of these two domains and the dsh1 mutant, the OFT defects in Dvl1−/−; Dvl2−/− double mutant were found to arise solely from disruption of the PCP, but not the canonical Wnt, pathway [67].
Since both the CNC and SHF lineages contribute to OFT formation, tissue-specific gene ablation was performed using a conditional Dvl2 BAC transgene, together with CNC-specific Wnt1-Cre and SHF-specific Islet1 (Isl1)-Cre. The results clearly indicated that Dvl1/2-mediated PCP activity is critically required only in the SHF, but not the CNC, for proper OFT formation. This finding is consistent with a more recent study by Ramsbottom et. al., showing that core PCP gene Vangl2 is also required only in the SHF, but not the CNC, lineage for OFT formation [74]. Finally, given that canonical Wnt signaling is also required in the SHF for OFT development [75–78], these results imply that in Dvl1−/−; Dvl2−/− mutants, the remaining Dvl3 is sufficient to mediate canonical Wnt but not PCP signaling in the SHF.
Requirement of PCP signaling for OFT lengthening and cardiac looping
A number of studies have now attributed the OFT malformation in PCP mutants to an early OFT lengthening defects that results in aberrant cardiac looping and OFT alignment. As described above, the OFT undergoes rapid lengthening from E8.5 to E9.5 in the mouse, resulting in a rightward looping of the heart and alignment of the OFT above the midline of the right and left ventricles. This alignment ensures that upon OFT septation, the aorta can be connected properly to the left ventricle. In Dvl1/2, Vangl2, Wnt5a and Wnt11 mutants, OFT shortening and aberrant cardiac looping was observed by E9.5, and by E11.5, the OFT is found to be aligned with only the right ventricle [65, 67, 69, 74, 79]. This alignment defect will affect OFT remodeling and cause a spectrum of anomalies such as DORV, TGA and over-riding aorta in the PCP mutants. Consistent with the genetic evidence that PCP signaling is required in the SHF for OFT formation, conditional deletion of Dvl, Vangl2 and Wnt11 in the SHF is sufficient to cause OFT shortening, cardiac looping and OFT alignment defects [65, 67, 74].
Current models for PCP signaling in OFT lengthening
Currently, there are two models to explain how the OFT shortening defects arise in PCP mutants. It is known that the myocardium in the early heart tube undergoes a temporary proliferation arrest, and that elongation of the OFT is driven largely by addition of cells from the SHF [7, 80]. Both models agree on that OFT shortening in PCP mutants originates from aberrant addition of SHF cells to the OFT, but differ in the specific location where the defect occurs and the cell behavior that causes the defect.
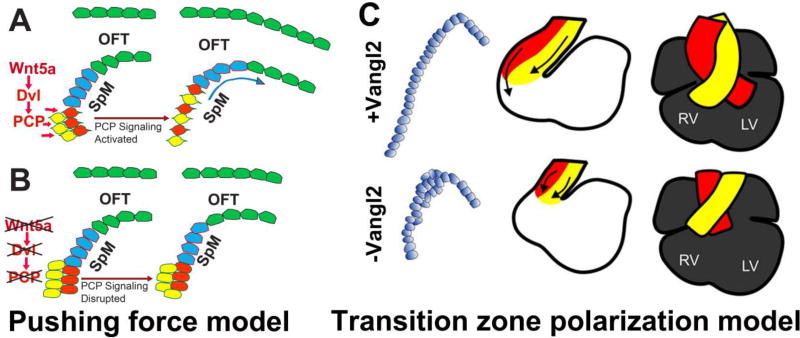
The first model, known as the “pushing force” model, argues that Wnt5a activates PCP signaling to promote CE-like oriented cell intercalation in the caudal SpM region of the SHF to generate a morphogenetic force that “push” SHF cell rostrally into the OFT (Figure 2A&B). This model is based on studies of Dvl1/2 and Wnt5a, in which Wnt5a mRNA and protein are found only in SHF cells in the caudal SpM surrounding the inflow tract, but devoid from the rostral SpM and the adjacent distal OFT [67, 79, 81]. Also, Dvl2 is expressed at much higher level in the SpM than in the OFT [67]. In wild-type E9.5 mouse embryos, SHF cells are largely organized into a pseudo-stratified epithelium in the rostral SpM, contiguous with the OFT myocardium. In the caudal SpM, however, large number of loosely-packed mesenchymal-like SHF cells are found behind the epithelial-like sheet. Immunostaining revealed that ZO1, a tight junction protein, is localized specifically at the apical cell-cell junction in SpM-SHF cells that have organized into an epithelial sheet. In the loosely-packed mesenchymal-like SHF cells in the caudal SpM, however, ZO1 expression is not found [67, 74, 79, 82, 83]. These data suggest that SHF cells may undergo a mesenchymal-to-epithelial transition (MET) at the caudal SpM and subsequently up-regulate ZO1 expression, and are deployed to the OFT as an epithelial sheet.
Figure 2. Current models on PCP signaling in OFT morphogenesis.
(A) The “pushing force” model argues that Wnt5a activates Dvl-mediated PCP signaling to promote CE-like oriented cell intercalation in the caudal SpM region of the SHF to generate a morphogenetic force that “push” SHF cell rostrally into the OFT. (B) Disruption of this morphogenetic force in Wnt5a and Dvl1/2 mutants reduced SHF cell deployment, leading to OFT shortening and subsequent morphogenesis defects. (C) The “transition zone polarization” model argues that PCP signaling maintains proper epithelial polarization and organization in SHF-derived cells prior to their terminal differentiation into myocardium at the distal region of the OFT. Disruption of Vangl2 perturbs polarization and epithelial organization of cells in the transition zone. These defects in turn cause OFT thickening at the expense of lengthening, and consequently, OFT shortening and mis-alignment with the ventricles.
Interestingly, in Dvl1/2 and Wnt5a null mutants, the mesenchymal-like SHF cells in the caudal SpM are aggregated into aberrant compact cluster. They lose the highly protrusive morphology normally observed in the wild-type, and display diminished actin polymerization. Moreover, whereas wild-type SHF cells are preferentially oriented along the dorso-ventral axis, perpendicular to the epithelial sheet, Wnt5a mutant cells display more random orientation [79]. Taken together, the data suggest that a Wnt5a→ Dvl PCP signaling cascade may regulate cytoskeletal dynamics, cell polarity and protrusive cell behavior to promote CE-like oriented cell intercalation that drive MET in the caudal SpM. The MET process continuously incorporates loosely-pack mesenchymal-like SHF cells into an epithelial-like sheet, providing the morphogenetic “pushing force” to deploy SHF cells rostrally into the OFT [67, 79].
The second model argues that PCP signaling functions in a “transition zone” to maintain proper epithelial polarization and organization in SHF-derived cells prior to their terminal differentiation into myocardium. The transition zone is proposed to be located at the distal OFT, containing SHF cells that have just entered the OFT and are differentiating to cardiomyocytes, based on their expression of myocardial markers, but also still express the progenitor marker Isl1. This “transition zone polarization” model is based on thorough tissue-specific gene ablation studies showing that the core PCP gene Vangl2 is not required in neither differentiated myocardial cells or endocardial cells in the OFT, but rather is required in SHF cells prior to or during myocardial differentiation [74]. This study also found that plasma membrane localization of Vangl2, which is though to be important for its function, is only found in Isl1-positive SHF progenitors in the distal OFT and the SpM. In the proximal OFT cells that have undergone further myocardial differentiation and lost Isl1 expression, Vangl2 localization becomes cytoplasmic [74].
Analyses of Vangl2 mutants showed that cells in the distal OFT/transition zone are abnormally polarized, and their normal epithelial organization also appears to be affected. These defects are proposed to cause OFT thickening at the expense of lengthening, and consequently, a shortened OFT [74].
There are a number of reasons that may account for the differences between the two models. First, the “pushing force model” is based on analyses of Dvl1/2 and Wnt5a mutants, while the “transition zone” model is based on Vangl2 mutant. As we explained above on the mechanism of PCP signaling, Vangl2 may exert dual positive and negative regulation on PCP during CE [62]. Therefore, loss of Vangl2 may cause mis-regulation of PCP signaling rather than solely loss of PCP signaling as in Dvl and Wnt5a mutants. Secondly, due to genetic redundancy in the mouse, none of the mutants analyzed is likely to completely disrupt PCP signaling. PCP signaling may have multiple roles in SHF cells as postulated in the two models, but each mutant may preferentially display only one of the defects described in the two studies [67, 74]. Finally, the “pushing force” model is based mainly on studies of the sub-population of SpM-derived SHF cells that give rise to only the inferior wall of the OFT [79, 84], whereas the “transition zone” model is based also on analyses of the superior-lateral wall of the OFT, which may arise from a distinct sub-population of SHF cells located in the pharyngeal arch mesoderm. PCP signaling may act differently in these two sub-populations of SHF cells.
Evidence for SHF cell deployment defects in PCP mutants
Both mouse genetic and chick experimental manipulation approaches have been taken to prove that PCP signaling indeed plays a crucial role in proper deployment of SHF cells to promote OFT morphogenesis. For the former approach, two SHF-specific enhancer trap transgenes were used to monitor SHF cell deployment in the mouse Wnt5a mutants. The first transgene, known as Mlc1v, harbors a nuclear LacZ inserted in the Fgf10 locus, and is expressed strongly in SHF progenitors in the SpM at E8.5–9.5 [7, 79, 85]. Subsequently at E10.5, after SHF deployment to the OFT, Mlc1v expression is found in the OFT but largely absent from the SpM-SHF. In Wnt5a null mutants, however, significant more Mlc1v expression is maintained in the SpM-SHF at E10.5, suggestive of aberrant retention of SHF progenitors in the SpM.
On the other hand, a second transgene, known as y96-16, marks specifically the SpM-SHF cells that have entered the OFT and contributed to the inferior OFT myocardium and its derivative, the sub-pulmonary myocardium [79, 84]. In Wnt5a null mutants, significant reduction of y96-16 positive cells occurred in the OFT from E9.5 onwards, consistent with reduced SHF cell deployment from the SpM. These results also imply that the PTA/CAT defects in Wnt5a null mutants may originate from pulmonary atresia, in which the sub-pulmonary myocardium is diminished due to the deployment defect [79]. A similar loss of sub-pulmonary myocardium was also proposed to explain the PTA phenotype in mouse mutant of Tbx1, a putative up-stream transcription regulator of Wnt5a [84, 86, 87].
As the above mouse studies inferred SHF cell deployment based on transgene expression, a complementary approach was also taken to directly label SHF cells using DiI and track their behavior in cultured chick embryo. Interestingly, like the mouse, chick embryos also display highly restricted Wnt5a expression in the caudal SpM [63]. When chick embryos at HH14 were injected with DiI to label the caudal SpM-SHF cells and cultured for 45 hours to HH21, the labeled cells were found in the inferior OFT myocardium. However, when a function-blocking anti-Wnt5a antibody was co-injected with DiI, the majority of the labeled cells were trapped in the SpM and very few were able to reach the inferior OFT. Together, these data strongly support a crucial role of Wnt5a-initiated signaling in deploying SHF cells from the SpM to the OFT to form specifically the sub-pulmonary myocardium [79].
Lineage tracing and 3D reconstruction reveal PCP-mediated polarized morphogenesis in the SpM-SHF
Mef2c-Cre lineage tracing
While the above studies may provide additional indirect evidence for the “pushing force” model, we wished to test the model more directly by examining morphogenesis of the SHF lineage per se, prior to their deployment into the OFT. To this end, we first used Mef2c-Cre [8] and Cre reporter Rosa26td-Tomato [88] to permanently label SHF cells genetically. The labeled embryos were serially sectioned and stained with myocardial markers to differentiate SHF progenitors from their descendants in the heart. Segmentation and volume rendering were then performed with Amira to generate 3D reconstruction models for the SHF and various structures in the heart (Li and Wang, unpublished).
These analyses revealed that the SpM-SHF first extends rostrally from E9.0 to 10.0, forming a small triangular shaped protrusion behind the OFT and the aortic sac. Subsequently from E10.0 to 11.5, it also protrudes caudally into the atria to form the dorsal mesenchymal protrusion (DMP). Together with the atrioventricular cushions (AVCs) and the primary atrial septum (PAS), the DMP will form the septal complex in the common atrium [11, 89, 90]. In Wnt5a null mutants, however, the SpM-SHF displays reduced lengthening, and fails to extend rostrally to form the triangular structure behind OFT by E10.0 or caudally into the atria to form the DMP by E11.5.
Morphometric analyses further revealed that SpM-SHF morphogenesis normally occurs in a highly polarized fashion in wild-type embryos. As the SpM-SHF grows, its length increases much more significantly than its width and thickness. Consequently, from E10.5–11.5, the length-to-width ratio (LWR) is increased by two folds and the length-to-thickness ratio (LTR) is increased by 22% in wild-type SpM-SHF. In Wnt5a−/− mutants, however, the SpM-SHF becomes significantly widened and thickened, and the LWR fails to show any increase while the LTR is decreased by 21% from E10.5–11.5.
In contrast to the significant change in the morphology of the SpM-SHF in Wnt5a−/− mutants, its total volume remains similar to that in the wild-type, consistent with the fact that there is no cell proliferation or apoptosis defects in Wnt5a−/− mutant SHF [67, 81]. Collectively, these data provide the first evidence that the SpM-SHF normally grows through concomitant lengthening and narrowing, reminiscent of CE. Loss of the putative PCP ligand Wnt5a disrupts the polarized, CE-like morphogenesis, causing the SpM-SHF to instead grow in an isotropic fashion.
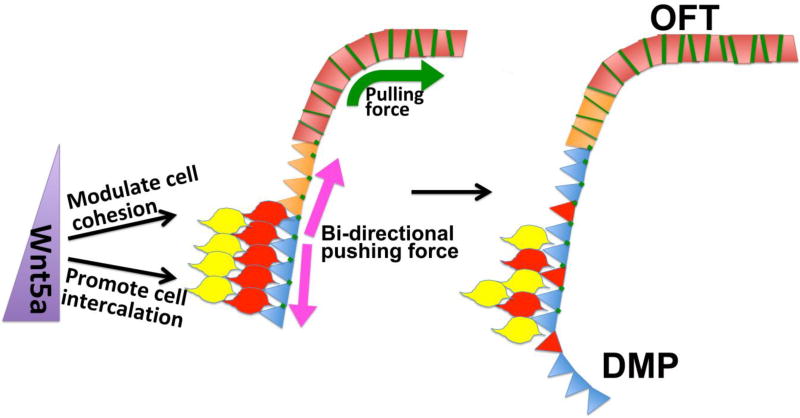
The failure of the SpM-SHF to extend efficiently along the AP axis in Wnt5a−/− mutant may cause not only OFT malformation at the arterial pole, but also atrial septation defect at the venous pole. By E13.5, Mef2cCre-labeled DMP can be observed to fuse with the PAS and AVCs to fully septate the common atrium in wild-type embryos. In Wnt5a−/− mutants, however, a shortened DMP fails to fuse with the PAS and AVCs, causing atrioventricular septal defects (AVCD). Together, these data suggest that Wnt5a-initiated PCP signaling may regulate polarized morphogenesis of the SpM-SHF to generate a bi-directional pushing force to deploy SHF cells to both the arterial and venous pole of the heart for OFT and atrial septation, respectively (Fig. 3).
Figure 3. An updated model for SHF cell deployment.
At the caudal SpM, Wnt5a-initiated PCP signaling may regulate both cell cohesion and oriented cell intercalation to promote polarized morphogenesis of the SpM-SHF, thereby generating a bi-directional pushing force to deploy SHF cells to both the arterial and venous pole of the heart for OFT and DMP formation, respectively. In the rostral SpM, loss of Wnt5a expression will allow cell cohesion to increase when SHF cells approach the OFT and undergo myocardial differentiation. This spatially regulated cell cohesion may in turn create a directional “pulling force”, which acts together with the caudally-generated pushing force to more efficiently deploy SHF cells rostrally into the OFT.
Wnt5a-CreER lineage tracing
Wnt5a may act both cell-autonomously and cell-non-autonomously to impact SHF morphogenesis and OFT formation. To more definitively examine the effect from cell-autonomous mode of action, a tamoxifen-inducible Wnt5a-CreER mouse line has been generated to specifically label and monitor the morphogenesis of Wnt5a-expressing cells and their descendants (Li and Wang, unpublished). Tamoxifen injection at E7.5 revealed that Wnt5a-expressing cells are located initially at the caudal SpM. Their descendants, however, gradually move anteriorly to enter the OFT from E10.0 onwards, and contribute only to the pulmonary trunk smooth muscle cells, but not the sub-pulmonary myocardium nor aorta. At the venous pole, Wnt5a-CreER labeled cells start to extend caudally into the atria from E10.5 to form the DMP.
In Wnt5a−/− mutant, very few Wnt5a-CreER labeled cells are able to enter or contribute to any structure of the OFT. This finding led to a new interpretation of the PTA/CAT defect in Wnt5a null mutant: the lack of Wnt5a-lineage, which contributes specifically to the pulmonary trunk, may result in an OFT vessel that is consisted of cells solely fated for the aorta and lacks the potential to form the pulmonary artery. This interpretation is consistent with the idea that the superior and inferior OFT wall are pre-specified in, and originated from, different pools of SHF cells, and that OFT septation may require the presence or juxtaposition of both sub-populations within the OFT [84, 91–93]. These results additionally indicate that Wnt5-expressing cells do not contribute to the sub-pulmonary myocardium directly, but Wnt5 may act cell-non-autonomously to push the progenitors of the sub-pulmonary myocardium into the OFT. The diminished sub-pulmonary myocardium in Wnt5a null mutant may arise from disruption of this cell-non-autonomous effect.
Genetic evidence that Wnt5a signals through the PCP pathway
While Wnt5a clearly acts as a PCP ligand for morphogenesis in zebrafish and Xenopus [54, 94], its function in mammals was controversial in the literature. Through phenotypic analyses and genetic interaction studies with known core PCP genes in mice, several publications have concluded that mammalian Wnt5a also function through the PCP pathway during neural tube closure, and cochlea and limb morphogenesis [45, 47, 48, 95]. Other studies, however, argued that mammalian Wnt5a acts other mechanisms, such as antagonizing or restraining canonical Wnt signaling, or activating calcium signaling [63, 96–99].
Using an epistasis approach, we tested more definitively whether Wnt5a signals through the PCP pathway to regulate SpM-SHF morphogenesis and OFT formation. As mentioned above, Daam1, a formin protein with potent actin polymerizing and bundling activities [100], was identified as a PCP effector downstream of Dvl in Xenopus [49, 50]. The current model proposes that Daam1 exists in an auto-inhibited form, due to intra-molecular bond between its N-terminal GBD (GTPase binding domain) and C-terminal DAD (diaphanous auto-regulatory domain) domains. PCP activation by non-canonical Wnt enables Dvl to bind to the DAD domain, disrupt the auto-inhibitory bond and activate Daam1 [50, 101]. ΔDAD, a Daam1 mutant lacking the DAD domain, is unable to form the auto-inhibitory bond and functions in a constitutively active fashion [50].
We therefore created a conditional-activating Rosa26-ΔDAD BAC transgene to study whether expressing ΔDAD in the SHF may rescue the defects in Wnt5a−/− mutants (Li and Wang, unpublished). When ΔDAD expression is activated in the SHF with Mef2c-Cre [8], it is able to restore SpM lengthening in Wnt5a−/− mutant at E10.5, and partially rescue the OFT septation defect at E18.5. These epistasis experiments indicate that endogenous Wnt5a functions, at least in part, through PCP effector Daam1 to regulate SHF morphogenesis and OFT formation.
A pulling force for rostral deployment of SpM-SHF cells into the OFT
The above ΔDAD rescue experiment also indicates that constitutively activating Daam1 throughout the entire SHF is sufficient to rescue the SpM-SHF morphogenesis defects in Wnt5a−/− mutants, implying that PCP signaling acts in a permissive rather than instructive fashion. This idea is also supported by prior studies, in which broadly expressing Wnt5a in the entire SHF using a Rosa26Wnt5aGOF allele (Wnt5a GOF) is able to rescue cell polarity, actin polymerization and SpM shortening defects in Wnt5a−/− mutants [81].
There are, however, important differences between ΔDAD and Wnt5a over-expression. In Wnt5a−/− background, ΔDAD over-expression throughout the SHF can rescue both SpM/DMP shortening and OFT septation defects, whereas Wnt5a over-expression rescues only SpM/DMP shortening but not the OFT defects. In wild-type embryos, ΔDAD over-expression has no deleterious effect, whereas Wnt5a over-expression causes OFT shortening and conotruncal defects [81]. Therefore, it appears while Wnt5a acts primarily through Daam1 to regulate SpM-SHF morphogenesis and DMP extension at the venous pole, it may signal through other effectors besides Daam1 to impact OFT formation.
Detailed analyses revealed that Wnt5a GOF results in ectopic Wnt5a expression in the rostral SpM and the adjacent distal OFT myocardium, which normally lack endogenous Wnt5a expression. This ectopic expression does not alter proliferation, differentiation or apoptosis in the SHF or OFT, but instead blocks SHF cells from entering the OFT, causing them to be trapped and form an aberrant bulge at the junction between the SpM and the OFT [81].
Further immunostaining demonstrated a reverse correlation between Wnt5a expression and the level of cell cohesion. In wild-type embryos, the cell surface level of N-cadherin and α-catenin is low in the caudal SpM where Wnt5a is highly expressed, but is elevated in the rostral SpM and distal OFT where Wnt5a expression is diminished. In Wnt5a GOF mutants, ectopic Wnt5a expression blocks the normal up-regulation of adherens junction in the rostral SpM. Conversely, in Wnt5a−/− mutants loss of Wnt5a expression causes aberrant, premature increase of adherens junction level in the caudal SpM. Furthermore, over-expression of mouse Wnt5a in Xenopus is able to reduce C-cadherin level on the plasma membrane without affecting its total protein level, suggesting that Wnt5a may have an evolutionarily conserved function in modulating cadherin-mediated cell cohesion during tissue morphogenesis.
Together, these data indicate that restricting Wnt5a expression to the caudal SpM-SHF is critically important for OFT formation. This could be due to at least two reasons. Firstly, by suppressing cell cohesion, Wnt5a may increase the dynamics of cell motility at the caudal SpM, thereby facilitating oriented cell interaction induced by its other downstream effectors like Daam1. This idea is consistent with the previously reported aberrant aggregation of SHF cells in the caudal SpM of Wnt5a and Dvl1/2 mutants [67]. Secondly, caudally restricted Wnt5a expression will also allow cell cohesion to increase rostrally when SHF cells approach the OFT and undergo myocardial differentiation. This spatially regulated cell cohesion may in turn create a directional “pulling force”, which acts together with the “pushing force” discussed above, to more efficiently deploy SHF cells rostrally into the OFT (Figure 3). In Wnt5a GOF mutants, this “pulling force” may be disrupted due to ectopic Wnt5a expression that inhibits the normal up-regulation of cell cohesion in the rostral SpM/distal OFT, thereby leading to reduced SHF cell deployment and OFT shortening [81]. These findings, along with a number of previous studies [102–107], uncover important and novel roles of cell-cell adhesion in mammalian heart formation, and warrant future investigation to elucidate its role in development and regeneration.
Conclusion remarks
Prior genetic studies have revealed that PCP, an evolutionally conserved morphogenetic engine, plays critical roles in mammalian OFT formation. Detailed investigation over the last few years have led to distinct models to explain how PCP signaling may exert its morphogenetic effect, either within the SHF or in the adjacent transitional zone in the OFT. Despite their differences, these studies all implicate a key function of PCP signaling in facilitating efficient addition of SHF cells to the forming heart tube, and provide a solid foundation for more in depth studies to further dissect temporal and spatial specific roles of PCP in mammalian heart development. Finally, the putative PCP ligand Wnt5a was identified as a transcriptional target of Tbx1, whose haplo-insufficient deletion leads to the highly increased rate of OFT malformation in humans with 22q11.2 deletion syndrome (DS) [87, 108, 109]. Determining how PCP signaling contributes to the Tbx1 network, and how PCP gene variants may impact the variable presentation of OFT malformations in 22q11.2DS, may help translate our insight from mouse studies into understanding of the pathogenic mechanisms of CHDs in humans in the future.
Acknowledgments
We thank Dr. Deborah Henderson for helpful discussion, and for the permission to adapt Fig. 8M in Ramsbottom et. al, PLoS Genetics 2014 10(12) doi: 10.1371/journal.pgen.1004871 in the current publication. This work was supported by grants HL109130 and HL138470 from the National Institute of Health, and 14GRNT20380467 from the American Heart Association.
Footnotes
Compliance with Ethical Standards
Ethical approval : All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest: None.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Samanek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young. 2000;10(3):179–85. doi: 10.1017/s1047951100009082. [DOI] [PubMed] [Google Scholar]
- 3.Harvey RP. Patterning the vertebrate heart. Nat Rev Genet. 2002;3(7):544–56. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- 4.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Developmental cell. 2003;5(6):877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Developmental biology. 2009;336(2):137–44. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circulation research. 2010;107(12):1428–44. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Developmental cell. 2001;1(3):435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 8.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Developmental biology. 2005;287(1):134–45. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Current topics in developmental biology. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 10.Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, et al. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circulation research. 2007;101(10):971–4. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- 11.Briggs LE, Phelps AL, Brown E, Kakarla J, Anderson RH, van den Hoff MJ, et al. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circulation research. 2013;112(11):1420–32. doi: 10.1161/CIRCRESAHA.112.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131(19):4651–64. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 13.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 14.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12(6):385–91. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Semin Cell Dev Biol. 2009;20(8):957–63. doi: 10.1016/j.semcdb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129(6):1051–63. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nature cell biology. 2010;12(2):170–6. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nature genetics. 2005;37(9):980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207(2):171–9. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nature cell biology. 2008;10(11):1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H, Smallwood PM, Williams J, Nathans J. The spatio-temporal domains of Frizzled6 action in planar polarity control of hair follicle orientation. Developmental biology. 2016;409(1):181–93. doi: 10.1016/j.ydbio.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 23.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 24.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132(20):4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 25.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11(1):63–9. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 26.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405(6782):76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 27.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nature cell biology. 2002;4(8):610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298(5600):1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 29.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180(1):221–32. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430(7000):689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 31.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405(6782):81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 32.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harb Perspect Biol. 2009;1(3):a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb Perspect Biol. 2012;4(5) doi: 10.1101/cshperspect.a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 2009;5(3):e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13(13):1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 37.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4(11):e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature genetics. 2001;28(3):251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Human molecular genetics. 2001;10(22):2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133(9):1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26(8):2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, Andelfinger G, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci U S A. 2008;105(9):3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430(6995):93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 44.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Developmental cell. 2011;20(2):163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Developmental biology. 2007;306(1):121–33. doi: 10.1016/j.ydbio.2007.03.011. Epub 2007/04/17. doi: S0012-1606(07)00194-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savory JG, Mansfield M, Rijli FM, Lohnes D. Cdx mediates neural tube closure through transcriptional regulation of the planar cell polarity gene Ptk7. Development. 2011;138(7):1361–70. doi: 10.1242/dev.056622. [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Human molecular genetics. 2011;20(2):271–85. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Current biology : CB. 2010;20(22):1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105(1):210–5. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17(2):295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. Embo J. 2008;27(4):606–17. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133(5):957–66. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- 54.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120(4):467–76. doi: 10.1016/s0925-4773(03)00004-2. Epub 2003/04/05. doi: S0925477303000042 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. The International journal of developmental biology. 2001;45(1):225–7. [PubMed] [Google Scholar]
- 56.Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133(6):1093–105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nature cell biology. 2013;15(9):1045–55. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307(5708):423–6. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Developmental cell. 2008;15(3):462–9. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109(3):371–81. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 61.Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137(21):3707–17. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seo HS, Habas R, Chang C, Wang J. Bimodal regulation of Dishevelled function by Vangl2 during morphogenesis. Human molecular genetics. 2017;26(11):2053–61. doi: 10.1093/hmg/ddx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, et al. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61(4):386–91. doi: 10.1203/pdr.0b013e3180323810. Epub 2007/05/23. [DOI] [PubMed] [Google Scholar]
- 64.Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nature genetics. 2007;39(10):1225–34. doi: 10.1038/ng2112. Epub 2007/09/04. doi: ng2112 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Vliet PP, Lin L, Boogerd CJ, Martin JF, Andelfinger G, Grossfeld PD, et al. Tissue specific requirements for WNT11 in developing outflow tract and dorsal mesenchymal protrusion. Developmental biology. 2017;429(1):249–59. doi: 10.1016/j.ydbio.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129(24):5827–38. doi: 10.1242/dev.00164. Epub 2002/11/08. [DOI] [PubMed] [Google Scholar]
- 67.Sinha T, Wang B, Evans S, Wynshaw-Boris A, Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Developmental biology. 2012;370(1):135–44. doi: 10.1016/j.ydbio.2012.07.023. Epub 2012/07/31. doi: S0012-1606(12)00407-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu H, Ye X, Guo N, Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development. 2012;139(23):4383–94. doi: 10.1242/dev.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henderson DJ, Conway SJ, Greene ND, Gerrelli D, Murdoch JN, Anderson RH, et al. Cardiovascular defects associated with abnormalities in midline development in the Loop-tail mouse mutant. Circulation research. 2001;89(1):6–12. doi: 10.1161/hh1301.092497. [DOI] [PubMed] [Google Scholar]
- 70.Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419(6908):726–9. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- 71.Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. Embo J. 2000;19(5):1010–22. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12(16):2610–22. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94(1):109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 74.Ramsbottom SA, Sharma V, Rhee HJ, Eley L, Phillips HM, Rigby HF, et al. Vangl2-regulated polarisation of second heart field-derived cells is required for outflow tract lengthening during cardiac development. PLoS Genet. 2014;10(12):e1004871. doi: 10.1371/journal.pgen.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, et al. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104(22):9319–24. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. The Journal of clinical investigation. 2007;117(7):1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(22):9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104(26):10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinha T, Li D, Theveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Human molecular genetics. 2015;24(6):1704–16. doi: 10.1093/hmg/ddu584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, et al. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circulation research. 2009;104(2):179–88. doi: 10.1161/CIRCRESAHA.108.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li D, Sinha T, Ajima R, Seo HS, Yamaguchi TP, Wang J. Spatial regulation of cell cohesion by Wnt5a during second heart field progenitor deployment. Developmental biology. 2016;412(1):18–31. doi: 10.1016/j.ydbio.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Developmental biology. 2005;281(1):78–90. doi: 10.1016/j.ydbio.2005.02.012. Epub 2005/04/26. doi: S0012-1606(05)00104-1 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Francou A, Saint-Michel E, Mesbah K, Kelly RG. TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field. Development. 2014;141(22):4320–31. doi: 10.1242/dev.115022. [DOI] [PubMed] [Google Scholar]
- 84.Theveniau-Ruissy M, Dandonneau M, Mesbah K, Ghez O, Mattei MG, Miquerol L, et al. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circulation research. 2008;103(2):142–8. doi: 10.1161/CIRCRESAHA.108.172189. Epub 2008/06/28. doi: CIRCRESAHA.108.172189 [pii] [DOI] [PubMed] [Google Scholar]
- 85.Kelly RG, Papaioannou VE. Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(3):821–8. doi: 10.1002/dvdy.21063. Epub 2007/01/24. [DOI] [PubMed] [Google Scholar]
- 86.Kirby ML. Pulmonary atresia or persistent truncus arteriosus: is it important to make the distinction and how do we do it? Circulation research. 2008;103(4):337–9. doi: 10.1161/CIRCRESAHA.108.174862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen L, Fulcoli FG, Ferrentino R, Martucciello S, Illingworth EA, Baldini A. Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet. 2012;8(3):e1002571. doi: 10.1371/journal.pgen.1002571. Epub 2012/03/23 PGENETICS-D-11-01700 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. doi: 10.1038/nn.2467. Epub 2009/12/22. doi: nn. 2467 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Developmental cell. 2012;23(2):280–91. doi: 10.1016/j.devcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, et al. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135(10):1887–95. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bertrand N, Roux M, Ryckebusch L, Niederreither K, Dolle P, Moon A, et al. Hox genes define distinct progenitor sub-domains within the second heart field. Developmental biology. 2011;353(2):266–74. doi: 10.1016/j.ydbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rochais F, Dandonneau M, Mesbah K, Jarry T, Mattei MG, Kelly RG. Hes1 is expressed in the second heart field and is required for outflow tract development. PloS one. 2009;4(7):e6267. doi: 10.1371/journal.pone.0006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Z, Wang J, Guo C, Chang W, Zhuang J, Zhu P, et al. Temporally Distinct Six2-Positive Second Heart Field Progenitors Regulate Mammalian Heart Development and Disease. Cell Rep. 2017;18(4):1019–32. doi: 10.1016/j.celrep.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126(6):1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 95.Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109(11):4044–51. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139(11):1931–40. doi: 10.1242/dev.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104(39):15436–41. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaiswal R, Breitsprecher D, Collins A, Correa IR, Jr, Xu MQ, Goode BL. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Current biology: CB. 2013;23(14):1373–9. doi: 10.1016/j.cub.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, et al. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133(21):4219–31. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- 102.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Developmental biology. 1997;181(1):64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 103.Linask KK. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Developmental biology. 1992;151(1):213–24. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- 104.Linask KK, Knudsen KA, Gui YH. N-cadherin-catenin interaction: necessary component of cardiac cell compartmentalization during early vertebrate heart development. Developmental biology. 1997;185(2):148–64. doi: 10.1006/dbio.1997.8570. [DOI] [PubMed] [Google Scholar]
- 105.Soh BS, Buac K, Xu H, Li E, Ng SY, Wu H, et al. N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res. 2014;24(12):1420–32. doi: 10.1038/cr.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, et al. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circulation research. 2015;116(1):70–9. doi: 10.1161/CIRCRESAHA.116.304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J, Miao L, Shieh D, Spiotto E, Li J, Zhou B, et al. Single-Cell Lineage Tracing Reveals that Oriented Cell Division Contributes to Trabecular Morphogenesis and Regional Specification. Cell Rep. 2016;15(1):158–70. doi: 10.1016/j.celrep.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104(4):619–29. doi: 10.1016/s0092-8674(01)00247-1. Epub 2001/03/10. doi: S0092-8674(01)00247-1 [pii] [DOI] [PubMed] [Google Scholar]
- 109.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nature genetics. 2001;27(3):286–91. doi: 10.1038/85845. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]