SUMMARY
The development of interventions to prevent congenital Zika syndrome (CZS) has been limited by the lack of an established nonhuman primate model. Here we show that infection of female rhesus monkeys early in pregnancy with Zika virus (ZIKV) recapitulates many features of CZS in humans. We infected 9 pregnant monkeys with ZIKV, 6 early in pregnancy and 3 later in pregnancy, and compared findings with uninfected controls. 100% (6 of 6) of monkeys infected early in pregnancy exhibited prolonged maternal viremia and fetal neuropathology, including fetal loss, smaller brain size, and histopathologic brain lesions, including microcalcifications, hemorrhage, necrosis, vasculitis, gliosis, and apoptosis of neuroprogenitor cells. High-resolution MRI demonstrated concordant lesions indicative of deep gray matter injury. We also observed spinal, ocular, and neuromuscular pathology. Our data show that vascular compromise and neuroprogenitor cell dysfunction are hallmarks of CZS pathogenesis, suggesting novel strategies to prevent and to treat this disease.
In Brief
ZIKV infection in early pregnant female rhesus monkeys resulted in fetal neuropathology with vascular compromise and neuroprogenitor cell dysfunction, similar to congenital ZIKV syndrome in human infants.

INTRODUCTION
Zika virus (ZIKV) is a flavivirus that has been associated with fetal microcephaly and multiple congenital abnormalities (Brasil et al., 2016; Honein et al., 2017; Johansson et al., 2016). The challenges associated with studying CZS in humans and the lack of an established model for CZS in nonhuman primates, however, have limited our understanding of the pathogenesis of this disease. Moreover, the lack of a nonhuman primate CZS model has slowed the development of vaccines and other interventions to prevent this condition.
Studies of CZS pathology in humans have been limited to autopsy studies from the most severe fatal cases (Chimelli et al., 2017; Martines et al., 2016; Sousa et al., 2017). Autopsy findings from fatal cases of infants with CZS have demonstrated altered migration of neuroprogenitor cells, neuronal apoptosis, and necrosis of periventricular gray matter leading to ventriculomegaly ex vacuo, abnormal cortical development, and gross structural deformities. Typical radiologic and histopathologic lesions include subcortical dystrophic calcifications associated with neuronal cell death (Aragao et al., 2017; Guillemette-Artur et al., 2016). ZIKV-associated lesions are also observed in highly vascularized deep gray matter structures such as the basal ganglia and thalamus (Chimelli et al., 2017; Shao et al., 2016). In vitro models using organoids and tissue explants have confirmed the neuropathologic effects of ZIKV in brain tissue (Bayer et al., 2016; Gabriel et al., 2017). Studies using cell lines and immunocompromised mice have also shown that ZIKV targets neuroprogenitor cells, placental trophoblasts, and endothelial cells (Adibi et al., 2016; Miner et al., 2016; Nowakowski et al., 2016; Sapparapu et al., 2016; Vermillion et al., 2017).
Microcephaly is a severe manifestation of CZS affecting approximately 2–4% of ZIKV-infected pregnant women in Brazil and in the United States (Brasil et al., 2016; Honein et al., 2017). More common manifestations of CZS include cerebral microcalcifications, hemorrhage, congenital abnormalities, ocular pathology, and spinal cord and peripheral nerve lesions, which contribute to neuromuscular dysfunction and arthrogryposis (Melo et al., 2016). Surviving infants may develop seizure disorders, developmental abnormalities, and postnatal microcephaly (Adebanjo et al., 2017; Mejdoubi et al., 2017). Perinatal magnetic resonance imaging (MRI) of ZIKV exposed infants reveal basal ganglia and cortico-white matter junction calcifications, ventriculomegaly, gyral simplification, and lissencephaly (Aragao et al., 2017; Moore et al., 2017). CZS pathology has been modeled in immunosuppressed mice (Miner et al., 2016; Sapparapu et al., 2016), but studies to date in ZIKV-infected pregnant female nonhuman primates have not shown consistent or severe fetal neuropathology (Adams Waldorf et al., 2016; Hirsch et al., 2018; Mohr et al., 2018; Nguyen et al., 2017; Waldorf et al., 2018).
In this study, we show that infection of female rhesus monkeys early in pregnancy with a Brazilian strain of ZIKV recapitulates many lesions that are characteristic of CZS, including consistent neuropathology in the fetal brain and spinal cord. Neuropathology was associated with neuroprogenitor cell apoptosis and vascular compromise. These data provide new insights into CZS pathogenesis and should accelerate the development of vaccines and other interventions for this disease.
RESULTS
ZIKV Infection of Pregnant Female Rhesus Monkeys Leads to Prolonged Viremia
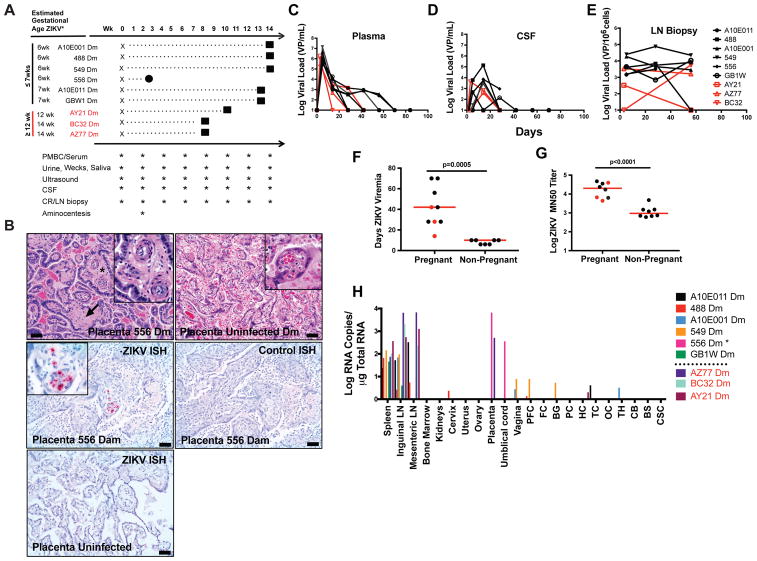
ZIKV infection of first trimester pregnant women has been associated with severe CZS (Brasil et al., 2016; Johansson et al., 2016). To assess whether infection of pregnant female rhesus monkeys would lead to similar fetal neuropathology, we infected 6 female monkeys early in pregnancy (weeks 6–7 of gestation) and 3 later in pregnancy (weeks 12–14 of gestation) with 103 PFU ZIKV from the recent Brazil epidemic (ZIKV-BR) (Fig. 1A) (Cugola et al., 2016; Larocca et al., 2016). One dam infected early in pregnancy (556 Dm) experienced early fetal loss, whereas the other 8 fetuses survived to term. At delivery, 66% (6 of 9) of dams (556 Dm, 549 Dm, GBW1 Dm, 488 Dm, A10E011 Dm, AZ77 Dm) exhibited significant placental pathology, including chorioamnionitis and deciduitis (Table 1, Fig. S1), consistent with prior studies (Hirsch et al., 2018; Mohr et al., 2018; Nguyen et al., 2017). Dam 556 also had a promiment villitis (Fig. 1B, C), and virus was detected by in situ hybridization in placental villi on day 21 (Fig. 1D–F).
Figure 1. ZIKV infection in pregnant female rhesus monkeys.
A, Study schema and procedures. One fetal loss (556) occurred at week 3 post-infection (circle). All other fetuses were delivered by caesarian section at term (squares). B, Placental vasculitis (asterisks, inset) and villar sclerosis (arrow) in infected dam 549. C, Placenta from uninfected dam. D, In situ hybridization (ISH) for ZIKV RNA in placenta of dam 556 that aborted. E, ISH for control RNA in the same tissue section from 556 Dm. F, ISH for ZIKV RNA in placenta from uninfected dam. G, Longitudinal ZIKV viral loads by RT-PCR in maternal plasma. H, Longitudinal ZIKV viral loads by RT-PCR in CSF. I, Longitudinal ZIKV viral loads by RT-PCR in lymph node biopsies. J, Duration of ZIKV viremia in pregnant versus non-pregnant female rhesus monkeys (p=0.0005, unpaired t-test). K, Log ZIKV microneutralization titers (MN50) in pregnant versus non-pregnant female rhesus monkeys at week 8 post-infection (p<0.0001, unpaired t-test). L, ZIKV viral loads in tissues from infected dams by RT-PCR at term, except for 556 Dm which was at time of fetal loss. Scale bars = 50 microns. H&E hematoxylin and eosin, PFC prefrontal cortex, FC frontal cortex, BG basal ganglia, PC parietal cortex, HC hippocampus, TC temporal cortex, OC occipital cortex, TH thalamus, CB cerebellum, BS brain stem, CSC cervical spinal cord. Black names, lines, and dots reflect animals infected early in pregnancy at ≤7 weeks gestation. Red names, lines, and dots reflect animals infected later in pregnancy at ≥12 weeks gestation. Horizontal red bar reflects mean responses. See also Figure S1.
Table 1. Summary of pathologic findings in ZIKV-infected infant monkeys.
Features highlighted in bold correspond to lesions described in human fetal autopsies and placenta pathology.
| ≥ 12 wks | ZIKV positive tissue | Gross Pathology | Histopathology | post-mortem MRI |
|---|---|---|---|---|
| AZ77 | heart | NSF | Neutrophils lumbar spinal cord; mild optic nerve gliosis; Dam placental pathology characterized by increased islands of intermediate trophoblasts extending along stem villi; increased calcifications associated with perivillous fibrin | ND |
| BC32 | None | NSF | NSF | ND |
| AY21 | Prefrontal cortex, frontal cortex, basal ganglia occipital lobe, spleen, inguinal LN, mes LN, ileum, kidney, testis, lung | NSF | Focal microcalcification and hemorrhage; gliosis | Streaks of gliosis in the BG and deep white matter as well as the cerebellar WM; Scattered gliosis in the brainstem, occipital and frontal WM |
| ≤ 7wks | ||||
| 549 | Liver, axillary LN | Hemorrhage and malacia in frontal cortex, thalamus, occipital cortex; unilateral absent occipital gyrus | Vascular necrosis in multiple regions of brain; hemorrhage in thalamus; focal astrocytosis; focal necrosis in cerebellum and parietal cortex, large neuroprogenitor cell aggregate; Dam placental pathology characterized by features of maternal malperfusion; thin placenta with multiple areas of infarction associated with maternal arterial thrombosis and decidual necrosis and acute inflammation (deciduitis) | Streaks of gliosis in the BG and deep white matter as well as the cerebellar WM; Scattered gliosis in the frontal WM |
| GBW1 | None | NSF | Fetus increased neuroprogenitors in frontal cortex and basal ganglia; Dam—placental pathology characterized by decidual necrosis and angiocentric acute inflammation (maternal vasculitis) | Minimal streaks of gliosis in the deep gray and frontal subcortical WM |
| 488 | None | NSF | Vascular necrosis in multiple tissues including multiple regions of brain; ganglion necrosis/apoptosis in multiple organs; Dam placenta pathology characterized by increased calcifications associated with perivillous fibrin; areas of infarction; deciduitis | Mild gliosis in the BG and deep white matter as well as the cerebellar WM |
| A10E001 | None | NSF | Focal spinal cord hemorrhage; large neuroprogenitor cell aggregate and neuroprogenitor apoptosis | Streaks of deep gray matter gliosis +/− |
| A10E011 | None | NSF | Increased neuroprogenitors in basal ganglia; placental pathology characterized by increased calcifications associated with perivillous fibrin; areas of infarction; deciduitis. Increased islands of intermediate trophoblasts extending along stem villi and onto ischemic terminal villi | Minimal streaks of gliosis in the basal ganglia and deep WM |
| 566 | Brain, kidney, spleen, spinal cord, umbilical cord, placenta, LN | Aborted | Placental pathology characterized by chronic villitis with destruction of villous capillaries and intervillositis; bacterial chorioamnionitis | ND |
NSF= No significant findings; ND= Not done; LN= lymph node: WM= white matter; BG= basal ganglia; Bold= features seen in human infection
Non-pregnant rhesus monkeys infected with ZIKV typically clear viremia in 7–10 days (Abbink et al., 2016; Aid et al., 2017; Dowd et al., 2016), coincident with the development of ZIKV-specific neutralizing antibodies (Aid et al., 2017). We previously showed that ZIKV persists for longer periods of time in the central nervous system, lymph nodes, and other immune privileged sites in infected rhesus monkeys (Aid et al., 2017). Pregnant dams infected early in pregnancy exhibited prolonged ZIKV viremia (28–70 days) compared with dams infected later in pregnancy (14–42 days) (Fig. 1G). Pregnant dams also demonstrated virus in cerebrospinal fluid (CSF) (Fig. 1H) and lymph nodes (LN) (Fig. 1I). ZIKV viremia was substantially longer in pregnant dams compared to non-pregnant monkeys (7–10 days; p=0.0005) (Fig. 1J). Moreover, pregnant dams developed remarkably robust ZIKV-specific neutralizing antibody responses at 8 weeks post-infection, as measured by microneutralization (MN50) assays (Abbink et al., 2016; Larocca et al., 2016). MN50 titers in the infected pregnant females were substantially higher than in infected non-pregnant females infected with the same challenge stock at week 8 (Fig. 1K, p<0.0001). Following delivery, 100% (9 of 9) of dams also exhibited persistent virus in spleen and lymph nodes, as well as sporadic virus in placenta and other tissues (Fig. 1L).
Radiographic and Neuropathologic Lesions in ZIKV-Infected Infant Monkeys
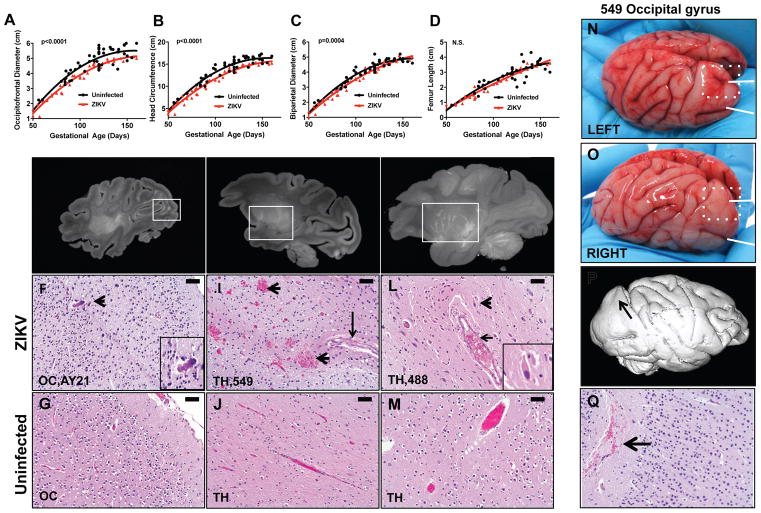
To evaluate fetal growth kinetics, we performed longitudinal fetal ultrasounds on the 6 rhesus monkeys infected with ZIKV early in pregnancy (≤7 wks) and a concurrent control group of 28 uninfected pregnant rhesus monkeys from the same breeding colony. ZIKV-infected fetuses demonstrated a 7.2% reduction in the average occipitofrontal diameter at term (p<0.0001), an 8.3% reduction in head circumference at term (p<0.0001), and modestly reduced biparietal diameter (p=0.0004) as compared with uninfected controls but exhibited comparable femur length (p=NS) (Fig. 2A–D). Biometric differences were evident by 3–4 weeks following ZIKV infection by gestational day 70. These data suggest that ZIKV infection led to reduced brain size, rather than overall growth restriction, although we did not observe gross microcephaly.
Figure 2. Radiographic-histopathologic correlations of selected lesions in ZIKV-infected infants.
Scatterplots of biometric measurements of occipitofrontal diameter (A), head circumference (B), biparietal diameter (C), and femur length (D) collected by transabdominal ultrasound every 2–4 weeks in 6 dams infected early in pregnancy (≤7 weeks) (red) and 28 concurrent uninfected controls from the same breeding colony (black); p-values reflect nonlinear regression with extra-sum of squares F test. Post-mortem, high resolution, sagittal T1 maximum intensity projection hemispheric brain magnetic resonance imaging (MRI) showing hyperintense foci. E, MRI showing hyperintense foci (box) in occipital lobe (OC, AY21) with associated histopathologic microcalcifications (arrowhead) (F). G, OC from age-matched uninfected fetus. H, MRI showing hyperintense foci (box) in the thalamus (TH, 549) and associated histopathologic hemorrhage (arrowheads) and vasculitis (arrow) (I). J, TH from age-matched uninfected fetus. K, MRI, maximum intensity projection (MIP), showing hyperintense foci (box) in the lenticulostriate vasculature of the thalamus (TH, 488) and associated histopathologic perivascular neuronal necrosis (arrowheads and inset) (L). M, TH vessel from age-matched uninfected fetus. N, Gross image of unfixed left brain hemisphere from fetus 549 showing presence of two occipital gyri (white arrows), upper gyrus delineated by white dashed box. O, Gross image of unfixed right brain hemisphere from fetus 549 showing absence of the upper occipital gyrus (white dashed box). P, MRI of fixed right hemisphere showing lissencephaly corresponding to absence of gyrus in the right hemisphere (arrow). Q, H&E showing focal hemorrhage (arrow) in right occipital lobe. Histopathology reflects hematoxylin and eosin (H&E) staining. Scale bars = 50 microns. See also Figure S2.
We performed caesarean sections to deliver the 8 ZIKV-infected infants that survived to term. Infant and maternal tissues were assessed for radiographic, pathologic, and virologic evidence of ZIKV infection. High-resolution, post-mortem magnetic resonance imaging (MRI) was performed on formalin fixed brain from the 5 infants infected earliest in pregnancy that survived to term (A10E001, 488, 549, A10E011, GBW1) and 1 infant infected at pregnancy week 12 (AY21). 66% (4 of 6) of the imaged brains showed hyperintense foci in white matter (Fig. 2E, Table 1) and deep gray matter nuclei (Fig. 2H, K), which was interpreted as gliosis and alterations of the lenticulostriate vasculature, similar to CZS radiographic lesions reported in human infants although we did not observe polymicrogyria (Guillemette-Artur et al., 2016).
Histopathologic evaluation of the anatomic regions corresponding to these radiographic lesions by two independent veterinary pathologists revealed microcalcifications in the occipital cortex (OC) of infant AY21 (Fig. 2F), hemorrhage and vasculitis in the thalamus (TH) of infant 549 (Fig. 2I), and perivascular neuronal necrosis and edema in the thalamus (TH) of infant 488 (Fig. 2L). Uninfected age-matched infant rhesus monkey brains, obtained from the Tulane National Primate Center Tissue Archive, were similarly analyzed and exhibited minimal or no neuropathology (Fig. 2G, J, M). Infant 549 also had gross structural brain defects, including unilateral absence of an occipital gyrus (focal lissencephaly) (Fig. 2N–P) and gray/brown discolorations in the OC and TH, consistent with subventricular malacia and hemorrhage (Fig. S2) that was also seen on histopathology (Fig. 2Q). In addition, dam 549 was the only animal with detectable ZIKV RNA in amniotic fluid at day 14 (data not shown).
We next performed comprehensive histopathology of the brains and spinal cords of all the infected infants, including analysis of cortical tissue (prefrontal, frontal, parietal, temporal, occipital lobes); deep white matter and gray matter (basal ganglia, thalamus, hippocampus, brainstem); and cerebellum (Fig. 3A; Table 1). A semi-quantitative scoring system (0–4) was developed based on the severity of pathologic lesions (Table S1). 100% (6 of 6) of the infants infected early in pregnancy (≤7wks) showed mild, moderate, or severe neuropathology (Fig. 3A). Neuropathologic lesions in infants infected early in pregnancy were more severe than those noted in infants infected later in pregnancy (p=0.0053; Fig. 3B) as well as uninfected age-matched controls (p=0.0053; Fig. 3B).
Figure 3. Comprehensive neuropathology in ZIKV-infected infants.
A, Summary of neuropathologic lesions in the central nervous system, peripheral nervous system, enteric nervous system, eyes, and muscles in ZIKV-infected infants (see also Table 1). Scoring system is defined in Table S1. B, Maximum neuropathology score in infants from dams infected early in pregnancy (≤7 weeks), infants from dams infected later in pregnancy (≥12 weeks), and uninfected control infants; p-values reflect one-way ANOVA with Tukey post-test for multiple comparisons; red bars reflect mean scores. C, Necrotic neurons (inset, arrowheads) near a damaged vessel (arrows) in infant 488; dashed arrow represents viable neuron. D, Perivascular neuronal necrosis (arrowhead) and vasculitis (arrow) in thalamus from infant 549. E, Necrosis within deep cerebellar nuclei (dashed region) of infant 549. F, Uninfected, age-matched control fetus CB. Scale bars = 50 microns (C, D) or 200 microns (E, F); Luxol Fast Blue and Cresyl Violet counter stain (C); Hematoxylin and eosin (D–F). PFC prefrontal cortex, FC frontal cortex, BG basal ganglia, TH thalamus, SN substantia nigra, HC hippocampus, OC occipital cortex, PC parietal cortex, TC temporal cortex, CB cerebellum, BS brain stem, CSC cervical spinal cord, TSC thoracic spinal cord, LSC lumbar spinal cord, DRG dorsal root ganglia, ENS enteric nervous system. See also Figure S2–S6.
In addition to the early fetal loss (556), two additional infants infected early in pregnancy (549, 488) exhibited severe neuropathology with prominent cerebral hemorrhage, perivascular neuronal necrosis and edema, vasculitis, and gliosis throughout the thalamus (Fig. 2I, L; Fig. 3C, D), basal ganglia (Figs. S2, S3), and deep cerebellar nuclei (Fig. 3E). Areas of cerebral necrosis were adjacent to vessels with hypertrophied endothelial cells and lightly basophilic and granular cytoplasm, indicative of vasculitis with early dystrophic mineralization. Perivascular neuronal necrosis was characterized by cytoplasmic hypereosinophilia by hematoxylin and eosin staining (Figs. 2L, 3D) and loss of nuclear basophilia by cresyl violet staining (Fig. 3C).
Moderately severe lesions were observed in two additional infants infected early in pregnancy (A10E001, A10E011), including focal necrosis and focal hemorrhage in the basal ganglia and spinal cord (Fig. 4A; Fig. S4). Focal cerebral microcalcifications were found in 44% (4 of 9) of the infants (549, GBW1, AY21, AZ77) (Fig. 2F; Figs. S2, S6). Moreover, 77% (7 of 9) of the infants showed lesions in the spinal cord (556, 549, A10E011, 488, A10E001, AZ77, AY21), which is a key pathologic feature of human CZS and is associated with arthrogryposis (Aragao et al., 2017). Spinal cord lesions were similar to brain lesions and consisted of vasculitis, perivascular neuronal necrosis, and foci of hemorrhage associated with local myelin degeneration (Fig. 4A–C; Fig. S3). Three infants also showed periorbital myositis and myodegeneration (A10E001, 549, GBW1; Fig. 4D, E; Figs. S2, S4, S6). Ocular pathology included uveitis, choroid thickening, hypercellularity of the optic nerve, and vasculitis of choroidal, scleral, and periocular vessels (Figs. S2–S4) (Fernandez et al., 2017; Mohr et al., 2018; Nguyen et al., 2017). In total, 100% (6 of 6) of the infants infected early in pregnancy developed neuropathology, and many also developed spinal cord, ocular, and neuromuscular pathology (Table 1).
Figure 4. Spinal cord, ocular, and neuromuscular pathology in ZIKV-infected infants.
A, Perivascular necrosis in thoracic spinal cord (TSC) of infant A10E001; call-out (*), higher magnification showing perivascular neuronal necrosis (arrowheads) and vasculitis (arrows); scale bar = 200 microns; H&E. B, Vasculitis and necrosis of vessels (dashed areas) in white matter of cervical spinal cord (CSC) with adjacent fragmentation of myelin; call-out (*), normal myelin with “beads on a string” appearance (dashed area, dashed arrow), fragmented myelin (dashed area, solid arrow). Luxol Fast Blue with Cresyl Violet counterstain, scale bar = 50 microns. C, Vasculitis (call-out *, arrows) in gray matter of lumbar spinal cord (LSC) near central canal; hematoxylin and eosin, scale bar = 200 microns. D, Semitendinosus muscle adjacent to sciatic nerve from infant 549 showing loss of myofiber cross striations and infiltrates of lymphocytes within the perimysium of the muscle fascicles consistent with inflammation and myodegeneration; H&E, scale bar = 50 microns. E, Focal lymphocyte infiltrate in periorbital muscle (arrow) and vasculitis of periorbital arteriole (call out *, arrowhead). See also Figure S2–S6.
Neuroprogenitor Cell Dysfunction and Neuronal Apoptosis
ZIKV is believed to infect neuroprogenitor cells, which may lead to premature maturation, apoptosis, and abnormalities in cell migration (Cugola et al., 2016; Miner et al., 2016; Oh et al., 2017; Qian et al., 2016; Tang et al., 2016; Waldorf et al., 2018). We observed increased numbers of neuroprogenitor cells in ZIKV infected infants (Fig. 5A–E). Two infants (549, A10E001) also showed disorganized neuroprogenitor cell migration in the frontal cortex, including large aggregates of periventricular neuroprogenitor cells that likely reflect persistence of the ganglionic eminence (Ulfig, 2002) (Fig. 5D; Fig. S4). In human infants, regression of the ganglionic eminence is almost complete at 36 weeks gestation (Del Bigio, 2011), and studies of migration of neurons from the ganglionic eminence in monkeys suggest similarities with humans (Petanjek et al., 2009; Zecevic and Rakic, 2001). ZIKV-infected infant monkeys had higher overall neuroprogenitor scores than uninfected controls (p=0.0028, Fig. 5F, Table S1) and 55% (5 of 9) of the infected infant monkeys showed significant irregularities in the localization and distribution of neuroprogenitor cells. ZIKV-infected infants had aggregates of neuroprogenitor cells more loosely organized along the subventricular zone and in multiple regions of the brain, as compared to uninfected control infants (Fig. 5C, E). In addition, the number of apoptotic cells was substantially increased in the neuroprogenitor-rich subventricular zone of infected animals (Fig. 5G) as compared with uninfected controls (Fig. 5H). ZIKV-infected infants had higher overall maximum apoptosis scores than uninfected infants (p=0.001, Fig. 5I, Table S1).
Figure 5. Proliferation and apoptosis of neuroprogenitor cells in ZIKV-infected infants.
Neuroprogenitor cells (arrows) in the basal ganglia (BG, A10E001) (A) and frontal cortex (FC, GBW1) (B) of infected infants compared with an age-matched uninfected infant (C); H&E; scale bars = 200 microns. Increased periventricular neuroprogenitor cells (arrow) in the frontal cortex (FC, 549) of an infected infant (D) compared with an age-matched control (E); dashed arrows represent the lateral ventricle, Luxol Fast Blue with Cresyl Violet counter stain; scale bars = 200 microns. F, Maximum neuroprogenitor score from prefrontal cortex (PFC), FC, and BG brain sections from ZIKV infants compared to control infants (p=0.0028, unpaired t-test). Immunohistochemistry for cleaved caspase 3 (Cl-Caspase 3) in region of neuroprogenitors in (A) (BG, A10E001) and adjacent neuropil (insets) (G) as compared with an age-matched uninfected infant (H); scale bar = 50 microns. I, Maximum apoptosis score from PFC, FC, and BG brain sections from ZIKV-infected compared with control infants (p= 0.001, unpaired t-test). Red bars reflect mean scores. See also Figures S4–S5. Scoring systems are defined in Table S1.
ZIKV Detection in Tissues
We next assessed ZIKV viral loads in fetal tissues by RT-PCR (Abbink et al., 2016; Aid et al., 2017). In the aborted fetus (556), high levels of ZIKV was detected in multiple central nervous system and peripheral tissues, including, brain, spinal cord, spleen, lymph nodes, and kidney (Fig. 6A). ZIKV staining was confirmed in renal tubular epithelium by immunohistochemistry (Fig. 6E–G). Infant AY21, which was the earliest of the late pregnancy infection group (12 weeks), also had virus detectable in multiple brain tissues by RT-PCR (Fig. 6A). This finding was confirmed in meninges by immunohistochemistry (Fig. 6B–D) and was associated with histiocytic meningitis as evidenced by the presence of CD68+ macrophages (Fig. S5). ZIKV was also detected in this animal in occipital cortex (OC) and frontal cortex (FC), which correlate with the observed microcalcification (Fig. 2F) and astrocytosis (Fig. S5). The other infants demonstrated sporadic virus in peripheral tissues, suggesting that virus may have been cleared in these fetal tissues by term.
Figure 6. Tissue Virus detection in ZIKV-infected infants.
A, Tissue ZIKV viral loads by RT-PCR in infants at term or at the time of fetal loss (556). Immunohistochemistry (IHC) for ZIKV envelope protein (ZIKV Env) (B) versus isotype control antibody (C) in the meninges of infant AY21. D, IHC for ZIKV Env in meninges from uninfected control infant. In situ hybridization (ISH) for ZIKV RNA in kidney from fetus 556 (E) compared to ISH for control RNA in same tissue section (F). G, ISH for ZIKV RNA in uninfected control infant. Scale bars= 20 microns.
DISCUSSION
By comprehensive radiographic, histopathologic, immunologic, and virologic analyses, we demonstrate that ZIKV infection during early pregnancy in female rhesus monkeys recapitulates many features of CZS in humans (Chimelli et al., 2017; Martines et al., 2016; Soares de Oliveira-Szejnfeld et al., 2016; Sousa et al., 2017; Strafela et al., 2017) (Table 1). 100% (6 of 6) of infants born to dams infected early in pregnancy (≤7wks) demonstrated neuropathology. Histopathologic lesions in the central nervous system included microcalcifications, hemorrhage, vasculitis, and neuronal necrosis, which correlated with hyperintense foci observed by high-resolution MRI. Fetal neuropathology was characterized by vascular compromise as well as neuroprogenitor cell proliferation, disorganization, and apoptosis. The coexistence of vascular changes in ZIKV-infected infants and in placentas of the dams suggests that both vascular insufficiency and neuroprogenitor cell dysfunction play key roles in the early pathogenesis of CZS, resulting in necrosis and gliosis of highly vascularized deep gray matter tissue and abnormal migration of neuroprogenitor cells, leading to focal defects and congenital abnormalities. These insights into the pathogenesis of CZS suggest novel strategies for prevention and treatment by targeting these key pathways. Moreover, this primate model should be useful for testing vaccines and other interventions that are currently in clinical development.
Mouse models of ZIKV infection during pregnancy have shown that ZIKV-induced damage to the placenta leads to placental insufficiency, intrauterine growth restriction, and fetal loss (Miner et al., 2016). Our data confirm and extend these findings by showing that 66% (6 of 9) of ZIKV-infected pregnant dams exhibited severe placental pathology (Table 1, Fig. 1, Fig. S1) including fetal loss, multiple placental infarctions, neutrophilic chorioamnionitis, and histiocytic villitis. Our findings further suggest that ZIKV targets vascular endothelial cells in the placenta, leading to reactive endothelial cells, vasculitis, thrombosis, and infarction with subsequent collapse and sclerosis of villar vessels (Fig. 1B). This process may contribute to the development of deciduitis and chorioamnionitis, possibly secondary to necrosis of infarcted tissues (Nguyen et al., 2017).
In a cohort of ZIKV-infected pregnant women in Brazil, congenital neurologic abnormalities were identified in 55% of first trimester infections and 29% of third trimester infections, with microcephaly in 3% and fetal demise in 7% of these pregnancies (Brasil et al., 2016). In the United States, congenital neurologic abnormalities were identified in 11% of first trimester infections but in no second and third trimester infections (Honein et al., 2017). Consistent with these studies, we detected high frequencies of fetal neuropathologic lesions in rhesus monkeys infected early in pregnancy. Further studies will be required to define fully the extent of neuropathology later in pregnancy. We did not detect gross microcephaly, presumably because of the rarity of this condition, but we did observe reduced fetal brain size (Fig. 2A–C) and agyria in one animal (Fig. 2N–Q). No gender-related differences in gross or histopathology of the infants were noted (5 males, 3 females).
Prior preclinical studies of congenital Zika infection in immunosuppressed mice (Miner et al., 2016; Sapparapu et al., 2016) and nonhuman primates (Adams Waldorf et al., 2016; Nguyen et al., 2017; Waldorf et al., 2018) did not demonstrate consistent or severe neuropathology. Subtle injury to the ependymal epithelium with underlying gliosis was observed in monkeys infected with a Cambodian ZIKV isolate (Adams Waldorf et al., 2016; Waldorf et al., 2018), and neutrophilic infiltration in the placenta and ocular pathology were reported in four rhesus monkeys infected with a Polynesian ZIKV isolate (Nguyen et al., 2017). It remains to be determined whether the substantially more extensive fetal neuropathology observed in the present study reflects the comprehensive analytic approaches in the present study, possible differences in ZIKV challenge strains, or differences in monkey species or cohorts.
ZIKV has been shown to infect neuroprogenitor cells in vitro and in mouse models (Cugola et al., 2016; Miner et al., 2016; Oh et al., 2017; Qian et al., 2016; Tang et al., 2016), and autopsy studies in humans have revealed a spectrum of abnormalities, including abnormal proliferation and migration of these cells (Chimelli et al., 2017). Consistent with these findings, we observed that 83% (5 of 6) of early pregnancy infected infants demonstrated proliferation, apoptosis, aggregation, and disorganization of neuroprogenitor cells (Table 1). These data suggest that dysregulation and dysfunction of these cells may underlie the diverse neuropathologic lesions that characterize CZS (Bhatnagar et al., 2017; Miner et al., 2016).
We also observed vasculitis and vascular compromise as hallmarks of the neuropathology observed in the ZIKV-infected infant monkeys, with overt vasculitis in 66% (4 of 6) of fetuses infected early in pregnancy (Table 1). Vasculitis and villitis associated with ZIKV RNA localization was observed in placental villi in one animal (556), and two fetuses (549, 488) demonstrated severe widespread central nervous system vasculitis that correlated with lesions in highly vascularized deep gray matter nuclei, suggesting vascular compromise. Vasculitis and vascular compromise have not been extensively studied in human CZS but have been suggested by similar lesions in vascularized deep gray matter structures (Chimelli et al., 2017; Shao et al., 2016). In addition, a ZIKV-associated stroke associated with viral vasculitis has been described in a pediatric case report (Landais et al., 2017), suggesting the generalizability of these observations.
In summary, we demonstrate that ZIKV infection of pregnant female rhesus monkeys early in pregnancy leads to extensive fetal neuropathologic lesions that are characteristic of CZS. Our data suggest that vascular compromise and neuroprogenitor cell dysfunction early during fetal development are critical early pathologic events that lead to placental insufficiency and neuropathology. These insights augment our current understanding of CZS pathogenesis and suggest novel approaches for the development of prophylactic and therapeutic strategies for this disease.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Dan Barouch (dbarouch@bidmc.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Outbred, healthy Indian-origin female rhesus monkeys (Macaca mulatta) were housed at Bioqual, Rockville, MD or Alphagenesis Inc., SC. Animals selected for this study were research naïve. Pregnant females were grouped housed within their respective breeding groups or individually housed in a room with other female animals to minimize stress. All animals were provided enrichment according to recommended guidelines. Nine female rhesus monkeys confirmed pregnant by ultrasound were challenged subcutaneously with 106 virus particles (VP) [103 plaque-forming units (PFU)] ZIKV-BR (Brazil ZKV2015). The time of challenge ranged from 6–14 weeks of gestation, reflecting early pregnancy (≤7 weeks) and later in pregnancy (≤12 weeks). Fetal development was monitored bi-weekly by ultrasound, and animals that survived to term were delivered by caesarian section. Five live male infants and three live female infants were delivered. All animal studies were approved by the Alphagenesis Institutional Animal Care and Use Committee (IACUC) or the Bioqual IACUC, as appropriate. All experiments conformed to regulatory standards outlined by the American Veterinary Medical Association (AVMA) and American Association of Laboratory Animal Medicine (AALAM).
METHOD DETAILS
ZIKV Challenge Stock Preparation
ZIKV-BR (Brazil ZKV2015) was propagated in Vero cells (World Health Organization, NICSC-011038011038) that were maintained in EMEM media supplemented with 10%FBS, 6mM L-glutamine and 1x pen/strep. Cells were passaged twice a week and incubated at 37°C, 10% CO2.
Ultrasonography
Ultrasounds were performed bi-weekly in the ZIKV-infected pregnant rhesus monkeys as well as in 28 concurrent uninfected pregnant rhesus monkeys in the same breeding facility. Animals were sedated with Telazol (5mg/kg), and a GE Logic E with a 8CRS Micro-convex transducer (FOV 132°, 3.6–10MHz) was used for multiparameter b iometric measurements, including biparietal diameter (BPD), occipitofrontal diameter (OFD), head circumference (HC), crown-rump length (CRL), abdominal circumference (AC), and femur length (FL) as shown in Figure 2.
Amniocentesis
Animals were sedated with Telazol HCL (4–7 mg/kg IM). The area on the abdomen was clipped and sterilely prepped with triple alternating applications of betadine and alcohol. Using sterile technique, a 22-gauge 3.34 inch needle on a 3 cc syringe was inserted into the ventral abdomen to the amniotic sac with ultrasound guidance. 2 cc of amniotic fluid was collected and frozen immediately.
RT-PCR
RT-PCR assays were utilized to monitor viral loads in plasma, CSF, lymph node biopsies, colorectal biopsies, colorectal weck samples, and urine longitudinally every 2–4 weeks as indicated in the experimental design (see Fig. 1A) and amniotic fluid collected by amniocentesis at day 14 post-ZIKV infection, and from tissues collected at necropsy, essentially as previously described (Abbink et al., 2016; Aid et al., 2017; Larocca et al., 2016). RNA was extracted with a QIAcube HT (Qiagen, Germany). Liquid samples were extracted using the Qiacube 96 Cador pathogen HT, and tissue samples were lysed in Qiazol, using the Tissuelyser II (Qiagen, Germany), chloroform treated and extracted with the Qiacube 96 RNeasy HT kit. The wildtype ZIKV BeH815744 Cap gene was utilized as a standard. RNA standards were generated using the AmpliCap-Max™ T 7 High Yield Message Maker Kit (Cell Script) and purified with RNA clean and concentrator kit (Zymo Research, CA, USA). RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per microgram of total RNA as measured on the NanoDrop (Thermo Scientific, Waltham, MA, USA) or as VP per million cells, as shown in Figures 1 and 6. Assay sensitivity was >100 copies/ml, >100 copies per million cells, and >3 copies/μg total RNA.
Neutralization Assay
A high-throughput, standardized ZIKV microneutralization (MN) assay was utilized for measuring ZIKV-specific neutralizing antibodies, essentially as previously described (Abbink et al., 2016; Aid et al., 2017; Larocca et al., 2016). Briefly, serum samples were serially diluted three-fold in 96-well micro-plates, and 100 μl of ZIKV-PR containing 100 PFU were added to 100 μl of each serum dilution and incubated at 35°C for 2 h. Supernatants were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 d, cells were fixed with absolute ethanol: methanol for 1 h at −20°C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from JT Roehrig, CDC) was then added to each well, incubated at 35°C for 2 h, and washed with PBS. Plates were washed, developed with 3,3′,5,5′–tetramethylbenzidine (TMB) for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be ≤ 0.5, and virus-only control wells had to be ≥ 0.9. Normalized absorbance values were calculated, the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized ≥ 50% of ZIKV, and seropositivity was defined as a titer ≥ 10, with the maximum measurable titer 7,290, as shown in Figure 1.
Tissue Collection and Histopathology
Within 14 days of estimated term gestation (26 weeks), dams and fetuses were euthanized with intravenous sodium pentobarbital, and delivery was by caesarian section. Complete necropsies were performed by a veterinarian on dams and fetuses immediately following euthanasia, utilizing standard necropsy procedures with standard sterile surgical grade necropsy instruments and dissection blades Briefly, peripheral lymphoid tissues were collected, followed by the gastrointestinal tract and abdominal organs. The pleural cavity was opened and the tongue, pharynx, trachea, esophagus, heart and lungs (“pluck”) were removed en masse. Reproductive organs were collected, followed by brain, spinal cord, and eyes with minimal use of a strycker saw to prevent tissue artifacts. Ruskin-liston bone cutting forceps were used to expose the spinal cord to the level of the cauda equina. Fresh tissues were collected utilizing sterile blades for viral RT-PCR in RNAlater (Ambion). Frozen tissue for histopathology was prepared by trimming tissue, placing tissue samples into cryomolds with optimal cutting temperature medium (OCT, Tissue-Tek), and flash freezing on-site. Additional tissues were fixed in 10% neutral buffered formalin (NBF) for histopathology. Formalin-fixed tissues were trimmed, processed and embedded in paraffin, sectioned and stained with hematoxylin and eosin, and evaluated independently by two veterinary pathologists (A.J.M., R.B.) and gynecologic pathologist (J.L.H) as shown in Figures 1–6 and reported in Table 1.
Special Stains, Immunohistochemistry, and In Situ Hybridization
Myelination was evaluated using Luxol-Fast Blue counterstained with Cresyl Violet. Immunohistochemistry was performed for cleaved caspase-3 (Cl-Caspase 3, Cell Signaling, clone Asp175; dilution 1:100), CD68 (Dako, clone kP1, dilution 1:410), and ZIKV envelope (BioFront Technologies, 1:200). Briefly, tissue sections were deparaffinized in xylene and rehydrated through graded ethanol solutions to distilled water. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide followed by heat induced epitope retrieval (HIER) in citrate buffer (Vector Labs) using a slide steamer (IHC World). Tissues were treated for nonspecific protein binding (Protein Block, DAKO) followed by application of primary antibodies for 30 minutes at room temperature (RT, Cl-Caspase 3), or 1 hour overnight at 4 °C (CD68 and ZIKV Env). Biotinylated goat-anti rabbit IgG (Cl-Caspase 3) or horse anti-mouse IgG (CD68) were applied for 30 min at RT at 1:200 (Vector Labs). Antigen-antibody complexes were localized by application of streptavidin-horseradish peroxidase (HRP) conjugate (ABC Elite, Vector Labs) followed by development in chromogenic 3,3′-diaminobenzidine (DAB, DAKO) for Cl-Caspase 3 and CD68. For ZIKV envelope (Env), a biotin-free polymer-based alkaline phosphatase kit with Permanent Red was used to detect antigen-antibody complexes (Polink-1 AP, Golden Bridge International Labs) as shown in Figure 6. In situ detection of ZIKV RNA was performed using RNAscope (ACDBio) technology as shown in Figure 1 and 6. The ZIKV Asian probe (formerly O4) and red detection kit were used according to the manufacturer’s instructions as seen in Figure 1 and 6. Tissue scoring was developed by AJM and RB as described in Table S1.
Magnetic Resonance Imaging
Formal fixed hemispheric brain was rocked with 1% PBS for 24 hours at room temperature to eliminate formalin and air bubbles. The specimen was placed in a custom fit, sealed plastic bag (Food Saver) filled Galden HT-270 (Kurt Lesker CO), a dense, proton-free liquid that prevents MR signal, and decreases susceptibility artifact. The bagged specimen was vigorously rocked for 24 h to release air bubbles that contribute to MR susceptibility artifact and residual PBS from the sample. After removal of residual PBS, the specimen is sealed for MR acquisition. MR acquisitions were achieved on a 3 Tesla Siemens Skyra scanner with 64-channel head coil and a 7 mm loop coil. Three different MRI sequences were used to generate different contrasts: 1) 0.3 mm isotropic resolution T1-weighted gradient echo acquisition with 4 averages (total scan time~3 hours). 2) T2-weigted fast spin echo (T2-FSE) sequence with 200 micron in-plane resolution and 800micron slice thickness, 8 averages with a total scan time ~3 hours. 3) Phase sensitive inversion recovery sequence with matching resolution to T2-FSE with 8 averages and total scan time of 3 hours. Images are based on Sag T1-weighted sequences where “k” is a maximum intensity projection” of the sagittal T1-weighted image for that subject as shown in Figure 2. Images were evaluated by a pediatric radiologist (E.Y.) on a Fuji Synapse PACS (Picture Archiving and Communication System) with reformats generated using a Synapse 3D thin client workstation.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of virologic and immunologic data was performed using GraphPad Prism v6.03 (GraphPad Software, CA, USA). Comparisons of groups were performed using unpaired Student-test or one-way ANOVA for groups of three or more, followed by Tukey post-test for multiple comparisons. A p value cut-off of 0.05 was considered statistically significant. Fetal biometry curves were evaluated using non-linear regression and extra-sums F test.
DATA AND SOFTWARE AVAILABILITY
All data generated and analyzed in this study are available from the corresponding author upon reasonable request.
Supplementary Material
A, Arterial thrombosis in placenta from Dam 549 (arrow) and downstream infarcted, necrotic placental tissue (arrowhead); Scale bar= 500 microns. B, Villar sclerosis (arrowheads) and loss of fetal vessels within villi in placenta from Dam A10E011; Scale bar= 500 micron. C, Intervillous inflammatory cells extending from decidua in placenta from Dam GBW1; Scale bar= 100 microns. D, Intervillositis (arrow) in Dam 556 that had concomitant bacterial chorioamnionitis; Scale bar= 50 microns. E, Islands of intermediate trophoblasts extending along stem villi (arrow) in placenta from Dam AZ77; Scale bar= 500 microns. F, Deciduitis (arrow) in placenta from Dam 488; Scale bar= 50 microns.
A, Gross brain specimen (unfixed) from infant 549 showing focal asymmetry between the two brain hemispheres with absence of one of two occipital gyri in the right hemisphere. B, Sectioned gross brain specimen from left hemisphere of infant 549 showing hemorrhage in the thalamus (upper) and periventricular malacia (softening of the brain, lower) in the occipital lobe of fetus 549. C, Myocyte degeneration in skeletal muscle. D, Vasculitis (arrowhead) and hemorrhage (arrow) in the lumbar spinal cord (LSC). E, Focal microhemorrhage (arrow) and microcalcification in basal ganglia (arrowhead). F, Vasculitis (arrowhead) of a blood vessel within the ganglion cell layer of the retina. G, Thickening and hypercellularity of the choroid with vasculitis in choroidal vessels (call out *, arrowheads). Scale bars = 50 microns.
A, Vasculitis in lumbar spinal cord (LSC) (arrow) with neuronal necrosis (arrowhead); H&E, scale bar = 50 microns. B, IHC for cleaved caspase 3 in basal ganglia (BG) showing increased periventricular apoptosis (inset, apoptotic glial cell), scale bar = 50 microns. C, IHC for CD68 showing increased presence perivascular microglial cells in BG, scale bar = 50 microns. D, vasculitis (arrows) in choroidal vessels of the eye; H&E, scale bar = 50 microns. E, Vasculitis in scleral vessels of the eye; H&E, scale bar = 20 microns. F, Focal infiltrate of inflammatory cells (arrowhead) within the uvea (uveitis, eye); H&E, scale bar = 50 microns. G, Focal edema and disruption of myelin within the white matter of the temporal cortex (TC); LFB/CV, scale bar = 200 microns, call out (*) = Higher magnification showing separation of the myelin fibers (arrowheads) from panel G. H&E = hematoxylin and eosin, LFB/CV = Luxol fast blue with cresyl violet counterstain.
A, Focal microhemorrhage (call-out *, arrowhead) in the frontal cortex of A10E001; H&E, scale bar = 200 microns. B, Large neuroprogenitor cell aggregate in the frontal cortex (FC) of A10E001; H&E, scale bar = 200 microns. C, IHC for glial fibrillary acidic protein (GFAP) within neuroprogenitor aggregate from B in A10E001; scale bar = 50 microns. D, Infiltrates of lymphocytes (arrowhead) in periorbital muscle of A10E001; H&E, scale bar = 50 microns. E, Hypercellularity (inset, arrowhead) of optic nerve of A10E001; H&E, scale bar = 200 microns. F, Focal hemorrhage (call-out *, arrowhead) in cervical spinal cord (CSC) of A10E011. G, Periganglionic vasculitis (arrowhead) in pancreatic connective tissue in A10E011; H&E, scale bar = 50 microns.
A, Glial nodule in cortex near basal ganglia (BG); H&E, scale bar = 50 microns. B, Higher magnification of glial nodule with CD68 immunohistochemistry on serial section from A. C, Thoracic spinal cord (TSC) showing microhemorrhage; H&E, scale bar = 50 microns. D, Immunohistochemistry for glial fibrillary acidic protein (GFAP) in occipital cortex (OC) showing increased numbers of astrocytes (call-out *, arrowhead). E, Immunohistochemistry for GFAP in OC of uninfected age-matched control. F, Immunohistochemistry for CD68 in meninges from Figure 6B showing infiltrates of macrophages (call-out *) consistent with mild histiocytic meningitis.
A, Focal microcalcification in basal ganglia of AZ77; scale bar = 50 microns. B, Higher magnification of glial nodule in basal ganglia from infant GBW1; scale bar = 50 microns. C, Periorbital myositis in GBW1, scale bar = 50 microns. D, Focal myodegeneration and inflammation in skeletal muscle of GBW1; scale bar = 50 microns. E, Focal microhemorrhage (arrow) and microcalcification (arrowhead) in prefrontal cortex of GBW1; scale bar = 20 microns. All figures are stained with hematoxylin and eosin.
Highlights.
ZIKV infection in early pregnancy leads to fetal neuropathology in rhesus monkeys
Placental pathology in ZIKV infected dams is associated with severe fetal pathology
Key features include vascular compromise and neuroprogenitor cell proliferation
Acknowledgments
We thank J. Estes, S. Westmoreland, M. Boyd, R. Nityanandam, N. Mercado, Z. Li, M. Kirilova, J. LeSuer, M. Kamath, S. Khatiwada, A. Agarwal, P. Gandhi, A. Chandrashekar for generous advice, assistance, and reagents. We acknowledge support from the National Institutes of Health (AI096040, AI100663, AI124377) and the Ragon Institute of MGH, MIT, and Harvard.
Footnotes
AUTHOR CONTRIBUTIONS
A.J.M., P.A., R.A.L., and D.H.B. designed the studies. A.J.M., O.A., A.P., R.D., E.Y., J.L.H, and S.W. led the pathologic and radiologic analyses. P.A. and R.D.B. led the virologic assays. E.N.B. led the study organization and specimen processing. W.R., M.F., P.D., D.W., and M.G.L. led the animal work. A.J.M. and D.H.B. wrote the paper with all co-authors.
DECLARATION OF INTERESTS
The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, Walke H, Oduyebo T, Polen K, Peacock G, et al. Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection - United States, October 2017. MMWR Morbidity and mortality weekly report. 2017;66:1089–1099. doi: 10.15585/mmwr.mm6641a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Marques ET, Jr, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta. Lancet. 2016;387:1587–1590. doi: 10.1016/S0140-6736(16)00650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, Martinot AJ, Moseley ET, Blass E, Borducchi EN, et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell. 2017;169:610–620. e614. doi: 10.1016/j.cell.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragao M, Brainer-Lima AM, Holanda AC, van der Linden V, Vasco Aragao L, Silva MLM, Junior, Sarteschi C, Petribu NCL, Valenca MM. Spectrum of Spinal Cord, Spinal Root, and Brain MRI Abnormalities in Congenital Zika Syndrome with and without Arthrogryposis. AJNR American journal of neuroradiology. 2017;38:1045–1053. doi: 10.3174/ajnr.A5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerging infectious diseases. 2017;23:405–414. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimelli L, Melo ASO, Avvad-Portari E, Wiley CA, Camacho AHS, Lopes VS, Machado HN, Andrade CV, Dock DCA, Moreira ME, et al. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta neuropathologica. 2017;133:983–999. doi: 10.1007/s00401-017-1699-5. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain: a journal of neurology. 2011;134:1344–1361. doi: 10.1093/brain/awr052. [DOI] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Parra Saad E, Ospina Martinez M, Corchuelo S, Mercado Reyes M, Herrera MJ, Parra Saavedra M, Rico A, Fernandez AM, Lee RK, et al. Ocular Histopathologic Features of Congenital Zika Syndrome. JAMA ophthalmology. 2017;135:1163–1169. doi: 10.1001/jamaophthalmol.2017.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell. 2017;20:397–406. e395. doi: 10.1016/j.stem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Guillemette-Artur P, Besnard M, Eyrolle-Guignot D, Jouannic JM, Garel C. Prenatal brain MRI of fetuses with Zika virus infection. Pediatric radiology. 2016;46:1032–1039. doi: 10.1007/s00247-016-3619-6. [DOI] [PubMed] [Google Scholar]
- Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nature communications. 2018;9:263. doi: 10.1038/s41467-017-02499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2017;317:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016;375:1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais A, Cesaire A, Fernandez M, Breurec S, Herrmann C, Delion F, Desprez P. ZIKA vasculitis: A new cause of stroke in children? Journal of the neurological sciences. 2017;383:211–213. doi: 10.1016/j.jns.2017.10.045. [DOI] [PubMed] [Google Scholar]
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388:898–904. doi: 10.1016/S0140-6736(16)30883-2. [DOI] [PubMed] [Google Scholar]
- Mejdoubi M, Monthieux A, Cassan T, Lombard C, Flechelles O, Adenet C. Brain MRI in Infants after Maternal Zika Virus Infection during Pregnancy. N Engl J Med. 2017;377:1399–1400. doi: 10.1056/NEJMc1612813. [DOI] [PubMed] [Google Scholar]
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, Batista AG, Ferreira T, Dos Santos MP, Sampaio VV, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA neurology. 2016;73:1407–1416. doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr EL, Block LN, Newman CM, Stewart LM, Koenig M, Semler M, Breitbach ME, Teixeira LBC, Zeng X, Weiler AM, et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS ONE. 2018;13:e0190617. doi: 10.1371/journal.pone.0190617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, Ribeiro EM, Ventura LO, Neto NN, Arena JF, et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA pediatrics. 2017;171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13:e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Zhang F, Wang Y, Lee EM, Choi IY, Lim H, Mirakhori F, Li R, Huang L, Xu T, et al. Zika virus directly infects peripheral neurons and induces cell death. Nature neuroscience. 2017;20:1209–1212. doi: 10.1038/nn.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cerebral cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;15:443–447. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, Chen JF. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143:4127–4136. doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, Tanuri A, Aguiar RS, Malinger G, Ximenes R, et al. Congenital Brain Abnormalities and Zika Virus: What the Radiologist Can Expect to See Prenatally and Postnatally. Radiology. 2016;281:203–218. doi: 10.1148/radiol.2016161584. [DOI] [PubMed] [Google Scholar]
- Sousa AQ, Cavalcante DIM, Franco LM, Araujo FMC, Sousa ET, Valenca-Junior JT, Rolim DB, Melo MEL, Sindeaux PDT, Araujo MTF, et al. Postmortem Findings for 7 Neonates with Congenital Zika Virus Infection. Emerging infectious diseases. 2017;23:1164–1167. doi: 10.3201/eid2307.162019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafela P, Vizjak A, Mraz J, Mlakar J, Pizem J, Tul N, Zupanc TA, Popovic M. Zika Virus-Associated Micrencephaly: A Thorough Description of Neuropathologic Findings in the Fetal Central Nervous System. Archives of pathology & laboratory medicine. 2017;141:73–81. doi: 10.5858/arpa.2016-0341-SA. [DOI] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N. Ganglionic eminence of the human fetal brain--new vistas. The Anatomical record. 2002;267:191–195. doi: 10.1002/ar.10104. [DOI] [PubMed] [Google Scholar]
- Vermillion MS, Lei J, Shabi Y, Baxter VK, Crilly NP, McLane M, Griffin DE, Pekosz A, Klein SL, Burd I. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nature communications. 2017;8:14575. doi: 10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf KMA, Nelson BR, Stencel-Baerenwald JE, Studholme C, Kapur RP, Armistead B, Walker CL, Merillat S, Vornhagen J, Tisoncik-Go J, et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med. 2018 Feb 5; doi: 10.1038/nm.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Arterial thrombosis in placenta from Dam 549 (arrow) and downstream infarcted, necrotic placental tissue (arrowhead); Scale bar= 500 microns. B, Villar sclerosis (arrowheads) and loss of fetal vessels within villi in placenta from Dam A10E011; Scale bar= 500 micron. C, Intervillous inflammatory cells extending from decidua in placenta from Dam GBW1; Scale bar= 100 microns. D, Intervillositis (arrow) in Dam 556 that had concomitant bacterial chorioamnionitis; Scale bar= 50 microns. E, Islands of intermediate trophoblasts extending along stem villi (arrow) in placenta from Dam AZ77; Scale bar= 500 microns. F, Deciduitis (arrow) in placenta from Dam 488; Scale bar= 50 microns.
A, Gross brain specimen (unfixed) from infant 549 showing focal asymmetry between the two brain hemispheres with absence of one of two occipital gyri in the right hemisphere. B, Sectioned gross brain specimen from left hemisphere of infant 549 showing hemorrhage in the thalamus (upper) and periventricular malacia (softening of the brain, lower) in the occipital lobe of fetus 549. C, Myocyte degeneration in skeletal muscle. D, Vasculitis (arrowhead) and hemorrhage (arrow) in the lumbar spinal cord (LSC). E, Focal microhemorrhage (arrow) and microcalcification in basal ganglia (arrowhead). F, Vasculitis (arrowhead) of a blood vessel within the ganglion cell layer of the retina. G, Thickening and hypercellularity of the choroid with vasculitis in choroidal vessels (call out *, arrowheads). Scale bars = 50 microns.
A, Vasculitis in lumbar spinal cord (LSC) (arrow) with neuronal necrosis (arrowhead); H&E, scale bar = 50 microns. B, IHC for cleaved caspase 3 in basal ganglia (BG) showing increased periventricular apoptosis (inset, apoptotic glial cell), scale bar = 50 microns. C, IHC for CD68 showing increased presence perivascular microglial cells in BG, scale bar = 50 microns. D, vasculitis (arrows) in choroidal vessels of the eye; H&E, scale bar = 50 microns. E, Vasculitis in scleral vessels of the eye; H&E, scale bar = 20 microns. F, Focal infiltrate of inflammatory cells (arrowhead) within the uvea (uveitis, eye); H&E, scale bar = 50 microns. G, Focal edema and disruption of myelin within the white matter of the temporal cortex (TC); LFB/CV, scale bar = 200 microns, call out (*) = Higher magnification showing separation of the myelin fibers (arrowheads) from panel G. H&E = hematoxylin and eosin, LFB/CV = Luxol fast blue with cresyl violet counterstain.
A, Focal microhemorrhage (call-out *, arrowhead) in the frontal cortex of A10E001; H&E, scale bar = 200 microns. B, Large neuroprogenitor cell aggregate in the frontal cortex (FC) of A10E001; H&E, scale bar = 200 microns. C, IHC for glial fibrillary acidic protein (GFAP) within neuroprogenitor aggregate from B in A10E001; scale bar = 50 microns. D, Infiltrates of lymphocytes (arrowhead) in periorbital muscle of A10E001; H&E, scale bar = 50 microns. E, Hypercellularity (inset, arrowhead) of optic nerve of A10E001; H&E, scale bar = 200 microns. F, Focal hemorrhage (call-out *, arrowhead) in cervical spinal cord (CSC) of A10E011. G, Periganglionic vasculitis (arrowhead) in pancreatic connective tissue in A10E011; H&E, scale bar = 50 microns.
A, Glial nodule in cortex near basal ganglia (BG); H&E, scale bar = 50 microns. B, Higher magnification of glial nodule with CD68 immunohistochemistry on serial section from A. C, Thoracic spinal cord (TSC) showing microhemorrhage; H&E, scale bar = 50 microns. D, Immunohistochemistry for glial fibrillary acidic protein (GFAP) in occipital cortex (OC) showing increased numbers of astrocytes (call-out *, arrowhead). E, Immunohistochemistry for GFAP in OC of uninfected age-matched control. F, Immunohistochemistry for CD68 in meninges from Figure 6B showing infiltrates of macrophages (call-out *) consistent with mild histiocytic meningitis.
A, Focal microcalcification in basal ganglia of AZ77; scale bar = 50 microns. B, Higher magnification of glial nodule in basal ganglia from infant GBW1; scale bar = 50 microns. C, Periorbital myositis in GBW1, scale bar = 50 microns. D, Focal myodegeneration and inflammation in skeletal muscle of GBW1; scale bar = 50 microns. E, Focal microhemorrhage (arrow) and microcalcification (arrowhead) in prefrontal cortex of GBW1; scale bar = 20 microns. All figures are stained with hematoxylin and eosin.