Abstract
Background
A major obstacle in the treatment of individuals with cocaine addiction is their high propensity for relapse. Although the clinical scenario of acute stress-induced relapse has been well studied in animal models, few pre-clinical studies have investigated the role of chronic stress in relapse or the interaction between chronic stress and other relapse triggers.
Methods
We tested the effect of chronic restraint stress on cocaine seeking in rats using both extinction- and abstinence-based animal relapse models. Rats were trained to press a lever for I.V. cocaine infusions (0.50 mg/kg/infusion) paired with a discrete tone + light cue in daily 3-hr sessions. Following self-administration, rats were exposed to a chronic restraint stress procedure (3 h/day) or control procedure (unstressed) during the first seven days of a 13-day extinction period during which lever presses had no programmed consequences. This was followed by cue- and cocaine priming-induced drug seeking tests. In a separate group of rats, cocaine seeking was assessed during forced abstinence both before and after the same chronic stress procedure.
Results
A history of chronic restraint stress was associated with increased cocaine priming-induced drug seeking, an effect attenuated by co-administration of SCH-23390 (10.0 μg/kg; i.p.), a dopamine D1-like receptor antagonist, with daily restraint. Repeated SCH-23390 administration but not stress during extinction increased cue-induced reinstatement.
Conclusions
Exposure to chronic stress during early withdrawal may confer lasting vulnerability to some types of relapse, and dopamine D1-like receptors appear to mediate both chronic stress effects on cocaine seeking and extinction of cocaine seeking.
Keywords: abstinence, cocaine, dopamine, reinstatement, relapse, stress
1. Introduction
Cocaine addiction is a chronic disorder characterized by high rates of relapse, even after long periods of abstinence. Relapse prevention, therefore, is a primary goal—and a difficult challenge—in the treatment of cocaine addiction. Although chronic stress has long been associated with relapse vulnerability in the clinical literature (Sinha, 2008; Sinha et al., 2011), relatively few pre-clinical studies have used models of relapse that incorporate a chronic stressor. Such models are needed because, although it is known that chronic stress induces lasting structural changes in brain regions implicated in relapse, namely medial prefrontal cortex (mPFC) (for review see Holmes and Wellman, 2009), the functional significance of these changes as they relate to relapse is unknown. Given the overlapping circuitry, it is possible that exposure to chronic stress during drug abstinence increases one’s vulnerability to relapse. In support of this possibility, a few recent studies from our lab and others have shown that chronic or repeated stressors, including food restriction (D’Cunha et al., 2013), social defeat (Holly et al., 2016), the pharmacological stressor yohimbine (Ball et al., 2015), and restraint (Glynn et al., 2016), all increase relapse-like behaviors in rats.
In the present study, we explored the relationship between chronic stress and relapse to cocaine seeking by modifying two contemporary animal models of relapse: the classical reinstatement model (Shaham et al., 2003; Stewart and de Wit, 1987) and the forced abstinence model (Fuchs et al., 2006; Grimm et al., 2001). Both models begin with operant self-administration of a drug and end with non-reinforced responding (the operational definition of relapse) following a drug-free period. The reinstatement model, which requires extinction training before testing, has several strengths, including reliable behavioral effects and evidence for predictive validity (Epstein et al., 2006; Sinha et al., 2011); the forced abstinence model, however, may better reflect the typical human situation in which the drug-free period does not include explicit extinction training. Importantly, there are partially dissociable neural mechanisms underlying drug seeking following extinction training vs. forced abstinence (Fuchs et al., 2006; Marchant et al., 2013). Therefore, it is possible that the effects of chronic stress on relapse-like behavior are different depending on the animal model that is used. With this in mind, we tested the effect of chronic stress during early withdrawal from cocaine self-administration on later cocaine seeking following both explicit extinction training and forced home-cage abstinence.
Given that chronic stress has been shown to cause changes in mesocortical dopamine transmission (Moore et al., 2001) and D1R signaling (Mizoguchi et al., 2008; Mizoguchi et al., 2000), another goal was to determine the role of dopamine D1-like receptors (D1Rs) in any chronic stress-related effects on cocaine seeking. Indeed, D1Rs play a critical role in several types of cocaine-seeking behaviors, including reinstatement triggered by acute stress, cocaine cues, and cocaine priming injections (Capriles et al., 2003; McFarland et al., 2004; McFarland and Kalivas, 2001; Park et al., 2002; Sun and Rebec, 2005). Moreover, we reported that the D1R antagonist, SCH-23390, when combined with chronic stress, attenuated the effects of stress on subsequent reinstatement of food seeking after extinction (Ball et al., 2015; Ball et al., 2016) and during forced abstinence (Ball et al., 2017). Thus, in the present study, we tested the hypothesis that SCH-23390 combined with repeated restraint stress would attenuate any effects of stress on subsequent cocaine-seeking behavior.
2. Materials and methods
2.1. Subjects and apparatus
Adult male, Sprague-Dawley rats weighing 300–360 g at the commencement of experiments were obtained from Envigo (Indianapolis, IN). Rats were housed individually under standard laboratory conditions (12-hr light cycle from 7:00 AM to 7:00 PM) with ad libitum access to food and water. All testing was conducted between 7:00 AM and 7:00 PM and occurred in standard modular operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) that were housed in sound-attenuating, ventilated cubicles and connected to a PC with the Graphic State 4 software interface system (Coulbourn Instruments). Each chamber was equipped with an active and an inactive response lever. Responses on the inactive lever were recorded, but had no programmed consequences. Chambers also included a house light, a row of multicolored LED cue lamps (above active lever), a tone generator, and a fluid swivel with spring leash assembly connected to a counterbalanced arm assembly. A motor-driven syringe pump (Coulbourn Instruments) for drug delivery was located outside of each cubicle. All experiments were in compliance with NIH guidelines and were approved by the Bloomsburg University Institutional Animal Care and Use Committee.
2.2. Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Park, NC). SCH-23390 HCl was purchased from Sigma (St. Louis, MO). Cocaine and SCH-23390 were dissolved in 0.9% sterile saline. The dose of SCH-23390 was chosen based on our previous work (Ball et al., 2017; Ball et al., 2015; Ball et al., 2016) and the doses of cocaine were chosen based on pilot data. All doses refer to weight of salt.
2.3. Catheter surgery
After an injection of atropine sulfate (0.05 mg/kg; s.c.), animals were anesthetized with ketamine HCl (90 mg/kg; i.m.) and xylazine HCl (10 mg/kg; i.m.), with supplemental injections as needed. A catheter, made of silastic tubing (0.51 m I.D. × 0.94 mm O.D.; Dow Corning, Midland, USA) attached to a modified 22-gauge cannula-guide connector assembly (Plastics One, Roanoke, VA), was inserted into the right jugular vein. The catheter was routed subcutaneously and mounted to the skull with dental cement. Catheters were flushed with heparinized physiological saline (30 U/ml heparin) twice daily, and 0.1 ml (10 mg/ml; i.v.) of gentamycin (Lonza, Walkersville, MD) was administered once daily. Rats were given one week of recovery before self-administration sessions began. During the period of cocaine self-administration, catheter patency was evaluated by injecting 0.1 ml Brevital (1%) as necessary. Loss of muscle tone within five seconds after injection indicates a patent catheter. Any rats with faulty catheters were removed from the study and euthanized.
2.4. Cocaine self-administration training
Rats were trained to press the lever for cocaine in daily 3-hr sessions. During these sessions rats responded on a fixed ratio-1 schedule under which responses on the active lever resulted in an intravenous infusion of 0.50 mg/kg of cocaine accompanied by conditioned stimuli (CS), which consisted of a tone + flashing cue light compound stimulus presented for five seconds. Drug concentration (5.0 mg/ml) and pump delivery rate (10 μl/s) were kept constant, and dose of drug (0.50 mg/kg/infusion) was controlled by varying the duration of pump action (i.e., volume of injected solution) based on body weight. Delivery of the drug and CS was followed by a 20 second time-out period signaled by illumination of the house light. During cocaine infusions and time-outs responses were recorded but had no programmed consequences. The criterion of 12 cocaine infusions in one session was used to indicate acquisition. After the acquisition criterion was reached, rats self-administered cocaine for 9 more daily sessions if the number of infusions varied by < 20% on at least two of the last three sessions; otherwise, sessions continued until this criterion was met. Any rats that did not acquire cocaine self-administration following 10 sessions were removed from the study and euthanized.
2.5. Experiment 1: Effects of chronic stress and SCH-23390 on cue- and cocaine priming-induced cocaine seeking
2.5.1. Extinction and chronic stress treatment
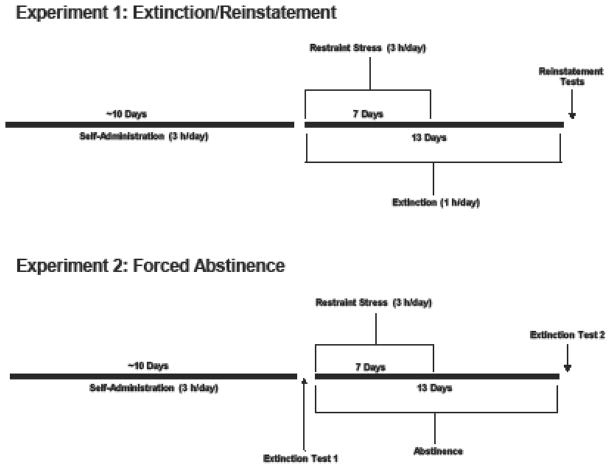
On the day following the last self-administration session, daily 1-hr extinction sessions began and continued for 13 days. During the extinction sessions, responses were recorded but had no programmed consequences (i.e., no CS or cocaine infusion). Beginning on the first day of extinction, stressed rats were exposed to daily restraint stress beginning approximately one hour after daily extinctions sessions. Each day, rats were placed in plastic semi-cylindrical restrainers (8.57 cm diameter × 21.59 cm length; Braintree Scientific) in an isolated room, separate from other rats for three hours for seven consecutive days. Chronic restraint stress is a well-established procedure that produces significant increases in plasma corticosterone levels (Kant et al., 1985; Kvetnansky et al., 1979). To assess dopaminergic involvement in chronic stress effects, rats received injections of SCH-23390 (0.0 or 10.0 μg/kg; i.p.), a D1R antagonist, immediately prior to placement in restrainers. Unstressed rats were injected daily with SCH-23390 or vehicle and then returned to their home cages. See Fig. 1 (top) for a schematic of experimental design.
Fig. 1.
Schematic representation of the experimental design for assessing the effects of chronic restraint stress on cocaine seeking using the extinction-reinstatement model (top) and the forced abstinence model (bottom).
2.5.2. Cue-induced reinstatement testing
Following the extinction sessions, animals underwent one day of one hour CS-induced reinstatement sessions. Sessions began with one non-contingent CS presentation; during the remainder of the session, conditions were identical to those of self-administration training, except that lever presses did not lead to cocaine infusions.
2.5.3. Cue + cocaine priming-induced drug seeking testing
Immediately following cue-induced reinstatement testing, animals underwent within-session cue + cocaine priming-induced drug seeking testing. The testing consisted of three, one hour sessions (separated by five minutes) that were each identical to the cue-induced reinstatement sessions except that rats were given 0.0, 5.0, or 10.0 mg/kg cocaine injections (i.p.) immediately before the start of each session. Doses of cocaine were given in an ascending order to minimize carry-over effects of residual cocaine. This within-session procedure is based on previous studies with cocaine priming (Deroche et al., 1999; Lu et al., 2004).
2.6. Experiment 2: Effects of chronic stress on cocaine seeking during forced abstinence
2.6.1. Extinction test 1
On the day following the last self-administration session, a 30-minute extinction test was conducted. Contingencies during the extinction tests were identical to self-administration sessions except that cocaine was not delivered.
2.6.2. Chronic stress treatment
On the day following the 30-minute extinction test, stressed rats in Exp. 2 were exposed to daily restraint stress as described in Exp. 1. Unlike Exp. 1, however, groups exposed to SCH-23390 were not included because preliminary data indicated no effect of chronic stress on cocaine seeking following forced abstinence.
2.6.3. Extinction test 2
For six days following treatment, rats were left undisturbed in their home cages, except for daily weighing. On Day 7, a second extinction test was conducted that was identical to the first extinction test, except that it was three hours in length. We chose the 1-week, post-stress time-point for the second extinction test because our previous work showed that chronic restraint stress caused an increase in palatable food seeking 7 days, but not one day, after the last restraint (Ball et al., 2017). See Fig. 1 (bottom) for a schematic of experimental design.
2.7. Statistical analyses
Data from Exps. 1 and 2 were analyzed separately using mixed factorial ANOVAs. The main dependent variable was lever pressing during cocaine seeking tests. Change in body weight across treatment days also was used as a dependent variable. Within-subjects factors included chronic treatment day (1 through 7), session (last extinction session and cue-induced reinstatement session; Exp. 1), cocaine dose (0.0, 5.0, and 10.0 mg/kg; Exp. 1), abstinence day (extinction sessions 1 and 2; Exp. 2), and lever (active and inactive). Between-subjects factors included stress condition (stressed or unstressed) and SCH-23390 dose (0.0 or 10.0 μg/kg; Exp. 1). Because the factorial ANOVAs resulted in multiple main and interaction effects, we report only significant effects that are important for interpretation. All ANOVAs were followed by Bonferroni post-tests.
3. Results
3.1. Experiment 1: Effects of chronic stress and SCH-23390 on cue- and cocaine priming-induced cocaine seeking
3.1.1. Self-administration and extinction
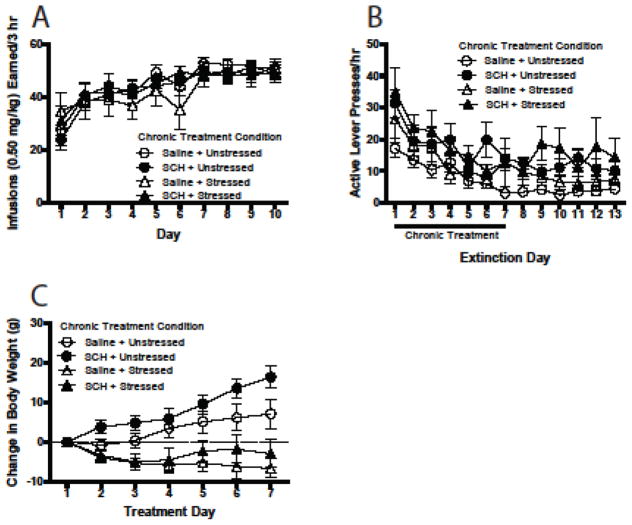
As shown in Fig. 2A, number of cocaine infusions increased over days [F(9, 315) = 14.70, p = .000] following acquisition (Day 1) and response rates were similar across groups destined for saline + unstressed (n = 11), SCH-23390 + unstressed (n = 8), saline + stressed (n = 10), and SCH-23390 + stressed (n = 7) treatments. A 3-way ANOVA (extinction day X stress condition X SCH-23390 dose) was used to analyze extinction responding. As shown in Fig. 2B, extinction responding decreased over days [main effect of extinction day, F(12, 384) = 10.19, p = .000] for all groups; however, overall responding was higher in the groups treated with SCH-23390 compared to saline [main effect of SCH-23390 dose, F(1, 32) = 11.81, p = .002].
Fig. 2.
Experiment 1. (a) Acquisition of cocaine self-administration in rats assigned to receive one of four daily treatments during the first 7 days of subsequent extinction training. Rats were trained on an fixed ratio-1 schedule of reinforcement in daily 3-h sessions. Delivery of cocaine (0.50 mg/kg) was accompanied by a tone + flashing cue light conditioned stimulus (CS) presented for 5 s, which was followed by a 20-s time-out period signaled by illumination of the house light. Day 1 represents responding on the first day that acquisition criterion was met. (b) Active lever responses across 13 days of extinction. Responses had no programmed consequences (i.e., no CS or cocaine). Rats received one of four treatments [saline + unstressed, SCH-23390 (10.0 μg/kg) + unstressed, saline + stressed, or SCH-23390 + stressed] once each day (~ 2 hr after extinction session) for the first 7 days of extinction training (n = 7–11/treatment condition). (c) Weight change in chronically stressed and unstressed rats receiving vehicle or SCH-23390 injection. Injections were given immediately prior to placement in restrainers (3 h/day) or transport back to home cages. All data in figure are represented as mean ± SEM.
3.1.2. Body weight during chronic treatment
A 3-way ANOVA (treatment day X stress condition X SCH-23390 dose) was used to analyze body weight data. Chronic stress significantly attenuated weight gain [main effect of stress, F(1, 32) = 22.32, p = .000; see Fig. 2C]. There also was a main effect of day [F(6, 192) = 6.73, p = .000] and a day x stress interaction [F(6, 192) = 13.97, p = .000]. Finally, there was a day X SCH-23390 dose interaction in that SCH-23390-treated rats gained weight at a faster rate across days compared to saline-treated rats.
3.1.3. Cue-induced reinstatement test
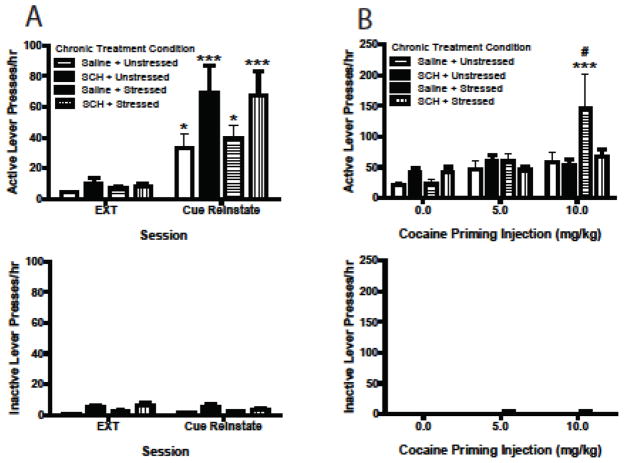
A 4-way ANOVA (session X lever X stress condition X SCH-23390 dose) was used to analyze responding during cue-induced reinstatement tests. As shown in Fig. 3A, active but not inactive lever responding increased significantly during cue-induced reinstatement tests compared to extinction for all treatment groups [main effect of session, F(1, 32) = 62.81, p = .000; session x lever interaction, F(1, 32) = 71.03, p = .000]. However, the magnitude of reinstatement responding was greater in groups treated previously with SCH-23390 compared to those treated with saline [session x SCH-23390 dose interaction, F(1, 32) = 5.97, p = .020].
Fig. 3.
Experiment 1. Effect of repeated restraint stress and repeated SCH-23390 on subsequent active (top) and inactive (bottom) lever pressing during cue- (a) and cocaine priming-induced (b) cocaine seeking. During both cue- and cue + cocaine priming-induced seeking tests, 1-hr sessions began with one non-contingent CS presentation; during the remainder of each session, conditions were identical to those of self-administration training, except that lever presses did not lead to cocaine infusions. EXT indicates the extinction session before seeking tests. *p < .05 and ***p < .001 compared to extinction (a) or 0.0 mg/kg priming condition (b), and #p < .05 compared to saline + unstressed and SCH + unstressed in the 10 mg/kg priming condition, Bonferroni post-test. All data in figure are represented as mean + SEM.
3.1.4. Cue + cocaine priming-induced drug seeking test
A 4-way ANOVA (cocaine dose X lever X stress condition X SCH-23390 dose) was used to analyze responding during cue + cocaine priming-induced drug seeking tests. As shown in Fig. 3B, active but not inactive lever responding increased as a function of cocaine priming dose [main effect of priming dose, F(2, 64) = 6.16, p = .004; priming dose x lever interaction, F(2, 64) = 5.37, p = .007]. Bonferroni post-tests indicated, however, that the 10 mg/kg cocaine-priming dose increased responding over baseline (0 mg/kg dose) to a larger degree in the saline + stressed chronic treatment group relative to the other treatment groups. Moreover, mean responding following the 10.0 mg/kg priming dose was significantly greater for the saline + stressed group compared to both unstressed groups.
3.2. Experiment 2: Effects of Chronic Stress on Cocaine Seeking During Forced Abstinence
3.2.1. Self-administration
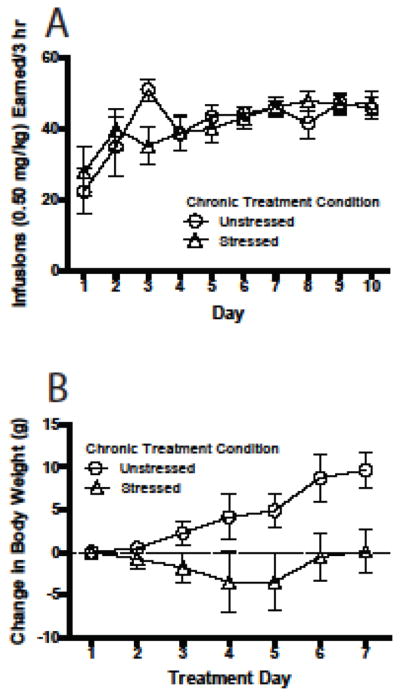
As in Exp. 1, number of cocaine infusions increased over days [F(9, 144) = 4.95, p = .000; see Fig. 4A] and response rates were similar across groups destined for unstressed (n = 8) and stressed (n = 9) treatments.
Fig. 4.
Experiment 2. (a) Acquisition of cocaine self-administration in rats assigned to receive one of two daily treatments (stressed or unstressed) during Days 2 to 8 of subsequent forced abstinence from cocaine self administration. See Fig. 2a for other details. (b) Weight change in chronically stressed and unstressed rats (n = 8–9/treatment condition). All data in figure are represented as mean ± SEM.
3.2.2. Body weight during chronic treatment
A 2-way ANOVA (treatment day X stress condition) was used to analyze body weight data. As in Exp. 1, chronic stress significantly attenuated weight gain [main effect of stress, F(1, 15) = 4.94, p = .042; see Fig. 4B]. There also was a main effect of day [F(6, 90) = 4.48, p = .001] and a day x stress interaction [F(6, 90) = 3.91, p = .002].
3.2.3. Cocaine seeking during forced abstinence
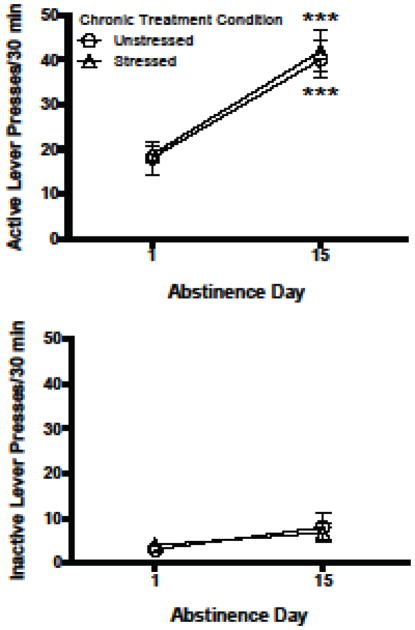
A 3-way ANOVA (extinction session X lever X stress condition) was used to analyze responding during extinction sessions 1 and 2. As shown in Fig. 5, unstressed and stressed groups displayed similar time-dependent increases in responding for cocaine cues, or incubation of craving (Grimm et al., 2001). There was a significant main effect of abstinence day [F(1, 15) = 31.89, p = .000] and lever [F(1, 15) = 143.55, p = .000], and an abstinence day x lever interaction [F(1, 15) = 46.45, p = .000].
Fig. 5.
Experiment 2. Active (top) and inactive (bottom) lever pressing during extinction tests given before and after the 7-day treatment period. Contingencies during the extinction tests were identical to self-administration sessions except that cocaine was not delivered. Extinction Test 1 occurred on Day 1 of abstinence, and Extinction Test 2 occurred on Day 15 of abstinence, or 7 days after the last chronic treatment. Lever pressing during the entire 30-min of Extinction Test 1 was compared to the first 30 min of Extinction Test 2. ***p < .001 compared to abstinence Day 1, Bonferroni post-test. All data in figure are represented as mean ± SEM.
4. Discussion
We tested the effect of chronic restraint stress during early withdrawal on later cocaine seeking using both extinction- and abstinence-based animal relapse models. Results showed that stress was associated with increased responding following the higher-dose (10 mg/kg) cocaine prime, but had no effect on responding for drug-associated cues in the absence of cocaine priming following either extinction training or forced abstinence. Co-administration of SCH-23390 with daily restraint attenuated the effect of stress on subsequent cue + cocaine priming-induced drug seeking, an effect that does not appear to be due to a reduction in the “stressfulness” of the manipulation because there was a significant decrease in weight gain in the SCH + stressed group, similar to that observed in the saline + stressed group. Moreover, SCH-23390-treated rats displayed increased cue-induced reinstatement, implicating dopamine D1Rs in both chronic stress effects on cocaine seeking and extinction of cocaine seeking behavior.
These results support and extend our previous findings showing that rats with a history of exposure to the pharmacological stressor yohimbine displayed increased acute yohimbine- and pellet priming-induced reinstatement of palatable food seeking after extinction (Ball et al., 2016). We did not conduct cue-induced reinstatement testing in our previous studies of palatable food seeking, so it is not known whether the differential effects of chronic stress on cue- vs priming-induced drug seeking generalize to food seeking. The finding that co-administration of SCH-23390 with daily restraint attenuated the effect of stress on cocaine priming-induced drug seeking also is in line with our food seeking studies in that co-administration of SCH-23390 with stress prevented or attenuated all stress effects in our previous studies, resulting in behavior that looked more like unstressed rats (Ball et al., 2017; Ball et al., 2015; Ball et al., 2016). This supports the hypothesis that excessive dopamine D1R stimulation due to repeated stress contributes to increased relapse-like behavior.
Considering potential mechanisms of SCH-23390’s effects, blockade of D1Rs during stress may have prevented either stress-induced changes in mesocorticolimbic dopaminergic transmission (Mizoguchi et al., 2008; Mizoguchi et al., 2000) or cross-sensitization between stress and cocaine (Kalivas and Stewart, 1991). Given that SCH-23390 blocks the D1 family of receptors, it is possible that our results are due, at least in part, to antagonism of D5 receptors. This is relevant because D5 receptors may influence internal calcium stores and protein kinase C signaling (Paspalas and Goldman-Rakic, 2004), a pathway implicated in stress-induced changes in mPFC structure and cognition (Hains et al., 2009). Finally, because SCH-23390 also has an affinity for serotonin 1C and 2C receptors (albeit less than for D1Rs (Millan et al., 2001; Wamsley et al., 1991)), we cannot rule out the possibility that altered serotonergic transmission contributed to our results.
It is noteworthy that SCH-23390 does not attenuate chronic stress-induced potentiation of reinstatement of extinguished 3,4-mehylenedioxymethampheatmine (MDMA) seeking induced by an acute yohimbine prime (Ball et al., 2015). This difference between cocaine and MDMA is somewhat surprising, because although the drugs have different mechanisms of action (blockade vs. reversal of monoamine reuptake transporters), both drugs induce relatively large increases in extracellular levels of dopamine and serotonin (Bradberry et al., 1993; Gough et al., 1991; Kankaanpaa et al., 1998; Teneud et al., 1996). Moreover, exposure to both cocaine and MDMA is associated with similar and enduring morphological adaptations in mPFC, dorsal striatum, and nucleus accumbens, including increases in spine density and dendritic branching as observed in Golgi-stained samples (Ball et al., 2009; Ball et al., 2010; Robinson and Kolb, 2004). We did not conduct MDMA priming-induced reinstatement of MDMA seeking in our previous study, so it is possible that the discrepancy is due to the use of different priming stimuli. In any case, the question of whether chronic stress increases vulnerability to relapse through different mechanisms depending on the type of self-administered drug or priming stimulus is worthy of further investigation.
Although it is well established that either stress or cocaine exposure alone cause structural changes in mPFC, to our knowledge, the effect of chronic stress on mPFC structure in animals with a history of cocaine or other drug self-administration has not been investigated. In drug-naïve animals, chronic stress causes dendritic retraction in mPFC pyramidal cells (Cook and Wellman, 2004; Radley et al., 2004). It is noteworthy that microinjections of SCH-23390 into the prelimbic region of medial PFC during chronic restraint was shown to prevent dendritic retraction (Lin et al., 2014), suggesting a possible mechanism of SCH-23390’s effect in the present study. Given recent 3D imaging showing that the self-administration of cocaine also causes atrophy in mPFC (Radley et al., 2015), stress during withdrawal may compound the deficits in mPFC-dependent cognition, contributing to maladaptive drug seeking. Complicating interpretation, however, is the dynamic nature of stress-induced structural changes during recovery from stress; thus, a recent time-course analysis of dendritic changes following chronic stress revealed dendritic outgrowth in male rats seven days post-stress (Moench and Wellman, 2017), or the approximate time-point at which reinstatement tests were conducted in the present study. Moreover, chronically stressed rats in our palatable food seeking studies displayed increased food seeking for seven days, but not one day, after the last restraint (Ball et al., 2017). Thus, future studies should assess the time course of structural changes following stress in drug-experienced animals.
It is important to note that the effects of chronic stress are sex dependent. For example, male and female rats display opposite stress-induced dendritic changes in medial PFC pyramidal neurons immediately after stress (Garrett and Wellman, 2009), as well as differential dendritic remodeling during the first 10 days of recovery from stress (Moench and Wellman, 2017). Sex differences in stress effects, moreover, are not limited to neural structure: chronic stress is associated with deficits in spatial working memory tasks in males and improvements in females (Bowman et al., 2003; Luine et al., 2007). Given the clinical evidence suggesting that women may be more vulnerable to cocaine craving and relapse, especially during stressful events or depression (Elman et al., 2001; McKay et al., 1996), a critical question to be addressed in future studies is whether chronic stress differentially affects cocaine seeking in male vs. female subjects.
In contrast to the finding of enhanced cocaine priming-induced drug seeking in the present study, prior chronic stress did not result in increased cue-induced reinstatement. A possible explanation for this discrepancy is that the neural circuitry of discrete cue- and cocaine priming-induced reinstatement are partially dissociable. For example, activation of the basolateral amygdala is critical for discrete cue-induced reinstatement of cocaine seeking, but appears less important for cocaine priming-induced reinstatement (for review see Feltenstein and See, 2008). It may be relevant that chronic stress produces increases in dendritic length and spine density in pyramidal cells of the basolateral amygdala one day post-stress (Mitra et al., 2005; Vyas et al., 2006), changes that are opposite of those observed in mPFC pyramidal cells at the same time-point. Note, however, that because cocaine-associated discrete cues were present during cocaine priming in the present study, it is possible that the circuitry of cue-induced reinstatement was engaged during priming tests. Indeed, it has been argued that drug priming increases drug seeking by enhancing the saliency or incentive motivational properties of reward-associated cues (Mueller and Stewart, 2000; Stewart et al., 1984).
Another main finding in the present study was that repeated post-extinction SCH-23390 administration resulted in increased responding during both extinction training and cue-induced reinstatement testing. These results are in line with those of our previous studies assessing the effect of chronic stress on palatable food seeking, in which repeated post-extinction SCH-23390 treatment resulted in increased extinction responding (Ball et al., 2017; Ball et al., 2016). Although post-training SCH-23390 has previously been shown to impair extinction of cocaine conditioned place preference (Fricks-Gleason et al., 2012), to our knowledge, the present results are the first to show that D1R blockade impairs extinction of operant responding for cocaine. Our results suggest that SCH-23390-treated animals had an impairment in consolidation of extinction memories, implicating the infralimbic (IL) mPFC. Indeed, this region is well known to mediate consolidation of extinction of both aversive and appetitive memories (Peters et al., 2009). Highlighting the importance of dopamine in this process, post-training intra-IL injections of SCH-23390 were shown to impair consolidation of extinction of fear conditioning (Hikind and Maroun, 2008). Particularly relevant to the present results, pharmacological inactivation of IL mPFC was shown to impair the consolidation of extinction of cocaine self-administration (LaLumiere et al., 2010), and chemogenic activation of IL was shown to reduce cue-, but not cue + cocaine-, induced reinstatement (Augur et al., 2016). This latter finding suggests a possible explanation for SCH-23390’s selective effect on cue-induced reinstatement in the present study.
Our results using the forced abstinence model contrast with a recent report showing that repeated restraint stress during early cocaine withdrawal accelerated incubation of craving (Glynn et al., 2016). There are some relevant methodological differences between the Glynn et al. (2016) study and the present study (e.g., length of daily restraint, pattern of stress, and number of recovery days before testing) that could account for the discrepancy; however, we speculate that a more important consideration is the possibility of a ceiling effect. Although comparisons across studies should be done with caution, it is noteworthy that in Glynn et al.’s (2016) study unstressed and stressed rats displayed ~24 and ~30 presses/30 minutes on test day, respectively, compared to 40.00 and 41.89 presses/30 minutes in the present study. When rats’ responding in Glynn et al’s (2016) study reached maximal levels at withdrawal day 48, responding was not different between stressed and unstressed groups and was similar to the levels of responding we observed in the present study. Also supporting the possibility of a ceiling effect, we found previously that chronic stress caused incubation of craving for highly palatable food, but in that study, unstressed rats showed no incubation at all (Ball et al., 2017). Collectively, these findings suggest that chronic stress may increase relapse to cocaine and palatable food seeking only under conditions of otherwise low vulnerability.
In conclusion, the present results suggest that the effects of chronic stress on subsequent cocaine seeking may depend on factors such as priming stimulus, baseline vulnerability, and/or animal model of relapse utilized. Importantly, we found that the ability of chronic stress to potentiate later cocaine priming-induced drug seeking was attenuated by co-administration of SCH-23390, supporting and extending our previous findings suggesting an important role for D1Rs in the effects of chronic stress on food seeking behavior (Ball et al., 2017; Ball et al., 2015; Ball et al., 2016). Our findings also support a role for D1Rs in the consolidation of extinction learning, a type of learning that may reduce future cocaine seeking behavior. Elucidating the mechanisms underlying chronic stress effects on relapse vulnerability in both males and females under a variety of conditions may aid the development of more targeted treatment options for addiction.
Highlights.
The effect of chronic restraint stress on subsequent cocaine seeking was tested.
Both extinction- and abstinence-based animal relapse models were used.
Chronic restraint stress caused increase in cocaine priming-induced reinstatement.
A dopamine D1-like receptor antagonist, combined with stress, attenuated this effect.
Prior antagonist treatment resulted in increased cue-induced reinstatement.
Acknowledgments
This work was supported by the National Institutes of Health (R15 DA035432 to KTB). The authors thank the National Institute on Drug Abuse for providing cocaine.
Abbreviations
- CS
conditioned stimuli
- D1R
D1-like receptor
- FR
fixed ratio
- IL
infralimbic
- MDMA
3,4-methylenedioxymethamphetamine
- mPFC
medial prefrontal cortex
Footnotes
Contributors
KTB was responsible for the study concept and design and drafted the manuscript. All authors performed experiments and analyzed and interpreted data. All authors approved the final version for publication.
Conflict of Interest
None declared.
Role of Funding Source
The sponsor had no role in study design, data collection, analysis, or interpretation, in the writing of the report, or in the decision to publish.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci. 2016;36:10174–10180. doi: 10.1523/JNEUROSCI.0773-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Best O, Luo J, Miller LR. Chronic restraint stress causes a delayed increase in responding for palatable food cues during forced abstinence via a dopamine D1-like receptor-mediated mechanism. Behav Brain Res. 2017;319:1–8. doi: 10.1016/j.bbr.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Jarsocrak H, Hyacinthe J, Lambert J, Lockowitz J, Schrock J. Yohimbine reinstates extinguished 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats with prior exposure to chronic yohimbine. Behav Brain Res. 2015;294:1–6. doi: 10.1016/j.bbr.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Miller L, Sullivan C, Wells A, Best O, Cavanaugh B, Copus T, Corrigan N, Hawkins S, Kobbe K, Schoener A, Steiger J, Vieweg L. Effects of repeated yohimbine administration on reinstatement of palatable food seeking: Involvement of dopamine D1 -like receptors and food-associated cues. Addict Biol. 2016;21:1140–1150. doi: 10.1111/adb.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neurosci. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Miller BR, Rebec GV. Electrophysiological and structural alterations in striatum associated with behavioral sensitization to (+/−)3,4-methylenedioxymethamphetamine (ecstasy) in rats: Role of drug context. Neurosci. 2010;171:794–811. doi: 10.1016/j.neuroscience.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacol. 2013;225:241–250. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart JYS. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacol. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: An overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Khalaj AJ, Marshall JF. Dopamine D1 receptor antagonism impairs extinction of cocaine-cue memories. Behav Brain Res. 2012;226:357–360. doi: 10.1016/j.bbr.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: A critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neurosci. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn RM, Rosenkranz JA, Wolf ME, Caccamise A, Shroff F, Smith AB, Loweth JA. Repeated restraint stress exposure during early withdrawal accelerates incubation of cue-induced cocaine craving. Addict Biol. 2018;23:80–89. doi: 10.1111/adb.12475. Epub 2016 Nov. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough B, Ali SF, Slikker W, Jr, Holson RR. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol Biochem Behav. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. Epub 2008 Apr 20. [DOI] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA. Episodic social stress-escalated cocaine self-administration: Role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci. 2016;36:4093–4105. doi: 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. Epub 2008 Dec 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, McCarty R, Thoa NB, Lake CR, Kopin IJ. Sympatho-adrenal responses of spontaneously hypertensive rats to immobilization stress. Am J Physiol. 1979;236:H457–462. doi: 10.1152/ajpheart.1979.236.3.H457. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GL, Borders CB, Lundewall LJ, Wellman CL. D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinol. 2014;51C:101–111. doi: 10.1016/j.psyneuen.2014.1009.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: Different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacol. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacol. 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol Biochem Behav. 2008;91:170–175. doi: 10.1016/j.pbb.2008.07.002. Epub 2008 Jul 13. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Wellman CL. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neurosci. 2017;357:145–159. doi: 10.1016/j.neuroscience.2017.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacol. 2001;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: Reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT. The contingency of cocaine administration accounts for structural and functional medial prefrontal deficits and increased adrenocortical activation. J Neurosci. 2015;35:11897–11910. doi: 10.1523/JNEUROSCI.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neurosci. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacol. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacol. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacol. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Teneud LM, Baptista T, Murzi E, Hoebel BG, Hernandez L. Systemic and local cocaine increase extracellular serotonin in the nucleus accumbens. Pharmacol Biochem Behav. 1996;53:747–752. doi: 10.1016/0091-3057(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neurosci. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Hunt ME, McQuade RD, Alburges ME. [3H]SCH39166, a D1 dopamine receptor antagonist: binding characteristics and localization. Exp Neurol. 1991;111:145–151. doi: 10.1016/0014-4886(91)90001-s. [DOI] [PubMed] [Google Scholar]