Abstract
Associations between cannabis use and psychotic disorders suggest that cannabis may be a contributory risk factor in the neurobiology of psychosis. In this study, we examined brain structure characteristics, total and regional gray matter density (GMD), using Voxel Based Morphometry, in psychotic individuals, stratified by history of cannabis use (total n=109). We also contrasted GMD estimates in individual diagnostic groups (schizophrenia/bipolar I disorder) with and without history of adolescent cannabis use (ACU). Individuals with psychosis as a whole, both with and without history of ACU, had lower total and regional GMD, compared to healthy controls. ACU was associated with attenuated GMD reductions, compared to non-users, especially in the schizophrenia cases, who showed robust GMD reductions in fronto-temporal and parietal cortex, as well as subcortical regions. Notably, total and regional GMD estimates in individuals with psychosis and ACU were not different from controls with no ACU. These data indicate that the history of ACU in psychotic individuals is associated with attenuated GMD abnormalities. Future investigations targeting potential unique etiological and risk factors associated with psychosis in individuals with ACU may help in understanding of the neurobiology of psychotic disorders and novel treatment options for these individuals.
Keywords: Schizophrenia, Psychotic Bipolar Disorder, Cannabis, Gray Matter Density, Voxel Based Morphometry
1. INTRODUCTION
According to the UNO World Drug Report, about 3.8 percent of the global population used cannabis in the past year, roughly the same proportion as in the last decade (United Nations Office on Drugs and Crime, World Drug Report 2017), making cannabis among the most frequently used illicit drugs in the world. In the US, the prevalence of cannabis use is on the rise and has reached 13.5% of the population (United Nations Office on Drugs and Crime, World Drug Report 2017). Cannabis use disorders are a common comorbidity for schizophrenia and related psychotic disorders, with a recent meta-analysis estimating current cannabis use at 16%, and lifetime cannabis use at 27%, with higher rates in males and in first episode schizophrenia individuals (Koskinen et al., 2010). Cross-sectional and longitudinal studies show a consistent relationship between cannabis use and psychotic disorders (Gage et al., 2016). Cannabis use prior to onset of psychosis (Semple et al., 2005) is associated with earlier onset of illness (Tosato et al., 2013). In addition, onset of cannabis use at younger ages (Large et al., 2011) and the frequent use of more potent strains of cannabis are associated with higher risk of developing psychosis (Di Forti et al., 2014). This temporal and dose response relationship between cannabis use and psychosis (Kraan et al., 2016) suggests that cannabis can be a contributory risk factor in the neurobiology of psychotic disorders. This clinical observation can also be found in basic neuroscience studies showing that the adolescent brain responds differently to cannabis use than the adult brain, making it susceptible to cannabis use and resulting in lasting effects on brain circuitry and morphology (Grigorenko et al., 2002; Kittler et al., 2000; Quinn et al., 2008; Realini et al., 2011; Rubino and Parolaro, 2016; Rubino et al., 2009).
An unexpected and intriguing observation is that premorbid cannabis use in schizophrenia is associated with better cognitive function, compared to individuals with schizophrenia without history of cannabis use (Hanna et al., 2016; Schnell et al., 2012; Yucel et al., 2012), though some studies have not supported this observation (Ringen et al., 2010). Schnell et al. (2012) have found less severe cognitive impairments and gray matter reductions in the middle frontal regions in patients with schizophrenia and concurrent cannabis use (Schnell et al., 2012). Two meta-analyses confirm the observation that previous cannabis use in schizophrenia is associated with less cognitive impairment (Rabin et al., 2011; Yucel et al., 2012). This association is more consistent in studies that focused on adolescent cannabis use (ACU), which was found to be associated with milder cognitive impairment compared to non-users (Hanna et al., 2016; Yucel et al., 2012), contrary to the observation of poorer cognition associated with ACU in otherwise healthy individuals (Meier et al., 2012).
We recently reported that in individuals with psychosis (schizophrenia/schizoaffective disorder [SZ] and psychotic bipolar I disorder [BP]), ACU was associated with less impaired global cognitive function, as measured by the Brief Assessment of Cognition in Schizophrenia (BACS), compared to cannabis non-users (Hanna et al., 2016). Further, we found that this effect on cognition was specifically driven by differences between the SZ with and without history of ACU, and not the BP, subgroups.
Here, we extended our work by examining brain structure characteristics—whole brain and regional gray matter density (GMD)—in the same cohort of psychosis cases, stratified by cannabis use history. First, we tested GMD characteristics in the psychosis group as a whole, stratified by history of ACU. Then, we contrasted GMD estimates in individual diagnostic groups (SZ, BP) with and without history of ACU. We hypothesized that individuals with psychosis and history of ACU would have higher GMD estimates, more similar to healthy controls (HC), compared to the non-ACU psychosis group. Further, based on our previous findings with the same sample demonstrating preserved cognitive function in individuals with psychosis following adolescent exposure to cannabis (Hanna et al., 2016), we predicted that the effect of ACU on GMD would be more evident in SZ compared to BP. In addition, we explored associations between GMD and cognitive performance in the overall psychosis group as well as in individual diagnostic subgroups.
2. METHODS
2.1 Study Sample
Whole brain and regional GMD measures were assessed in 109 volunteers from the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) sample (Tamminga et al., 2013) (Dallas site only, where more extensive data about cannabis use was collected). The B-SNIP logistics, overall sample characteristics, and clinical measures have been previously described (Tamminga et al., 2013). Individuals with psychosis included in this sample were stable, medicated outpatients; HC did not have any personal or family history of psychotic or recurrent mood disorders. Both patient and HC volunteers with organic brain disorders, history of brain insults accompanied by a loss of consciousness ≥ 30min, decompensated medical conditions, or substance abuse within 1 months or dependence within 3 months of enrollment were excluded. The drug-free status was confirmed by qualitative urine toxicology screens (Reditest Panel-Dip, substance abuse screening device; Redwood Toxicology Laboratory); each subject was screened at least twice, at the initial intake appointment and then again at the beginning of the study. Pregnant women, subjects with claustrophobia, those with irremovable ferromagnetic medical and non-medical objects lodged in body were also excluded.
For hypotheses-testing purposes, volunteers were grouped either by presence of lifetime psychosis [(i) psychosis (PSY), n=63; (ii) HC, n=46] or by specific DSM-IV diagnoses [(i) schizophrenia or schizoaffective disorder (SZ), n=44; (ii) psychotic bipolar I disorder (BP), n=19; (iii) HC, n=46]. Furthermore, within each group volunteers were stratified by history of cannabis use as previously described (Hanna et al., 2016): (i) adolescent-onset cannabis users (ACU), with cannabis use on 5 or more occasions before age 18 (89% used >50 occasions); or (ii) non-users (NonACU), with cannabis use on less than 5 occasions over their lifetime). Cannabis use assessment was done according to information collected using two primary tools. One is a semi-structured clinical interview, where information on age of first use, age of last use, and frequency of use was collected (Table 1). In addition, the DSM-IV diagnosis-level information pertinent to cannabis and other substance use was acquired using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P) (First et al., 1997), to define the diagnosis of cannabis abuse or dependence in all subjects. This information combined was used to divide the subjects into the different groups. Descriptive demographic, clinical, and cognitive characteristics of the study sample are detailed in Table 2 and Supplementary Table 1.
Table 1.
Substance use characteristics of the study sample
| a. Substance use characteristics in the Psychosis Groups | |||||
|---|---|---|---|---|---|
| HC-NonACU (n=32) | HC-ACU (n=14) | PSY-NonACU (n=35) | PSY-ACU (n=28) | Statistical Test** | |
| Lifetime Substance Use Disorder Diagnosis | |||||
| Cannabis use - Age of first use | 21.11 (17–35) | 15.57 (14–17) | 17.83 (15–20) | 14.48 (5–17) | F(3,60)=13.79, p<0.001 |
| Cannabis use - Age of last use | 21.00 (10–37) | 24.90 (16–31) | 41.67 (27–55) | 36.60 (15–55) | F(3,29)=0.52, NS |
| Cannabis use - Amount of use | χ2(6)=107.27, p<0.001 | ||||
| Less than 5 times over lifetime | 100% | 0% | 100% | 0% | |
| 5–50 times over lifetime | 0% | 25% | 0% | 4% | |
| More than 50 times over lifetime | 0% | 75% | 0% | 96% | |
| Cannabis Abuse | 0% | 7% | 0% | 4% | χ2(3)=3.91, NS |
| Cannabis Dependence | 0% | 14% | 0% | 32% | χ2(3)=22.8, p=0.001 |
| Other Substance Abuse/Dependence* | 6% | 43% | 26% | 89% | χ2(3)=47.07, p<0.001 |
| b. Substance use characteristics in the Diagnosis Groups | |||||||
|---|---|---|---|---|---|---|---|
| HC-NonACU (n=32) | HC-ACU (n=14) | BP-NonACU (n=12) | BP-ACU (n=7) | SZ-NonACU (n=23) | SZ-ACU (n=21) | Statistical Test* | |
| Lifetime Substance Use Disorder Diagnosis | |||||||
| Cannabis use - Age of first use | 21.11 (17–35) | 15.57 (14–17) | 18.67 (18–20) | 12.64 (5–17) | 17.56 (15–20) | 15.10 (12–17) | F(5,58)=9.26, p<0.001 |
| Cannabis use - Age of last use | 21.00 (10–37) | 24.90 (16–31) | n\a | 32.50 (32–43) | 41.67 (27–55) | 37.23 (15–55) | F(5,27)=0.48, NS |
| Cannabis use - Amount of use | χ2(10)=108.09, p<0.001 | ||||||
| Less than 5 times over lifetime | 100% | 0% | 100% | 0% | 100% | 0% | |
| 5–50 times over lifetime | 0% | 25% | 0% | 14% | 0% | 0% | |
| More than 50 times over lifetime | 0% | 75% | 0% | 86% | 0% | 100% | |
| Cannabis Abuse | 0% | 7% | 0% | 0% | 0% | 5% | χ2(5)=4.57, NS |
| Cannabis Dependence | 0% | 14% | 0% | 29% | 0% | 33% | χ2(5)=22.93, p<0.001 |
| Other Substance Abuse/Dependence* | 6% | 43% | 33% | 86% | 22% | 90% | χ2(5)=47.57, p<0.001 |
Other Substance Abuse/Dependence diagnoses included Alcohol, Cocaine, and Amphetamine use disorders.
Post hoc pairwise comparisons (Tukey HSD or chi square, as appropriate); only statistically significant (p<0.05) differences are reported:
Psychosis Groups:
Cannabis use–Age of first use: HC-NonACU > HC-ACU (p<0.001), PSY-NonACU (p<0.05); PSY-ACU (p<0.001); PSY-NonACU > PSY-ACU (p<0.01).
Cannabis use–Amount of use: There was a higher proportion of heavy users among HC-ACU compared to HC-NonACU (χ2(2)=44.0, p<0.001) and PSY-NonACU (χ2(2)=46.0, p<0.001); and among PSY-ACU compared to HC-NonACU (χ2(2)=56.0, p<0.001) and PSY-NonACU (χ2(2)=58.0, p<0.001).
Cannabis Dependence: There was a higher proportion of Cannabis Dependence among HC-ACU compared to HC-NonACU (χ2(1)=4.78, p=0.029) and PSY-NonACU (χ2(1)=5.21, p=0.022); and among PSY-ACU compared to HC-NonACU (χ2(1)=12.1, p=0.001) and PSY-NonACU (χ2(1)=13.13, p<0.001).
Other Substance Use: There was a higher proportion of Other Substance Use among HC-ACU compared to HC-NonACU (χ2(1)=9.08, p=0.003); and among PSY-ACU compared to HC-ACU (χ2(1)=10.41, p=0.001) and HC-NonACU (χ2(1)=41.6, p<0.001) and PSY-NonACU (χ2(1)=25.31, p<0.001); and among PSY-NonACU compared to HC-NonACU (χ2(1)=4.62, p=0.032).
Diagnosis Groups:
Cannabis use–Age of first use: HC-NonACU > HC-ACU (p<0.001), SZ-ACU (p<0.001); BP-ACU (p<0.001); BP-ACU < SZ-NonACU (p<0.05); BP-NonACU (p<0.05).
Cannabis use–Amount of use: There was a higher proportion of heavy users among HC-ACU compared to HC-NonACU (χ2(2)=44.0, p<0.001) and SZ-NonACU (χ2(2)=34.0, p<0.001) and BP-NonACU (χ2(2)=24.0, p<0.001); and among SZ-ACU compared to HC-NonACU (χ2(2)=49.0, p<0.001) and SZ-NonACU (χ2(2)=39.0, p<0.001) and and BP-NonACU (χ2(2)=29.0, p<0.001); and among BP-ACU compared to HC-NonACU (χ2(2)=39.0, p<0.001) and SZ-NonACU (χ2(2)=29.0, p<0.001) and and BP-NonACU (χ2(2)=19.0, p<0.001).
Cannabis Dependence: There was a higher proportion of Cannabis Dependence among HC-ACU compared to HC-NonACU (χ2(1)=4.78, p=0.029); and among SZ-ACU compared to HC-NonACU (χ2(1)=12.29, p<0.001) and SZ-NonACU (χ2(1)=9.12, p=0.003) and BP-NonACU (χ2(1)=5.08, p=0.024); and among BP-ACU compared to HC-NonACU (χ2(1)=9.64, p=0.002) and SZ-NonACU (χ2(1)=7.04, p=0.008) and BP-NonACU (χ2(1)=3.83, p=0.05).
Other Substance Use: There was a higher proportion of Other Substance Use among HC-ACU compared to HC-NonACU (χ2(1)=9.08, p=0.003); and among SZ-ACU compared to HC-ACU (χ2(1)=9.33, p=0.002) and HC-NonACU (χ2(1)=37.6, p<0.001) and SZ-NonACU (χ2(1)=20.92, p<0.001) and BP-NonACU (χ2(1)=11.81, p=0.001); and among BP-ACU compared to HC-NonACU (χ2(1)=22.24, p<0.001) and SZ-NonACU (χ2(1)=9.46, p=0.002) and BP-NonACU (χ2(1)=4.87, p=0.027); and among BP-NonACU compared to HC-NonACU (χ2(1)=5.44, p=0.02).
HC: healthy controls; PSY: volunteers with psychosis; SZ: volunteers with schizophrenia or schizoaffective disorder; BP: volunteers with psychotic bipolar I disorder, HC: healthy controls; ACU: adolescent onset cannabis use; Non-ACU: cannabis non-users.
Table 2.
Socio-demographic and cognitive characteristics of the study sample
| a. Socio-demographic and cognitive characteristics of the study sample by Psychosis Group | |||||
|---|---|---|---|---|---|
| HC-NonACU (n=32) | HC-ACU (n=14) | PSY-NonACU (n=35) | PSY-ACU (n=28) | Statistical Test* | |
| Socio-Demographic Characteristics | |||||
| Gender (% Female) | 59% | 36% | 66% | 36% | χ2(3)=7.8, p=0.05 |
| Age | 41.78 (18–63) | 38.21 (18–55) | 40.49 (17–62) | 38.25 (21–55) | F(3,105)=0.63, NS |
| Ethnicity (% Hispanic) | 9.00% | 21.00% | 9% | 4% | χ2(3)=3.59, NS |
| Race | χ2(12)=13.33, NS | ||||
| Caucasian | 69% | 79% | 54% | 54% | |
| African American | 25% | 14% | 37% | 46% | |
| Asian | 3% | 0% | 6% | 0% | |
| Multiracial | 0% | 0% | 3% | 0% | |
| Other | 3% | 7% | 0% | 0% | |
| Years of Education | 13.72 | 14.54 | 13.77 | 12.71 | F(3,104)=2.81, p=0.043 |
| Handedness (% Right-Handed) | 88% | 100% | 83% | 100% | χ2(3)=7.33, NS |
| Cognitive Assessments | |||||
| WRAT | 99.13 (72–134) | 104.86 (74–138) | 95.66 (69–126) | 95.00 (72–119) | F(3,105)=1.77, NS |
| BACS Composite | −0.41 (−3.65–1.55) | 0.05 (−1.83–1.46) | −1.77 (−4.00–0.94) | −1.11 (−3.84–3.55) | F(3,105)=8.93, p<0.001 |
| BACS Verbal Memory | −0.52 (−2.83–1.85) | −0.03 (−1.05–1.28) | −1.17 (−3.68–1.33) | −0.40 (−3.82–2.47) | F(3,105)=3.13, p<0.05 |
| BACS Digit Sequencing | −0.39 (−3.90–1.59) | −0.17 (−1.92–1.63) | −1.14 (−3.71–1.35) | −0.84 (−2.82–1.33) | F(3,105)=3.1, p<0.05 |
| BACS Token Motor | −0.25 (−4.00–1.73) | 0.32 (−1.04–1.87) | −1.48 (−4.00–0.72) | −1.43 (−3.72–0.42) | F(3,104)=17.19, p<0.001 |
| BACS Verbal Fluency | −0.12 (−2.37–1.92) | 0.19 (−1.47–2.40) | −0.64 (−3.27–1.64) | −0.22 (−2.26–2.25) | F(3,105)=2.42, p=0.071 |
| BACS Symbol Coding | −0.15 (−2.17–1.86) | 0.17 (−1.44–1.27) | −1.48 (−3.82–1.09) | −0.99 (−2.76–3.56) | F(3,105)=12.1 p<0.001 |
| BACS Tower of London | −0.20 (−2.45–1.79) | −0.18 (−3.23–1.36) | −0.96 (−4.00–0.77) | −0.31 (−4.00–2.76) | F(3,105)=3.17, p<0.05 |
| SFS (Total score) | 146.60 (115–171) | 142.08 (109–167) | 117.03 (59–174) | 115.25 (54–167) | F(3,100)=11.32, p<0.001 |
| b. Socio-demographic and cognitive characteristics of the study sample by Diagnosis Group | |||||||
|---|---|---|---|---|---|---|---|
| HC-NonACU (n=32) | HC-ACU (n=14) | BP-NonACU (n=12) | BP-ACU (n=7) | SZ-NonACU (n=23) | SZ-ACU (n=21) | Statistical Test* | |
| Socio-Demographic Characteristics | |||||||
| Gender (% Female) | 59% | 36% | 83% | 29% | 57% | 38% | χ2(5)=10.26, NS |
| Age | 41.78 (18–63) | 38.21 (18–55) | 39.83 (22–60) | 37.14 (22–45) | 40.83 (17–62) | 38.62 (21–55) | F(5,103)=0.4, NS |
| Ethnicity (% Hispanic) | 9.00% | 21.00% | 0.00% | 0% | 13% | 5% | χ2(5)=5.34, NS |
| Race | χ2(20)=16.93, NS | ||||||
| Caucasian | 69% | 79% | 58% | 71% | 52% | 48% | |
| African American | 25% | 14% | 33% | 29% | 39% | 52% | |
| Asian | 3% | 0% | 8% | 0% | 4% | 0% | |
| Multiracial | 0% | 0% | 0% | 0% | 4% | 0% | |
| Other | 3% | 7% | 0% | 0% | 0% | 0% | |
| Years of Education | 13.72 (12–18) | 14.54 (12–18) | 14.58 (11–18) | 13.14 (9–16) | 13.35 | 12.57 | F(5,102)=2.38, p=0.043 |
| Handedness (% Right-Handed) | 88% | 100% | 92% | 100% | 78% | 100% | χ2(5)=9.04, NS |
| Cognitive Assessments | |||||||
| WRAT | 99.13 (72–134) | 104.86 (74–138) | 98.17 (72–120) | 101.43 (89–118) | 94.35 (69–126) | 92.86 (72–119) | F(5,103)=1.54, NS |
| BACS Composite | −0.41 (−3.65–1.55) | 0.05 (−1.83–1.46) | −1.05 (−2.81–0.94) | −1.18 (−3.84–0.95) | −2.15 (−4.00–0.68) | −1.09 (−3.25–3.55) | F(5,103)=6.64, p<0.001 |
| BACS Verbal Memory | −0.52 (−2.83–1.85) | −0.03 (−1.05–1.28) | −0.62 (−2.78–1.24) | −0.37 (−2.73–1.40) | −1.46 (−3.68–1.33) | −0.40 (−3.82–2.47) | F(5,103)=2.52, p<0.05 |
| BACS Digit Sequencing | −0.39 (−3.90–1.59) | −0.17 (−1.92–1.63) | −0.66 (−2.50–1.35) | −1.10 (−2.82–1.05) | −1.40 (−3.71–0.29) | −0.75 (−2.50–1.33) | F(5,103)=2.53, p<0.05 |
| BACS Token Motor | −0.25 (−4.00–1.73) | 0.32 (−1.04–1.87) | −1.32 (−2.70–0.12) | −1.19 (−2.50–0.15) | −1.55 (−4.00–0.72) | −1.51 (−3.72–0.42) | F(5,102)=10.39, p<0.001 |
| BACS Verbal Fluency | −0.12 (−2.37–1.92) | 0.19 (−1.47–2.40) | −0.03 (−0.92–1.44) | −0.18 (−2.26–1.78) | −0.96 (−3.27–1.64) | −0.24 (−1.16–2.25) | F(5,103)=2.73, p<0.05 |
| BACS Symbol Coding | −0.15 (−2.17–1.86) | 0.17 (−1.44–1.27) | −0.88 (−2.09–0.39) | −1.05 (−2.76–0.05) | −1.80 (−3.82–1.09) | −0.96 (−2.56–3.56) | F(5,103)=8.69, p<0.001 |
| BACS Tower of London | −0.20 (−2.45–1.79) | −0.18 (−3.23–1.36) | −0.49 (−2.45–0.44) | −0.17 (−1.76–0.96) | −1.21 (−4.00–0.77) | −0.35 (−4.00–2.76) | F(5,103)=2.59, p<0.05 |
| SFS (Total score) | 146.60 (115–171) | 142.08 (109–167) | 131.83 (84–174) | 122.71 (62–167) | 108.95 (59–162) | 112.76 (54–152) | F(5,98)=8.71, p<0.001 |
Post hoc pairwise comparisons (Tukey HSD or chi square, as appropriate); only statistically significant (p<0.05) differences are reported:
Psychosis Groups:
Gender: There was a higher proportion of females among PSY-NonACU compared to HC-ACU (χ2(1)=3.68, p=0.055) and PSY-ACU (χ2(1)=5.61, p=0.018).
Years of Education: PSY-ACU < HC-ACU (p<0.05).
BACS Composite: PSY-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); PSY-ACU < HC-ACU (p<0.05).
BACS Verbal Memory: PSY-NonACU < HC-ACU (p<0.05).
BACS Digit Sequencing: PSY-NonACU < HC-ACU (p=0.07, trend), HC-NonACU (p=0.07, trend).
BACS Token Motor: PSY-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); PSY-ACU < HC-ACU (p<0.001), HC-NonACU (p<0.001).
BACS Symbol Coding: PSY-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); PSY-ACU < HC-ACU (p=0.01), HC-NonACU (p<0.05).
BACS Tower of London: PSY-NonACU < HC-NonACU (p<0.05).
SFS Total score: PSY-NonACU < HC-NonACU (p<0.001), HC-ACU (p<0.05); PSY-ACU < HC-NonACU (p<0.001), HC-ACU (p<0.05).
Diagnosis Groups:
Years of Education: SZ-ACU < HC-ACU (p=0.072), BP-NonACU (p=0.074, trend).
BACS Composite: SZ-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001).
BACS Verbal Memory: SZ-NonACU < HC-ACU (p<0.05).
BACS Digit Sequencing: SZ-NonACU < HC-ACU (p<0.05), HC-NonACU (p<0.05).
BACS Token Motor: SZ-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); SZ-ACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); BP-NonACU < HC-ACU (p<0.01), HC-NonACU (p<0.05); BP-ACU < HC-ACU (p<0.05).
BACS Verbal Fluency: SZ-NonACU < HC-ACU (p<0.05), HC-NonACU (p=0.05).
BACS Symbol Coding: SZ-NonACU < HC-ACU (p<0.001), HC-NonACU (p<0.001); SZ-ACU < HC-ACU (p<0.05).
BACS Tower of London: SZ-NonACU < HC-NonACU (p<0.05).
SFS Total score: SZ-NonACU < HC-NonACU (p<0.001), HC-ACU (p<0.01); SZ-ACU < HC-NonACU (p<0.001), HC-ACU (p<0.05).
HC: healthy controls; PSY: volunteers with psychosis; SZ: volunteers with schizophrenia or schizoaffective disorder; BP: volunteers with psychotic bipolar I disorder, HC: healthy controls; ACU: adolescent onset cannabis use; Non-ACU: cannabis non-users; WRAT: Wide Range Achievement Test (reading subscale); BACS: Brief Assessment of Cognition in Schizophrenia; SFS: The Birchwood Social Functioning Scale
2.2 Cognitive Assessment
Cognitive assessments used in these analyses included (i) premorbid general intelligence estimate from the Wide Range Achievement Test-4 (WRAT-4) Reading subtest (Wilkinson and Robertson, 2006), and (ii) the BACS battery (Keefe et al., 2004), including overall composite score and 6 subdomains and corresponding subtests scores: verbal memory/list learning, working memory/digit sequencing, motor speed/token motor, verbal fluency/category instances and letter fluency, attention and speed of information processing/symbol coding, and executive function/tower of London. These subdomains have been extensively validated and shown to be affected in psychotic disorders (Keefe et al., 2004).
2.3 MRI Acquisition and Data Processing
T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) images based on parameters from the Alzheimer’s Disease Neuroimaging Initiative (ADNI1) protocol (http://adni.loni.usc.edu/methods/documents/mri-protocols/) were acquired on a 3-Tesla Philips Achieva magnet at the Advanced Imaging Research Center, UT Southwestern Medical Center. All volunteers were scanned on the same magnet for the duration of the study. Imaging acquisition parameters and quality control procedures are detailed elsewhere (Ivleva et al., 2013; 2017). Whole brain voxel-wise GMD analyses were carried out using the optimized Voxel-Based Morphometry (Ashburner and Friston, 2000) toolbox (VBM8) for Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8), and incorporated the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL), a high-dimensional nonlinear inter-subject registration tool (Ashburner, 2007). Image preprocessing included the standard steps in MATLAB2013a/SPM8/VBM8/DARTEL (Kurth et al., 2010): (1) individual T1 images were manually reoriented to anterior-posterior commissure and segmented into gray matter, white matter, and cerebrospinal fluid components via the standard SPM8 segmentation algorithm; (2) rigid body transformation parameters were extracted from the nonlinear deformations estimated by the segmentation algorithm and subsequently used to write out rigidly transformed versions of the tissue class images for each subject via DARTEL; (3) the mean of the study images serving as a study-specific template was created and subsequently refined via the cyclic DARTEL iterations to ensure accurate registration while conserving each subject’s anatomical features informative for registering over subjects; (4) gray matter and white matter intensity averages were generated from the template, and nonlinear warping of the subjects’ gray matter and white matter images was performed simultaneously to the tissue intensity averages; (5) warped gray matter images were used in the modulation step to ensure preservation of absolute amount of gray matter corrected for individual brain size (Good et al., 2001); (6) modulated normalized GM images were smoothed with an 8-mm full width at half maximum (FWHM) and selected for subsequent group-level statistical analyses.
2.4 Statistical Analyses
2.4.1 Clinical and cognitive measures
A one-way ANOVA with post-hoc Bonferroni or Tukey multiple comparison tests and Yates corrected chi-square test were used as appropriate for demographic, clinical and cognitive variables (Statistical Package for Social Sciences (SPSS), version 19.0) (IBM Corp, 2013).
2.4.2 Whole brain GMD estimates
Subject-level whole brain GMD estimates were extracted from modulated (pre-smoothed) gray matter images using an in-house MATLAB-based routines (Ivleva et al., 2017). Separate two-way ANCOVAs were used to examine between-group differences on total GMD in Psychosis vs. HC (Model1: HC-NonACU vs. HC-ACU vs. PSY-NonACU vs. PSY-ACU) and Diagnosis vs. HC (Model2: HC-NonACU vs. HC-ACU vs. SZ- NonACU vs. SZ-ACU vs. BP-NonACU vs. BP-ACU) (SPSS, version 19.0). The main effects of diagnosis and ACU status and the interaction between diagnosis and ACU were included as between-subject factors while controlling for a set of covariates known to affect brain structure [age, sex, handedness (Li et al., 2012; Renteria, 2012), lifetime substance use other than cannabis (yes/no, for all substances combined) (Nunez et al., 2016)]. Bonferroni corrections were used for multiple post-hoc comparisons.
2.4.3 Voxel-wise GMD analyses
Whole brain voxel-wise GMD analyses were carried out using a series of general linear models (i.e., full factorial design) in SPM12, conducted separately for Psychosis vs. HC (Model1) and for Diagnosis vs. HC (Model2) grouped based on presence/absence of ACU history, as detailed above. The same covariates (age, sex, handedness, lifetime substance use other than cannabis) were incorporated into all SPM models specified as continuous or categorical regressors, as appropriate. Absolute threshold masking was set at 0.1mm3/voxel to include voxels with lower GMD estimates, given the nature of our sample (i.e., patients with psychotic disorders). All GMD group-level outcomes are reported at p<0.05 using cluster-wise FWE-R (Family Wise Error based on random field theory) (Worsley et al., 1992) correction for multiple comparisons, with a cluster-defining threshold of p<0.001, uncorrected.
Effect sizes for between-group differences were estimated using standard Cohen’s d based on group means and pooled standard deviation for whole brain, and regional GMD estimates derived from 5mm radius spheres placed around each significant cluster’s peak voxel on unsmoothed modulated gray matter images.
2.4.4 Associations between total GMD and cognitive measures (exploratory analyses)
In addition, associations between total GMD estimates and (i) WRAT score, (ii) BACS total score and (iii) BACS subscales scores were explored using Pearson correlations (SPSS, version 19.0). Given the exploratory nature of the analysis, we did not implement correction for multiple comparisons.
3. RESULTS
a. Gray matter density outcomes in the psychosis and healthy control groups stratified by ACU
i. Whole brain Gray matter density
In the Psychosis vs. HC model [psychosis (HC/PSY) × cannabis use (ACU/NonACU)], a significant effect of group on whole brain GMD estimates was detected (F(1,101)=16.30, p≤0.001). There was no significant effect of ACU status, or group by ACU interaction. Subsequent pairwise comparisons revealed that PSY-NonACU had significantly lower total GMD than HC-NonACU (p=0.026) and HC-ACU (p=0.004); likewise, PSY-ACU had lower GMD compared to HC-ACU (p=0.022) (Fig. 1a).
Figure 1. Whole brain gray matter density estimates categorized by the Psychosis (a) or Diagnosis (b) approaches, stratified by adolescent cannabis use.
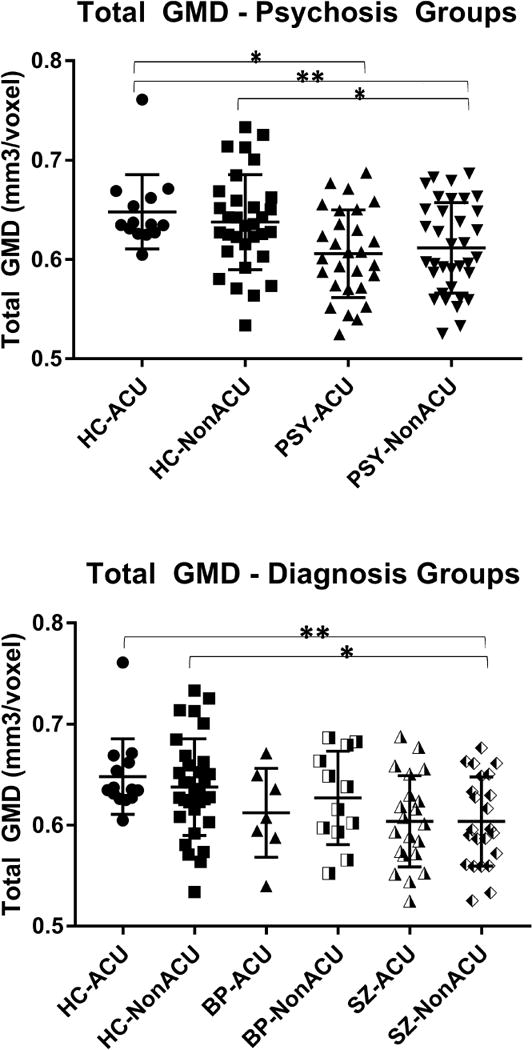
HC: healthy control; PSY: volunteers with psychosis; SZ: volunteers with schizophrenia; BP: volunteers with psychotic bipolar I disorder; ACU: adolescent onset cannabis use; GMD: gray matter density.
The bar graphs indicate group means, the error bars show standard deviations for total gray matter density estimates.
* p<0.05; ** p<0.01.
ii. Regional Gray matter density
Consistent with total GMD outcomes, voxel-wise GMD analysis detected a significant effect of group (F(3,100)=5.86, p<0.05, cluster-wise FWE, k≥875 voxels), but no significant interaction between the group and ACU status (Table 3).
Table 3.
Regional gray matter density characteristics categorized by the Psychosis or Diagnosis approach.
| Group Comparison | Hemisphere | Brain Region (Peak Voxel Location) | Cluster Size (Voxels) | Maximum t (x, y, z; MNI coordinates) | Regional GMD Estimates (Mean ± SD, mm3/voxel) | Effect Size (d) |
|---|---|---|---|---|---|---|
| Main effect of Group | ||||||
| Psychosis Model | Left | Posterior Cingulate | 11904 | 16.0 (−7.5, −63, 7.5) | HC-NonACU: 0.75±0.1; HC-ACU: 0.78±0.07; PSY-NonACU: 0.67±0.11; PSY-ACU: 0.68±0.09 | – |
| Left | Medial Frontal Gyrus | 2031 | 11.3 (−4.5, 52.5, 21) | HC-NonACU: 0.74±0.1; HC-ACU: 0.69±0.09; PSY-NonACU: 0.63±0.1; PSY-ACU: 0.64±0.08 | – | |
| Right | Middle Frontal Gyrus | 875 | 9.11 (30, 33, −4.5) | HC-NonACU: 0.47±0.06; HC-ACU: 0.69±0.09; PSY-NonACU: 0.63±0.1; PSY-ACU: 0.64±0.08 | – | |
| Diagnosis Model | Left | Posterior Cingulate | 6422 | 9.4 (−7.5, −63, 7.5) | HC-NonACU: 0.75±0.1; HC-ACU: 0.78±0.07; SZ-NonACU: 0.66±0.11; SZ-ACU: 0.68±0.09; BP-NonACU: 0.69±0.13; BP-ACU: 0.68±0.07 | – |
| Left | Medial Frontal Gyrus | 1558 | 7.8 (−7.5, 51, 25.5) | HC-NonACU: 0.76±0.08; HC-ACU: 0.72±0.1; SZ-NonACU: 0.64±0.11; SZ-ACU: 0.67±0.08; BP-NonACU: 0.67±0.1; BP-ACU: 0.70±0.07 | – | |
| Left | Parahippocampal Gyrus | 1097 | 8.4 (−16.5, −21, −18) | HC-NonACU: 0.38±0.03; HC-ACU: 0.37±0.04; SZ-NonACU: 0.34±0.04; SZ-ACU: 0.35±0.03; BP-NonACU: 0.38±0.03; BP-ACU: 0.39±0.03 | – | |
| Right | Inferior Frontal Gyrus | 1079 | 6.8 (30, 22.5, −4.5) | HC-NonACU: 0.76±0.12; HC-ACU: 0.76±0.09; SZ-NonACU: 0.66±0.07; SZ-ACU: 0.65±0.06; BP-NonACU: 0.76±0.09; BP-ACU: 0.65±0.12 | – | |
| Right | Inferior Parietal Lobule | 1058 | 7.5 (61.5, −31.5, 28.5) | HC-NonACU: 0.70±0.1; HC-ACU: 0.67±0.09; SZ-NonACU: 0.62±0.08; SZ-ACU: 0.61±0.1; BP-NonACU: 0.69±0.1; BP-ACU: 0.66±0.06 | – | |
| Right | Precuneus | 960 | 7.8 (16.5, −67.5, 34.5) | HC-NonACU: 0.69±0.13; HC-ACU: 0.73±0.16; SZ-NonACU: 0.57±0.1; SZ-ACU: 0.55±0.13; BP-NonACU: 0.58±0.1; BP-ACU: 0.59±0.11 | – | |
| Left | Medial Frontal Gyrus | 800 | 6.9 (−6, 34.5, −19.5) | HC-NonACU: 0.76±0.1; HC-ACU: 0.76±0.09; SZ-NonACU: 0.67±0.1; SZ-ACU: 0.72±0.09; BP-NonACU: 0.80±0.11; BP-ACU: 0.70±0.09 | – | |
| Pairwise comparisons | ||||||
| PSY-NonACU < HC-NonACU | Left Left Right Right Right Right |
Medial Frontal Gyrus Cuneus Precuneus Middle Occipital Gyrus Inferior Temporal Gyrus Superior Temporal Gyrus |
8550 6922 1803 1443 1088 971 |
5.4 (−4.5, 52.5, 21) 6.0 (−6, −67.5, 13.5) 4.5 (18, −69, 33) 4.3 (19.5, −94.5, −4.5) 4.0 (46.5, −67.5, −4.5) 4.5 (54, −27, 22.5) |
PSY-NonACU: 0.63±0.10; HC-NonACU: 0.74±0.10 PSY-NonACU: 0.65±0.10; HC-NonACU: 0.74±0.09 PSY-NonACU: 0.56±0.09; HC-NonACU: 0.67±0.12 PSY-NonACU: 0.42±0.05; HC-NonACU: 0.48±0.07 PSY-NonACU: 0.72±0.15; HC-NonACU: 0.86±0.21 PSY-NonACU: 0.69±0.10; HC-NonACU: 0.78±0.13 |
1.10 0.95 1.04 0.99 0.77 0.78 |
| PSY-ACU<HC-ACU | Right Right Left Right |
Cuneus Inferior Frontal Gyrus Lingual Gyrus Lingual Gyrus |
5622 2247 1631 1046 |
5.4 (7.5, −88.5, 18) 4.7 (28.5, 31.5, −6) 4.6 (−13.5, −64.5, 1.5) 5.0 (19.5, −93, −12) |
PSY-ACU: 0.53±0.07; HC-ACU: 0.64±0.08 PSY-ACU: 0.44±0.05; HC-ACU: 0.50±0.07 PSY-ACU: 0.61±0.11; HC-ACU: 0.77±0.14 PSY-ACU: 0.55±0.12; HC-ACU: 0.73±0.16 |
1.46 0.99 1.27 1.27 |
| PSY-NonACU<HC-ACU | Right Right Right Left |
Lingual Gyrus Parahippocampal Gyrus Middle Frontal Gyrus Parahippocampal Gyrus |
6387 1352 956 915 |
5.1 (16.5, −96, −12) 4.9 (37.5, −16.5, −18) 4.2 (30, 49.5, −7.5) 4.2 (−31.5, −16.5, −16.5) |
PSY-NonACU: 0.50±0.07; HC-ACU: 0.63±0.09 PSY-NonACU: 0.56±0.09; HC-ACU: 0.66±0.08 PSY-NonACU: 0.51±0.08; HC-ACU: 0.57±0.08 PSY-NonACU: 0.57±0.06; HC-ACU: 0.63±0.06 |
1.61 1.17 0.75 1.00 |
| SZ-NonACU<HC-NonACU | Left Left Right Right Right Right |
Superior Medial Gyrus Cuneus Superior Temporal Gyrus Superior Parietal Lobule Inferior Temporal Gyrus Inferior Frontal Gyrus |
9946 9645 2890 1620 1426 1185 |
5.7 (−4.5, 52.5, 22.5) 5.9 (−12, −70.5, 15) 5.4 (54, −28.5, 21) 4.6 (28.5, −75, 43.5) 4.2 (46.5, −69, −4.5) 4.5 (39, 21, 0) |
SZ-NonACU: 0.63±0.10; HC-NonACU: 0.75±0.09 SZ-NonACU: 0.62±0.12; HC-NonACU: 0.77±0.11 SZ-NonACU: 0.67±0.11; HC-NonACU: 0.78±0.14 SZ-NonACU: 0.48±0.07; HC-NonACU: 0.54±0.08 SZ-NonACU: 0.69±0.16; HC-NonACU: 0.82±0.19 SZ-NonACU: 0.62±0.09; HC-NonACU: 0.73±0.12 |
1.26 1.30 0.87 0.80 0.74 1.04 |
| SZ-ACU< HC-ACU | Right Right Right Left |
Cuneus Inferior Frontal Gyrus Inferior Occipital Gyrus Postcentral Gyrus |
2603 2260 923 895 |
5.0 (7.5, −88.5, 18) 4.7 (30, 31.5, −7.5) 4.9 (19.5, −93, −13.5) 4.3 (−66, −7.5, 21) |
SZ-ACU: 0.53±0.07; HC-ACU: 0.64±0.08 SZ-ACU: 0.46±0.04; HC-ACU: 0.55±0.06 SZ-ACU: 0.57±0.12; HC-ACU: 0.77±0.17 SZ-ACU: 0.45±0.07; HC-ACU: 0.57±0.07 |
1.46 1.77 1.36 1.71 |
| SZ-NonACU<HC-ACU | Left Right Right Right Right Left Left Left |
Parahippocampal Gyrus Superior Orbital Gyrus Lingual Gyrus Parahippocampal Gyrus Inferior Frontal Gyrus Superior Medial Gyrus Cingulate Gyrus Lingual Gyrus |
4778 3929 3883 3588 2408 1742 1593 1424 |
4.7 (−33, −16.5, −15) 5.0 (10.5, 24, −25.5) 4.7 (18, −97.5, −12) 5.1 (37.5, −18, −18) 4.5 (51, 40.5, −18) 4.2 (−3, 61.5, 7.5) 4.8 (−4.5, −33, 39) 4.9 (−10.5, −61.5, 4.5) |
SZ-NonACU: 0.43±0.04; HC-ACU: 0.47±0.03 SZ-NonACU: 0.78±0.14; HC-ACU: 0.91±0.12 SZ-NonACU: 0.48±0.06 HC-ACU: 0.59±0.07 SZ-NonACU: 0.55±0.09 HC-ACU: 0.65±0.08 SZ-NonACU: 0.58±0.07; HC-ACU: 0.68±0.10 SZ-NonACU: 0.52±0.08; HC-ACU: 0.59±0.08 SZ-NonACU: 0.70±0.10; HC-ACU: 0.82±0.09 SZ-NonACU: 0.67±0.12; HC-ACU: 0.82±0.10 |
1.13 1.00 1.69 1.17 1.16 0.88 1.26 1.36 |
| BP-NonACU<HC-NonACU | Left | Lingual Gyrus | 2182 | 4.3 (−3, −67.5, 12) | BP-NonACU: 0.57±0.06; HC-NonACU: 0.64±0.08 | 0.99 |
| BP-ACU<HC-ACU | Right | Cuneus | 3268 | 4.6 (7.5, −90, 21) | BP-ACU: 0.54±0.07; HC-ACU: 0.67±0.10 | 1.51 |
| BP-NonACU<HC-ACU | Right | Lingual Gyrus | 2857 | 4.1 (16.5, −94.5, −12) | BP-NonACU: 0.51±0.09; HC-ACU: 0.66±0.11 | 1.49 |
| SZ-NonACU<BP-NonACU | Right | Superior Temporal Gyrus | 1265 | 4.2 (42, −22.5, 0) | SZ-NonACU: 0.55±0.05; BP-NonACU: 0.66±0.11 | 1.29 |
HC: healthy control; PSY: psychosis; SZ: schizophrenia; BP: bipolar I disorder; ACU: adolescent cannabis use; GMD: gray matter density; SD: standard deviation; MNI: Montreal Neurological Institute
PSY-NonACU vs. HC-NonACU
Subsequent pairwise contrasts identified lower GMD in PSY-NonACU vs. HC-NonACU (t=3.17, k=971 voxels) in the left medial frontal gyrus (t=5.4, d=1.10), right inferior temporal gyrus (t=4.0, d=0.77), right Superior Temporal Gyrus (t=4.5, d=0.78), right precuneus (t=4.5, d=1.04), right middle occipital gyrus (t=4.3, d=0.99) and left cuneus (t=6.0, d=0.95) (Table 3, Fig. 2), consistent with typical GMD findings in psychosis samples (Honea et al., 2005; Ivleva et al., 2012; 2013).
Figure 2. Regional brain gray matter density outcomes across the Psychosis groups, stratified by adolescent cannabis use.
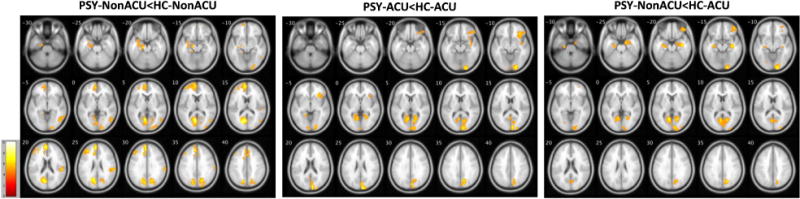
The images show voxel-wise spatial t maps for gray matter density reductions in PSY vs. HC, stratified by adolescent cannabis use. All between-group effects are reported at p<0.05, using cluster-wise Family Wise Error correction for multiple comparisons. The images are displayed in neurological convention; color bars indicate the ranges of t values for each between-group contrast.
PSY: volunteers with psychosis; HC: healthy controls; ACU: adolescent onset cannabis use; Non-ACU: cannabis non-users.
PSY-ACU vs. HC-ACU
Pairwise comparisons between HC vs. PSY with history of ACU, revealed attenuated between-group effects (relative to PSY vs. HC with non-ACU status), where PSY-ACU had lower GMD than HC-ACU (t=3.17, k=1046 voxels) in a few brain regions, including the right inferior frontal gyrus (t=4.7, d=0.99), right cuneus (t=5.4, d=1.46), right lingual gyrus (t=5.0, d=1.27) and left lingual gyrus (t=4.6, d=1.27) (Table 3, Fig. 2).
PSY-NonACU vs. HC-ACU
Likewise, PSY-NonACU, compared to HC-ACU, had significantly lower GMD (t=3.17, k=915 voxels) in several cortical regions, including the right middle frontal gyrus (t=4.2, d=0.75), right parahippocampal gyrus (t=4.9, d=1.17), left parahippocampal gyrus (t=4.2, d=1.00) and right lingual gyrus (t=5.1, d=1.61) (Table 3, Fig. 2).
PSY-ACU vs. HC-NonACU
Strikingly, no significant GMD differences were detected between PSY-ACU and HC-NonACU, i.e., voxel-wise GMD estimates in the psychosis group with history of ACU were not different from HC without lifetime cannabis exposure.
The within-group contrasts in the Psychosis and HC stratified by ACU status (HC-ACU vs. HC-NonACU; PSY-ACU vs. PSY-NonACU) showed no between-group differences.
b. Gray matter density outcomes in the DSM-IV diagnosis and healthy control groups stratified by ACU
i. Whole brain Gray matter density
In order to examine diagnosis-specific effects moderated by history of ACU on brain structure, we split the overall PSY group into two subgroups according to their DSM-IV diagnosis: SZ and BP. Whole brain GMD analyses [diagnosis (HC/SZ/BP) × cannabis use (ACU/Non-ACU)] revealed a significant effect of diagnosis (F(2,99)=9.36, p≤0.001). Similar to the Psychosis vs. HC model, there was no significant effect of ACU, or diagnosis by ACU interaction. Subsequent pair-wise comparisons showed that SZ had overall lower total GMD estimates than HC (p≤0.001), with GMD reductions observed in SZ-NonACU vs. HC-NonACU (p=0.017), SZ-NonACU vs. HC-ACU (p=0.002), and SZ-ACU vs. HC-ACU (p=0.062, trend) (Fig. 1b).
ii. Regional Gray matter density
Voxel-wise GMD analysis detected a significant effect of group (F(5,98)=4.49, p<0.05, FWE, k=800 voxels), but no diagnosis by ACU interaction (Table 3).
HC-NonACU vs. SZ-NonACU
In terms of pairwise contrasts, SZ-NonACU showed lower GMD relative to HC-NonACU (t=3.18, k=1185 voxels) in multiple fronto-temporo-parietal regions: the right inferior frontal gyrus (t=4.5, d=1.04), left superior medial gyrus (t=5.7, d=1.26), right inferior temporal gyrus (t=4.2, d=0.74), right superior temporal gyrus (t=5.4, d=0.87), right superior parietal lobule (t=4.6, d=0.80) and left cuneus (t=5.9, d=1.30) (Table 3, Supplementary Fig. 1a).
HC-ACU vs. SZ-ACU
When we contrasted HC-ACU vs. SZ-ACU, pairwise comparisons revealed a largely attenuated effect, where SZ-ACU had lower GMD estimates than HC-ACU (t=3.18, k=895 voxels) in a few regions: the right inferior frontal gyrus (t=4.7, d=1.77), right inferior occipital gyrus (t=4.9, d=1.36), left postcentral gyrus (t=4.3, d=1.71) and right cuneus (t=5.0, d=1.46) (Table 3, Supplementary Fig. 1a).
HC-ACU vs. SZ-NonACU
The SZ-NonACU vs. the HC-ACU contrast revealed GMD reductions in SZ-NonACU (t=3.18, k=1424 voxels) in the right inferior frontal gyrus (t=4.5, d=1.16), superior medial gyrus (t=4.2, d=0.88), right superior orbital gyrus (t=5.0, d=1.00), left cingulate gyrus (t=4.8, d=1.26), left parahippocampal gyrus (t=4.7, d=1.13), right parahippocampal gyrus (t=5.1, d=1.17), right lingual gyrus (t=4.7, d=1.69) and left lingual gyrus (t=4.9, d=1.36), with the largest clusters localized to frontal regions (Table 3, Supplementary Fig. 1a).
HC-NonACU vs. SZ-ACU
No significant GMD differences were observed in SZ-ACU vs. HC-NonACU, i.e., voxel-wise GMD estimates in SZ with history of ACU were not different from HC without lifetime cannabis exposure.
In contrast, when we compared BP vs. HC subgroups stratified by ACU status, the pattern of GMD changes remained consistent in magnitude and spatial distribution, with modest reductions in BP across comparisons localized to the posterior cortical regions: BP-NonACU vs. HC-NonACU, left lingual gyrus (t=3.18, k=2182 voxels); BP-ACU vs. HC-ACU, right cuneus (t=3.18, k=3268 voxels); and BP-NonACU vs. HC-ACU, right lingual gyrus (t=3.18, k=2857 voxels), (Table 3, Supplementary Fig. 1b).
Similar to the effect found in SZ, no significant GMD differences were observed in BP-ACU vs. HC-NonACU.
The within-diagnosis comparisons stratified by ACU status revealed no significant GMD differences when contrasting SZ-ACU vs. SZ-NonACU, BP-ACU vs. BP-NonACU or HC-ACU vs. HC-NonACU.
Cross-diagnostic groups contrasts (SZ vs. BP, stratified by ACU status) revealed lower GMD estimates in SZ-NonACU relative to BP-NonACU (t=3.18, k=1265 voxels) in the right superior temporal gyrus (t=4.2, d=1.29) (Table 3, Supplementary Fig. 1c), consistent with a typically observed SZ vs. BP effect. In contrast, no between-group differences in GMD were observed for SZ vs. BP with lifetime cannabis exposure (SZ-ACU vs. BP-ACU).
c. Associations between total gray matter density and cognitive measures
There was a significant direct association between premorbid general intelligence estimates (WRAT-4, reading subscale score) and total GMD in the PSY-ACU group (r=0.47, p<0.05). But, when this overall Psychosis group was split into individual diagnoses (SZ, BP), both with the history of ACU, no significant associations were observed.
There were also significant direct associations between WRAT and total GMD estimates in cannabis non-users: HC-NonACU (r=0.49, p<0.01) and SZ-NonACU (r=0.42, p<0.05). Finally, we found a significant association between BACS Digit Sequencing subscale score and whole brain GMD estimates in the HC-NonACU group (r=0.46, p<0.01).
4. DISCUSSION
We examined GMD characteristics in individuals across the SZ/BP psychosis continuum and healthy controls categorized by history of ACU status. We tested whole brain total and regional GMD outcomes, as well as the relationships between GMD and cognitive function. In the psychosis group as a whole, total and regional GMD estimates were lower than in HC, in both PSY-ACU and PSY-NonACU. This finding is consistent with previous reports on gray matter structure changes in individuals with psychotic disorders from our (Ivleva et al., 2012; 2013; 2017) and other groups (Honea et al., 2005). When the psychosis and HC groups were stratified by history of ACU, GMD reductions in both PSY-ACU vs. HC-ACU and PSY-NonACU vs. HC-ACU were attenuated relative to GMD differences between the psychosis and HC groups without adolescent cannabis exposure. The regional pattern of GMD changes spanned multiple cortical areas, consistent with prior reports of widespread gray matter changes in SZ and related psychotic disorders (Gupta et al., 2015; Ivleva et al., 2013; Ivleva et al., 2017; Rapp et al., 2012). This pattern of GMD reductions captured with in vivo imaging is thought to reflect postmortem tissue pathology observed in psychosis, including reduced interstitial neuropil (Selemon and Goldman-Rakic, 1999; Selemon and Rajkowska, 2003), dendritic spine density and length of dendrites (Konopaske et al., 2014; McDonald et al., 2017), pyramidal and interneuron cell size (Jeste and Lohr, 1989; Benes et al., 1991) and/or density (Benes et al., 1998; Bitanihirwe et al., 2009; Jeste and Lohr, 1989; Woo et al., 2004; Zhang et al., 2002); and perineuronal nets density (Berretta et al., 2015; Bitanihirwe and Woo, 2014; Mauney et al., 2013), albeit with an overall preservation of cortical neurons number (Selemon and Goldman-Rakic, 1999; Selemon and Rajkowska, 2003). Notably, total and regional GMD estimates in the PSY-ACU group were not different from those in the ‘typical’ (not exposed to cannabis) control group, HC-NonACU. These findings indicate that GMD is less reduced in individuals with psychotic disorders with a history of early cannabis exposure, and thus that early cannabis abuse may precipitate psychosis in those with less prominent cognitive and neuroanatomic risk factors for illness.
In order to test whether SZ or BP diagnoses associated with ACU have differential effects on these GMD changes, we split the PSY group into two subgroups according to their DSM-IV diagnosis, SZ (inclusive of schizophrenia and schizoaffective disorder) or BP. We found that the changes in GMD observed in PSY as a whole were more prominent in the SZ group, as it showed robust GMD reductions spanning fronto-temporo-parietal and subcortical regions. In contrast, BP-NonACU showed a few small clusters of GMD reduction in the left posterior cingulate and the right cuneus. Similar to SZ-ACU, BP-ACU cases had GMD not different from HC.
We did not observe a significant group by ACU status interaction in this sample, which supports a consistent pattern of association between gray matter structure and early cannabis exposure—higher GMD estimates associated with lifetime history of ACU—across both psychosis groups as well as HC, consistent with some recent reports (Filbey et al., 2015; Gilman et al., 2014; Matochik et al., 2005). In addition, we did not observe any within-group differences in direct comparison between ACU and NonACU, either among HC or the diagnostic groups, possibly due to the modest sample size. This will be examined further in the future in a larger sample.
Our findings demonstrate that individuals with psychosis and no ACU have significantly lower GMD compared to HC. With early cannabis exposure these GMD changes characteristic for psychosis are attenuated. In both SZ-ACU and BP-ACU individuals, GMD was not significantly different from controls. When considering the claim that cannabis use could be associated with more normal GMD, one possible explanation involves cannabis as a possible risk factor for developing psychosis (Kristensen and Cadenhead, 2007), especially at a younger age (Caspi et al., 2005; Konings et al., 2008). Certain individuals may have a genetic predisposition for psychosis, but require an additional environmental insult, such as cannabis use during adolescence, in order to develop psychosis (Hanna et al., 2016). GMD estimates and cognitive function in SZ with ACU are similar to those of HC, suggesting that they are innately more biologically and cognitively intact and have less vulnerability for psychosis, and perhaps had they not been exposed to cannabis, they would not have developed psychosis. These individuals may represent a clinically distinct subgroup, with novel illness mechanisms which might require novel therapies.
Another possible explanation for the association between ACU and higher GMD is that cannabis may have a sparing effect on brain structure. In humans, studies examining associations between cannabis use and brain structure are few, with mixed results, ranging from GMD/volume increases (Filbey et al., 2015; Gilman et al., 2014; Matochik et al., 2005), to no alteration (Jager et al., 2007), to volume reductions (Bangalore et al., 2008; Medina et al., 2007; Yucel et al., 2008), possibly due to modest sample sizes and differences in methodology. Gilman et al. (2014) found greater whole brain GMD following cannabis use in nondependent recreational cannabis users (18-25 years). Likewise, Filbey et al., (2015) reported that early cannabis use (onset of first use before age 16) was associated with increased cortical thickness and gray/white matter border contrast, and decreased local gyrification index in prefrontal regions. Also, Matochik et al. (2005) found greater GMD following heavy cannabis use in adult users (21-35 years). In preclinical studies, exposure to a cannabinoid agonist caused morphological changes in the structure of dendrites and dendritic spines in pyramidal neurons in the medial prefrontal cortex (Carvalho et al., 2016). In addition, Δ9–tetrahydrocannabinol (THC), the principal active component of cannabis, has been shown to alter structural morphology of dendrites in the central nervous system (Kolb et al., 2006). Kolb et al. (2006) found that chronic exposure to low doses of THC modified the structure of dendrites in the shell of the nucleus accumbens and the medial prefrontal cortex (though not in other brain regions), resulting in increased dendritic length and increased number of dendritic branches. These changes in neuronal morphology were evident long after drug exposure, and are therefore representative of a persistent reorganization of synapses in the affected brain regions (Kolb et al., 2006). This reorganization effect of THC may contribute to the sparing GMD effect observed in SZ-ACU individuals, whereby THC-induced lengthening and greater branching in dendrites might compensate for the disease-associated ‘primary’ GMD loss in psychosis.
Another possibility for the development of psychosis following ACU may involve the kind of cannabis that these individuals were exposed to. Cannabis contains more than 70 different cannabinoids (Mechoulam, 2005), the concentrations of which differ based on cannabis strain. Two of the more abundant cannabinoids are THC and cannabidiol (CBD). While THC is the main psychotomimetic ingredient, CBD has been shown to have antipsychotic properties (Zuardi et al., 2012). Therefore, exposure to cannabis strains with a low CBD and/or high THC content may be more likely to lead to psychosis.
We found that adolescent onset cannabis use was associated with higher GMD estimates, in line with an existing body of literature indicating higher GMD and cognitive function in individuals with early vs. late cannabis exposure (Filbey et al., 2015; Gruber et al., 2012; Jacobus et al., 2015). Several studies (DeRosse et al., 2010; Leeson et al., 2012; Meijer et al., 2012; Schnell et al., 2009; Stirling et al., 2005; Yucel et al., 2012), but not all (Mata et al., 2008; Power et al., 2015; Ringen et al., 2010), have linked prior cannabis use in individuals with schizophrenia to less impaired cognitive function, compared to individuals with schizophrenia without a cannabis use history. When prior cannabis use is defined as adolescent cannabis use, however, the results are highly consistent in associating it with improved cognitive function (Hanna et al., 2016; Jockers-Scherubl et al., 2007; Kumra et al., 2005; Yucel et al., 2012). These observations suggest that the adolescent brain may be particularly vulnerable to the effects of cannabis. Support for this comes from several independent animal studies reporting long term neurobehavioral effects of adolescent, but not adult, THC or synthetic cannabinoid treatments (O’Shea et al., 2004; 2006; Quinn et al., 2008; Rubino and Parolaro, 2015; Rubino et al., 2008; 2009; Schneider and Koch, 2003). In this sample, the overall modest sample size limited our ability to compare HC and SZ with early and late onset cannabis use; however, it will be important to examine in future studies.
When looking at correlations between whole brain GMD and WRAT/BACS, we found associations between cognitive ability and GMD in psychotic individuals with ACU. The same association emerged in healthy controls without ACU. Thus, there seemed to be an inverse pattern between HC and PSY in terms of how the relationship between GMD and WRAT scores is influenced by ACU status. Such relationships between GMD and cognitive function mediated by cannabis use require further investigation.
Another important aspect to consider involves the association between social adjustment and substance use. Previously it has been suggested that individuals who use drugs have higher social functioning (Salyers and Mueser, 2001). In addition, recent work highlights the link between socioeconomic status and brain structure (Noble et al., 2015). We did examine between-group differences in current psychosocial functioning based on the Birchwood Social Functioning scale (total score) and found no significant effects in either psychosis or diagnosis subgroups stratified by adolescent cannabis use (PSY-ACU vs. PSY-NonACU, SZ-ACU vs. SZ-NonACU, and BP-ACU vs. BP-NonACU) (Table 2). Likewise, no significant difference in social functioning was observed in HC with and without adolescent cannabis exposure. We did observe, however, significantly lower social functioning in the psychosis and SZ groups, compared to HC, consistent with previous findings in the overall B-SNIP sample (Tamminga et al., 2013). Based on these observations, it is unlikely that social functioning-associated effects can explain our GMD findings in ACU vs. NonACU groups. However, while the information on premorbid social adjustment is not available in our sample, it will be important to consider in future studies.
Several additional limitations should be noted. The sample size is modest introducing the possibility of subtle between-group GMD differences that were not detected with available power. The available information on lifetime cannabis use is limited, given the post hoc nature of these analyses. Future in-depth investigation of the effect of cannabis in psychosis on various brain structure and function measures is in progress. The psychosis volunteers included in this sample were chronically medicated, which could contribute to GMD differences observed in the psychosis vs. healthy control groups (Fusar-Poli et al., 2013; Ho et al., 2011; Vita et al., 2015). In the B-SNIP overall sample we previously tested for concomitant medication effects on brain structure (Ivleva et al., 2013; 2017) and robust associations were not observed. In this study, focused on adolescent cannabis use, the rates of concomitant psychotropic medication use among various classes were highly consistent in the psychosis- and diagnosis-based ACU and NonACU groups (Supplementary Table 1). Therefore, the medication effects are unlikely to explain different GMD characteristics observed in the ACU and NonACU groups, albeit the absence of lifetime medication data limits the interpretation of treatment-related effects.
In conclusion, we have found overall lower GMD in psychosis compared with HC, consistent with previous reports (Honea et al., 2005; Ivleva et al., 2012; 2013; 2017). However, when the psychosis group was stratified by ACU, we found that individuals with psychosis and ACU had GMD not different from healthy controls, consistent with previously reported higher cognitive functioning in these affected individuals (Hanna et al., 2016; Yucel et al., 2012). This intriguing observation of more spared cognitive function and GMD in psychotic individuals with ACU requires further investigation in order to better understand the effects of cannabis on the neurobiology of psychosis.
Supplementary Material
The images show voxel-wise spatial t maps for gray matter density reductions in SZ vs. HC (a), BP vs. HC (b), and SZ vs. BP (c), stratified by adolescent cannabis use. All between-group effects are reported at p<0.05, using cluster-wise Family Wise Error correction for multiple comparisons. The images are displayed in neurological convention; color bars indicate the ranges of t values for each between-group contrast.
SZ: volunteers with schizophrenia or schizoaffective disorder; BP: volunteers with psychotic bipolar I disorder; HC: healthy controls; ACU: adolescent onset cannabis use; Non-ACU: cannabis non-users.
Highlights.
Total and regional gray matter density (GMD) estimates were lower in individuals with psychosis, compared to healthy controls.
GMD reductions in individuals with psychosis who reported adolescent cannabis use were attenuated, compared to the psychosis group without adolescent cannabis use.
Total and regional GMD estimates in individuals with psychosis and adolescent cannabis use were not different from those in healthy controls without cannabis exposure.
Acknowledgments
We would like to thank Gunvant K. Thaker, M.D. formerly of Maryland Psychiatric Research Institute, University of Maryland, who was closely involved with the initial stages of the B-SNIP consortium development and its conceptual and methodological aspects. We also thank all clinicians for patients’ referral, patients themselves and their families for participation in this study.
FUNDING
This work was supported by the National Institute of Mental Health through MH077851 (CT), MH078113 (MK), MH077945 (GP), MH077852 (GT) and MH077862 (JS). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors have declared that there are no conflicts of interest in relation to the subject of this study.
Hila Abush, Subroto Ghose, Erin Van Enkevort, Brett A. Clementz, Matcheri S. Keshavan, and Elena I. Ivleva report no financial disclosures.
Godfrey D. Pearlson reports no potential conflicts of interest related to this manuscript. He reports the following financial disclosure: Astellas–Ad Hoc Consultant
John A. Sweeney reports no potential conflicts of interest related to this manuscript. He reports the following financial disclosures: Takeda Pharmaceutical Company
- Acadia Pharmaceuticals–Ad Hoc Consultant
- American Psychiatric Association–Deputy Editor
- Astellas–Ad Hoc Consultant
- The Brain & Behavior Foundation–Council Member
- Eli Lilly Pharmaceuticles–Ad Hoc Consultant
- International Congress on Schizophrenia Research–Organizer
- Intra-cellular Therapies (ITI, Inc.)–Advisory Board, drug development
- Institute of Medicine–Council Member
- Lundbeck, Inc–Ad Hoc Consultant
- NAMI–Council Member
- National Institute of Medicine–Council Member
- PureTech Ventures–Ad Hoc Consultant
Reference List
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bangalore SS, Prasad KMR, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia - A region of interest, voxel based morphometric study. Schiz Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Benes FM, Sorensen I, Bird ED. Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull. 1991;17:597–608. doi: 10.1093/schbul/17.4.597. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Soc Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167:18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neurosci Biobehav Rev. 2014;45:85–99. doi: 10.1016/j.neubiorev.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes BA, Ramalhosa F, Sousa N, Van Bockstaele E. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct. 2016;221(1):407–419. doi: 10.1007/s00429-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene × environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosse P, Kaplan A, Burdick KE, Lencz T, Malhotra AK. Cannabis use disorders in schizophrenia: effects on cognition and symptoms. Schizophr Res. 2010;120(1–3):95–100. doi: 10.1016/j.schres.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37(8):1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol psychiatry. 2016;79(7):549–556. doi: 10.1016/j.biopsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grigorenko E, Kittler J, Clayton C, Wallace D, Zhuang S, Bridges D, et al. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids. 2002;121:257–266. doi: 10.1016/s0009-3084(02)00161-5. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. 2012;511(2):89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophr Bull. 2015;41(5):1133–1142. doi: 10.1093/schbul/sbu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RC, Shalvoy A, Cullum CM, Ivleva EI, Keshavan M, Pearlson G, et al. Cognitive function in individuals with psychosis: moderation by adolescent cannabis use. Schizophr Bull. 2016;42(6):1496–1503. doi: 10.1093/schbul/sbw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxelbased morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. IBM Corporation; Armonk, NY: 2013. [Google Scholar]
- Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, et al. Brain Structure Biomarkers in the Psychosis Biotypes: Findings from the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) Biol Psychiatry. 2017;82(1):26–39. doi: 10.1016/j.biopsych.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, et al. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MML, Kahn RS, Van Den Brink W, Van Ree JM, et al. Effects of frequent cannabis use on hippocampal activity during an associative learning memory task. Eur Neuropsychopharmacol. 2007;17:289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Lohr JB. Hippocampal pathologic findings in schizophrenia. A morphometric study. Arch Gen Psychiatry. 1989;46:1019–1024. doi: 10.1001/archpsyc.1989.01810110061009. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gómez-Carrillo de Castro A, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1054–1063. doi: 10.1016/j.pnpbp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Grigorenko EV, Clayton C, Zhuang SY, Bundey SC, Trower MM, Wallace D, et al. Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics. 2000;3:175–185. doi: 10.1152/physiolgenomics.2000.3.3.175. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60(6):429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Konings M, Henquet C, Maharajh HD, Hutchinson G, Van Os J. Early exposure to cannabis and risk for psychosis in young adolescents in Trinidad. Acta Psychiatr Scand. 2008;118:209–213. doi: 10.1111/j.1600-0447.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan T, Velthorst E, Koenders L, Zwaart K, Ising HK, van den Berg D, et al. Cannabis use and transition to psychosis in individuals at ultra-high risk: review and meta-analysis. Psychol Med. 2016;46(4):673–681. doi: 10.1017/S0033291715002329. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151:151–154. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Thaden E, DeThomas C, Kranzler H. Correlates of substance abuse in adolescents with treatment-refractory schizophrenia and schizoaffective disorder. Schizophr Res. 2005;73(2–3):369–371. doi: 10.1016/j.schres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Kurth F, Luders Gaser C. BM8-Toolbox Manual 2010 [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull. 2012;38(4):873–880. doi: 10.1093/schbul/sbq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, van Tol MJ, Li M, Miao W, Jiao Y, Heinze HJ, et al. Regional specificity ofsex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp. 2012;35(1):238–247. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective Loss of Smaller Spines in Schizophrenia. Am J Psychiatry. 2017;174:586–594. doi: 10.1176/appi.ajp.2017.16070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata I, Rodríguez-Sánchez JM, Pelayo-Terán JM, Pérez-Iglesias R, González-Blanch C, Ramírez-Bonilla M, et al. Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol Med. 2008;38(9):1257–1266. doi: 10.1017/S0033291707002218. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146:913–915. doi: 10.1038/sj.bjp.0706415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007;48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Dekker N, Koeter MW, Quee PJ, van Beveren NJ, Meijer CJ, et al. Cannabis and cognitive performance in psychosis: a cross-sectional study in patients with non-affective psychotic illness and their unaffected siblings. Psychol Med. 2012;42(4):705–716. doi: 10.1017/S0033291711001656. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Ochoa S, Huerta-Ramos E, Baños I, Barajas A, Dolz M, et al. Cannabis use and cognitive function in first episode psychosis: differential effect of heavy use. Psychopharmacology (Berl) 2016;233(5):809–821. doi: 10.1007/s00213-015-4160-2. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20(5):611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18(4):502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Power BD, Dragovic M, Badcock JC, Morgan VA, Castle D, Jablensky A, et al. No additive effect of cannabis on cognition in schizophrenia. Schizophr Res. 2015;168(1–2):245–251. doi: 10.1016/j.schres.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, et al. Adolescent rats find repeated Delta(9)- THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Rapp C, Bugra H, Riecher-Rössler A, Tamagni C, Borgwardt S. Effects of cannabis use on human brain structure in psychosis: a systematic review combining in vivo structural neuroimaging and post mortem studies. Curr Pharm Des. 2012;18(32):5070–5080. doi: 10.2174/138161212802884861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res. 2011;128(1–3):111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Realini N, Vigano D, Guidali C, Zamberletti E, Rubino T, Parolaro D. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–243. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Renteria ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 2012;15(3):401–413. doi: 10.1017/thg.2012.13. [DOI] [PubMed] [Google Scholar]
- Ringen PA, Vaskinn A, Sundet K, Engh JA, Jónsdóttir H, Simonsen C, et al. Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychol Med. 2010;40(8):1337–1347. doi: 10.1017/S0033291709991620. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry. 2016;79:578–585. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Salyers MP, Mueser KT. Social functioning, psychopathology, and medication side effects in relation to substance use and abuse in schizophrenia. Schizophr Res. 2001;48(1):109–23. doi: 10.1016/s0920-9964(00)00063-3. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28(10):1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med. 2003;3(5):427–436. doi: 10.2174/1566524033479663. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Schnell T, Kleiman A, Gouzoulis-Mayfrank E, Daumann J, Becker B. Increased gray matter density in patients with schizophrenia and cannabis use: a voxel-based morphometric study using DARTEL. Schizophr Res. 2012;138(2–3):183–187. doi: 10.1016/j.schres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology (Berl) 2009;205(1):45–52. doi: 10.1007/s00213-009-1512-9. [DOI] [PubMed] [Google Scholar]
- Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005;75(1):135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tosato S, Lasalvia A, Bonetto C, Mazzoncini R, Cristofalo D, De Santi K, et al. The impact of cannabis use on age of onset and clinical characteristics in first-episode psychotic patients. Data from the Psychosis Incident Cohort Outcome Study (PICOS) J Psychiatr Res. 2013;47(4):438–444. doi: 10.1016/j.jpsychires.2012.11.009. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2017. United Nations publication, Sales No. E.17.XI.6. [Google Scholar]
- Vita A, De PL, Deste G, Barlati S, Sacchetti E. The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol Psychiatry. 2015;78(6):403–412. doi: 10.1016/j.biopsych.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, editors. Wide Range Achievement Test. 4th. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38(2):316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with heavy long-term cannabis use. Arch Gen Psychiatry. 2008;65(6):1–8. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun J, Reynolds GP. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin Med J (Engl) 2002;115:819–823. [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Bhattacharyya S, Atakan Z, Martin-Santos R, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The images show voxel-wise spatial t maps for gray matter density reductions in SZ vs. HC (a), BP vs. HC (b), and SZ vs. BP (c), stratified by adolescent cannabis use. All between-group effects are reported at p<0.05, using cluster-wise Family Wise Error correction for multiple comparisons. The images are displayed in neurological convention; color bars indicate the ranges of t values for each between-group contrast.
SZ: volunteers with schizophrenia or schizoaffective disorder; BP: volunteers with psychotic bipolar I disorder; HC: healthy controls; ACU: adolescent onset cannabis use; Non-ACU: cannabis non-users.


