Abstract
Purpose of Review
We review the underlying mechanisms and potential benefits of intermittent fasting (IF) from animal models and recent clinical trials.
Recent findings
Numerous variations of IF exist, and study protocols vary greatly in their interpretations of this weight loss trend. Most human IF studies result in minimal weight loss and marginal improvements in metabolic biomarkers, though outcomes vary. Some animal models have found that IF reduces oxidative stress, improves cognition and delays aging. Additionally, IF has anti-inflammatory effects, promotes autophagy, and benefits the gut microbiome. The benefit-to-harm ratio varies by model, IF protocol, age at initiation, and duration.
Summary
We provide an integrated perspective on potential benefits of IF as well as key areas for future investigation. In clinical trials, caloric restriction and IF result in similar degrees of weight loss and improvement in insulin sensitivity. Although these data suggest that IF may be a promising weight loss method, IF trials have been of moderate sample size and limited duration. More rigorous research is needed.
Keywords: intermittent fasting, fasting, obesity, calorie restriction, metabolism, insulin resistance, weight loss
Introduction
With more than 2 in 3 adults suffering with overweight or obesity, Americans are searching for effective weight loss methods [1]. Fasting, called “the next big weight loss fad” [2], has long been integral to many religious and ethnic cultures [3]. Intermittent fasting (IF) has many forms; the basic premise involves taking periodic breaks from eating. Common forms of IF include fasting for up to 24 hours once or twice a week with ad libitum (ad lib) food intake for the remaining days, which is known as periodic prolonged fasting (PF) or intermittent calorie restriction (ICR) [4]; time-restricted feeding (TRF), such as eating for only 8 hours then fasting for the other 16 hours of the day; and alternate-day fasting (ADF) [5,6]. Most ADF programs involve alternating feast (ad lib intake) and fast days (≤25% of energy needs) with some protocols allowing no caloric intake on fast days [4]. Thus, the degree of fasting varies in ADF based on the specific protocol.
IF continues to gain attention with new evidence from basic science research and clinical trials. This paper reviews these developments. First, we provide an overview of the key aspects of metabolism involved in fasting. Next, we review clinical trial data of IF outcomes including changes in weight loss and body composition, insulin sensitivity (Si), cardiovascular biomarkers, aging and cognition, psychosocial factors, and the gut microbiome. We review potential cellular mechanisms for these effects including modulation of oxidative stress, inflammation, and autophagy. Finally, we assess the clinical implications of these results and identify directions for future research.
Methods
We searched PubMed/MEDLINE and clinicaltrials.gov for relevant clinical trials and animal studies in English with the search terms: intermittent fasting, periodic, time-restricted, adipose, alternate-day fasting, ADF, and obesity, from 1970 to 2018. We reviewed references from key papers to identify additional articles. Reviews are cited to provide readers with more details and references than is possible here.
In order to provide depth in this review, studies were mostly limited to those on ADF and modified ADF. While TRF is another branch of IF, TRF-only studies were excluded from this review due to their variability, particularly in regards to the definition of TRF and whether breakfast- or dinner-skipping are considered types of TRF. Additionally, this review excludes papers on fasting mimicking diets (FMD), as we consider most FMD protocols to fall under the category of very low calorie diets (VLCD). Lastly, we excluded studies that focused on comorbidities of obesity, such as Type 2 diabetes (T2D), as these comorbidities add complexity beyond the scope of this paper. We focused on pertinent publications from the last 5 years that meet these criteria, but did not exclude older, high impact papers.
Overview of Human Metabolism: The Fed-Fast Cycle
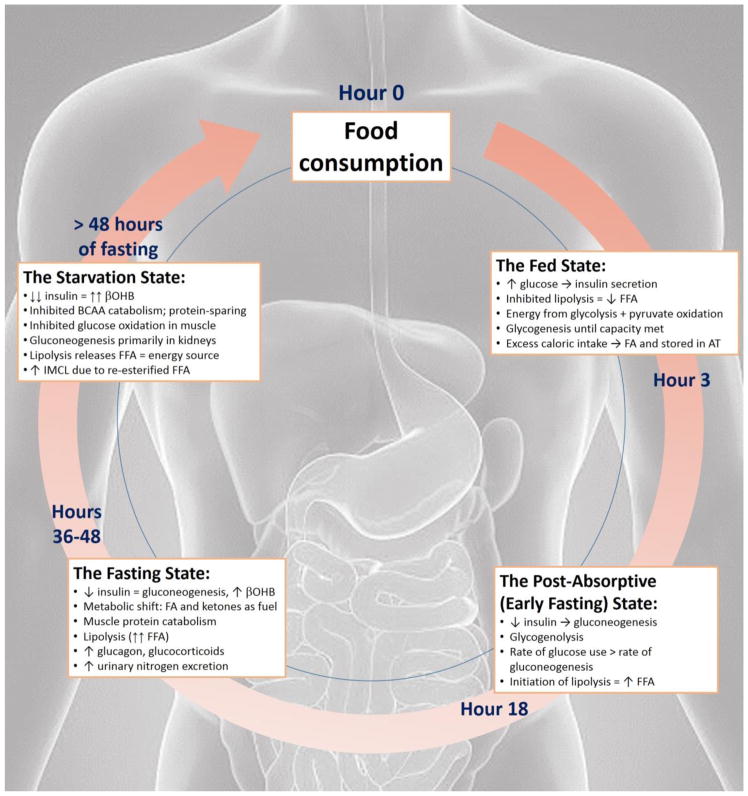
Glucose is the primary energy source for most tissues during the day. Fatty acids (FA) represent an alternative fuel source for the most metabolically active organs including the muscle, liver, and brain and rise overnight during fasting. In 1963, Randle proposed a theory of energy metabolism during feeding and fasting known as the “glucose-fatty acid cycle” whereby glucose and FA compete for oxidation [7]. Since 1963, this cycle and its underlying mechanisms have been elucidated [8]. The fed-fast cycle has four stages: the fed state, the post-absorptive or early fasting state, the fasting state, and the starvation or long-term fast state (Figure 1) [9].
Figure 1.
This figure illustrates the four stages of the fed-fast cycle. Only the fed and post-absorptive states are relevant to normal eating routines. Based on the IF regimen, an individual often goes through the fed, post-absorptive and fasting states. Additionally, while the figure is cyclic, it is possible to return to the food consumption point at any time.
The Fed-Fast Cycle, Circadian Rhythmicity of FFA and Intermittent Fasting
The circadian clock regulates gene expression and broadly affects various organs and the network of neurohormonal weight control signals [16]. Overnight fasting, or fasting during sleeping hours, is associated with a nocturnal rise in plasma free fatty acids (FFA), ghrelin, growth hormone, and increased hepatic gluconeogenesis [17]. Adipose tissue (AT) orchestrates the cycling of triglycerides (TG) by controlling the uptake, esterification, and release of FFA to meet the metabolic demands of the liver and muscle tissue. Hence, integration of circadian rhythms and eating may be beneficial.
The Effects of Intermittent Fasting
The following sections summarize the current literature on various effects of IF.
Alterations in Weight and Body Composition
Nearly all IF studies have resulted in some degree of weight loss, ranging from 2.5–9.9% [18,19], and associated fat mass loss. Numerous studies have been conducted on IF (Table 2), but IF protocol, duration, and baseline characteristics of the sample population have varied greatly.
Table 2.
This table highlights adult human RCTs studies that meet the following criteria: published between 2003 and 2018; IF evaluated as primary variable; IF regimen greater than 1 week. TRF and PF studies were excluded due to the scope of this paper. The studies are ordered by highest N and longest duration. Abbreviations include N (sample size of completers), F (females), M (males), mAge (mean age), bBMI (baseline BMI), IF (intermittent fasting), ADF (alternate-day fasting), mon (months), wk (weeks), d (day), yo (years old), combo (combination), LDL (low density lipoprotein cholesterol), HDL (high density lipoprotein cholesterol), NS (not statistically significant), FFM (fat fee mass), BP (blood pressure), NR (not reported).
| Study Population | Study Design | ||||
|---|---|---|---|---|---|
| First author, Yearab | N completers (Female/Male), inclusion criteriac, dropout rate (DR) | Groups and characteristics (N, mAged, bBMIe) | IF Regimen | Duration | Key Results |
| Harvie, 2013[33] | N = 115 Women only Age 20–69 BMI 24.0–45.0 and/or body fat >30% DR: 11 % in ICR group; 26% in ICR+PF and 33% in DER |
ICR (n=37, 45.6±8.3, 29.6±4.1) ICR+PF (n=38, 48.6±7.3, 31.0±5.7) CER (n=40, 47.9±7.7, 32.2±5.6) |
25% energy needs4 restricted in all, euenergetic groups ICR without ad lib protein, fat: CR and carb restriction on 2 consecutive d/wk ICR + protein, fat (ICR+PF): same as ICR but with unlimited protein and fat on restricted days CER: daily 25% CR |
3 mon weight loss, 1 mon weight maintenance |
|
|
| |||||
| Harvie, 2011[28] | N = 107 Women only Age 30–45 BMI 24.0–40.0 DR: 21% in ICR group; 13% in CER group |
ICR (n=53, 40.1±4.1, 30.7±5.0) CER (n=54, 40.0±3.9, 30.5±5.2) |
25% energy restriction4 as ICR (2d/wk) or CER (7d/wk) | 6 mon |
|
|
| |||||
| Bhutani, 2013[25] | N = 83 80F/3M Age 25–65 BMI 30.0–39.9 DR: 9 dropped out of ADF, 8 out of exercise. No dropouts in exercise or control groups. |
ADF + exercise (combo, n=18, 45±5, 35±1) ADF (n=25, 42±2yo) Exercise (n=24, 42±2, 35±1) Control (n=16, 49±2, 35±1) |
ADF: 25% energy needs2 fast day, ad lib feast day Exercise: moderate intensity exercise 3d/wk |
12 wk |
|
|
| |||||
| Trepanowski, 2017a [22] | N = 79 66F/13M Age 18–65 BMI 25.0–39.9 DR: 38% in ADF, 29% in CR, 26% in control |
ADF (n=25, 46±2, 34±1) CR (n=29, 44±2, 35±1) Control (n=25, 44±2, 34±1) |
ADF: 25% energy needs1 on fast days, 125% feast day with dietary counseling for first 12 wk CR: 75% needs1 daily + dietary counseling for first 12 wk only Control: 100% needs daily; no intervention |
4 wk baseline run-in period → 24-wk weight loss intervention period → 24-wk maintenance period |
|
|
| |||||
| Trepanowski, 2017b [23] | See Trepanowski, 2017a [22] | See Trepanowski, 2017a [22] | See Trepanowski, 2017a [22] | Only the first 28 weeks of Trepanowski, 2017a [22] |
|
|
| |||||
| Teng, 2013[90] | N = 56 Men only Age 50–70 BMI: 23.0–29.9 DR: NR |
ICR (n=28, 59.6±5.4, 26.8±1.7) Control (n=28, 59.1±6.2, 26.7±2.3 |
ICR: 300–500 kcal/d deficit with 2 d/wk Muslim Sunnah fasting. Fasting day included light meal before sunrise, no food and drink during the day (~13 h) and complete meal after sunset Control | 3 mon |
|
|
| |||||
| Byrne, 2017[19] | N = 51 Men only Age 25–54 BMI 30.0–45.0 DR: NR |
ICR (n=26, 39.9±9.2, 34.6±4.2) CER (n=25, 39.3±6.6, 34.4±3.3) |
During ER weeks, 67% energy needs3 for weight maintenance | 32 wk Interspersed: 8 x 2-week blocks of CR and 7 x 2- week blocks of energy balance |
|
|
| |||||
| Varady, 2011[91] | N = 49 40F/9M Age 35–65 BMI 25.0–39.9 DR: NR |
ADF (n=13, 47±2, 32±2) CR (n=12, 47±3, 32±2) Exercise (n=12, 46±3, 33±1) Control (n=12, 46±3, 32±2) |
ADF: 75% energy needs2 on fast days, ad lib feast days CR: 75% energy needs2 daily Exercise: moderate intensity 3d/wk Control: usual |
12 wk |
|
|
| |||||
| Keogh, 2014[29] | N = 36 Women only Age ≥18 BMI ≥27.0 Healthy or T2D managed by diet alone DR: 40% in first 8 wk, 20% between 8–52 wk |
ICR (n=19, 59.5 ± 8.7, 33.1±3.8) CER (n=17, 60.8 ± 12.5, 33.0 ± 7.5) |
ICR: 1 week ‘normal’ diet followed by 1 wk CR (5500 kJ) CER: every day CR (5500 kJ) |
8 wk weight loss intervention, 12 wk weight loss maintenance |
|
|
| |||||
| Hussin, 2013[92] | N = 31 Males only Age: 50–70 BMI: 23.0–29.9 DR: 0 subjects in ADF, 1 in control |
FCR (n=16, 59.7±6.6, 26.7±1.8) Control (n=15, 59.7±6.2, 26.8±2.6) |
IF: 300–500 kcal/d reduction from baseline6 + 2 d/wk of Muslim Sunnah fasting; with counseling Control: ad lib, no counseling |
3 mon |
|
|
| |||||
| Varady, 2013 [93] | N = 30 22F/8M Age 35–65 BMI 20.0–29.9 DR: 6.2% (2 of 32) |
ADF (n=15, 47±3, 26±1) Control (n=15, 48±2, 26±1) |
ADF: 25% energy needs2 on fast day (meals provided), ad lib feast day Control: ad lib daily |
12 wk |
|
|
| |||||
| Teng, 2011 [24] | N = 25 Men only Age 50–70 BMI: 23.0–29.9 DR: 14% (4 of 28) |
FCR (n=12, 59.3±3.4, 27.0±1.7a) Control (n=13, 58.3±6.3, 26.5±1.8) |
ICR: −300 to −500 kcal/day + 2 days of fasting/wk for 3 mon period Control: no intervention |
3 mon |
|
|
| |||||
| Catenacci, 2016[27] | N = 25 19F/6M Age: 18–55 BMI: ≥30.0 DR: 7 withdrew prior to randomization; all 25 completed intervention |
ADF (n=13, 36.9±9.5, 35.8±3.7) CR (n=12, 42.7±7.9, 39.5±6) |
ADF: 0% energy needs5 on fast day, ad lib feast day Control: 400 kcal/d deficit |
8 wk intervention 24 wk follow up |
|
|
| |||||
| Harder- Lauridsen, 2017[94] | N=20 Males only Age: ≥18 BMI: 18.5–2.05 DR: 10% in ADF, 0 in control |
ADF (n=10, 23±3.6, NR) Control (n=10, 24±1.8, NR) |
While on bed rest ADF: 25% energy needs2 on fast days (1 meal/d), 175% needs on feast days (4 meals/d) Control: 100% needs2 (3 meals/d) |
8 days |
|
Inclusion age in years and inclusion BMI in kg/m2. Mean age (mAge) presented as Mean ± SEM when available. Mean baseline BMI (bBMI) presented as Mean ± SEM when available.
Energy needs calculated by: 1doubly labeled water technique, 2Mifflin-St. Jeor equation, 3measured REE (indirect calorimetry) x self-reported physical activity level, 4calculated resting metabolic rate x activity factor, 5[(372 + 23.9 X FFM) X 1.5], 6Diet history questionnaire (DHQ)
The literature distinguishes between ADF and ICR regimens. Heilbronn et al. evaluated 22 days of ADF (0% intake on fast days, ad lib feast days) in 16 healthy subjects with normal BMI [18]. ADF resulted in minor weight loss (2.5%), fat loss (4%), and increased fat oxidation [18]. In contrast, Eshghinia and Mohammadzadeh evaluated 6 weeks of ADF (very low-calorie diet (VLCD) on fast days, ad lib feast days) in women with overweight or obesity [20]. ADF led to 7.1% weight loss and visceral fat mass loss (5.7%). Another study assessed the effects of 8 weeks of ADF with either a high- or low-fat diet in 32 women with obesity [21]. Weight, fat mass, and waist circumference decreased similarly in both groups.
When comparing ADF to no-intervention control, ADF resulted in 6.5% weight loss relative to the control group [5]. However, dietary satisfaction is also important to consider when evaluating weight loss methods. In the same study, hunger did not change in either group, but satisfaction and fullness increased in the ADF group only [5]. This is of particular clinical significance, as diets are often not sustainable due to dissatisfaction with dietary restrictions.
An RCT comparing ADF, CR, and no-intervention control found that mean weight loss was not significantly different at 6 or 12 months between ADF and CR, but dropout rate was significantly higher in the ADF group [22]. Mean LDL cholesterol levels were significantly elevated (+11.5mg/dL [95%CI, 1.9–21.1mg/dL]) in the ADF group at month 12 compared to the CR group. A preplanned secondary analysis compared changes in body composition and fat distribution measured by DEXA and MRI at week 24 [23]. There was no significant difference between ADF and CR in the relative amounts of fat mass, FFM, or visceral and subcutaneous fat loss.
In a study comparing ICR and CER in men with obesity, greater weight loss was seen in the ICR group (12.6% vs. 7.2%) [19]. Fat mass loss was also greater in the ICR group (12.3 kg vs. 6.6 kg), but changes in fat free mass (FFM) were similar [19]. Teng et al. evaluated ICR compared to no-intervention control in men (BMI 18.5–29.9 kg/m2) [24]. The ICR group had decreased weight and fat mass, but weight and fat mass increased in the CER group. FFM was similar pre- and post-intervention in both groups. In contrast to these two studies, Bhutani et al. found that only subjects in the ADF plus exercise group experienced decreased fat mass, not those in the ADF or exercise groups alone [25]. To further add complexity to the mixed results, other studies comparing IF and CER have seen similar weight effects in both groups [26–29]. For example, Catenacci et al. observed 8.8% weight loss in the ADF group and 6.2% in the CER group after 8 weeks, though the between group difference was only marginally significant [27].
While weight and fat mass decreased in most studies, it is important to consider protocol adherence and dropout rates in IF interventions. Some studies have found that the ADF group ate more than prescribed on fasting days and less than prescribed on feast days [22]. Based on these findings, two questions arise. First, does IF, or simply the intervention itself, lead to weight loss? Secondly, does the ADF intervention become CER in the real-world setting due to difficulty following the protocol? Furthermore, dropout rates have been as high as 40%. Thus, despite the statistical significance of weight loss results, the clinical significance and practicality of sustaining an IF regimen are questionable.
Effects on glucose metabolism and insulin sensitivity
Si may be assessed by several methods, including HOMA-IR and hyperinsulinemic euglycemic clamp [30]. Halberg et al. evaluated Si by hyperinsulinemic euglycemic clamp in 8 heathy men (BMI 25.7 ± 0.4 kg/m2) pre- and post-ADF with 20 hours of fasting [31]. Insulin-mediated glucose uptake was assessed by glucose infusion rate (GIR). The final clamp was performed after a 36 hour fast. Although subjects maintained stable weight, Si improved, as indicated by significant increases in GIR, adiponectin, and inhibition of insulin-mediated lipolysis. Soeters et al. sought to replicate these results in a crossover study of 8 healthy men who followed a standard diet or ADF with 20 hours of fasting for 2 weeks [32]. Weight remained unchanged. Unlike the Halberg study, glucose uptake and Si were unchanged during the clamp performed 14-hours post-fasting [32]. Thus, it is unknown when Si improves the most post-fasting and if this is a sustainable change, particularly in healthy men. However, a more clinically relevant question is whether IF can benefit subjects with impaired baseline Si.
In a study comparing ADF and CER, there were no between group differences in lipids or Si [27]. This differs from results of a larger randomized controlled trial (RCT), which found that Si and fasting insulin improved more in the ICR group, compared to the CER group, despite similar effects on weight and other biomarkers [28]. A preplanned secondary analysis of a 2017 RCT [22] found that serum leptin decreased similarly in both CR and ADF groups, and HOMA-IR decreased more in the ADF group (−42%) than in the CR group (−18%) [23].
Another RCT reinforced these findings [33]. Subjects were randomized to one of three CR protocols: CER; carbohydrate- and energy-restricted ICR with ad lib protein and fat (IECR+PF); or carbohydrate- and energy-restricted ICR without ad lib protein and fat (IECR) [33]. Both IECR groups experienced greater improvements in Si than the CER group at 3 months. Both groups also experienced a larger reduction in body fat than the CER group, although total weight loss at 3 and 4 months was not significantly different.
Because IF in animal studies were associated with decreased serum glucose and insulin, these beneficial effects were anticipated in humans. However, human trials have shown only stable or decreased fasting insulin with no change in fasting glucose [20,28,33–36], which is a difficult endpoint to translate to the clinical setting. Thus, while some animal studies [37] suggest an association between IF and Si, the results may not be extrapolated to humans.
Cardiovascular Effects
Limited literature exists on the cardiovascular effects of IF in humans. A 2010 study in rats found that IF compared to daily CR improves glycemic control and protects the myocardium against ischemia-induced cellular damage and inflammation [38]. ADF in male C57BL/6 mice for 4-weeks was associated with a decreased proportion of visceral fat, increased adiponectin, decreased resistin, and improved lipid profiles [39]. IF also resulted in increased adiponectin prior to and after induced myocardial infarctions [38]. A 2016 crossover study of 10 healthy participants (BMI 25–45 kg/m2) found significant alterations in postprandial glucose and lipid metabolism [40]. The study, which evaluated total (100%) and partial (75%) CR compared to no CR, suggests that CER could alter cardiometabolic risk, independent of weight change [40].
Fasting is part of the Latter-Day Saints (LDS) religious practice. A meta-analysis of two observational studies on a predominantly LDS population found that those who routinely fasted were 35% less likely to develop coronary artery disease (95% CI, 0.46–0.94) and 44% less likely to develop T2D (95% CI, 0.36–0.88) compared to those who followed normal eating patterns [41]. Routine fasting was also associated with a lower BMI. This population had a lower prevalence of smoking, however, which may confound the association between fasting and clinical outcomes. Nonetheless, the findings suggest that IF could also alter cardiometabolic risk factors. Additionally, a randomized comparison of IF and CER found comparable reductions in leptin, free androgen index, C-reactive protein, total and LDL cholesterol, TG, and blood pressure as well as similar increases in sex hormone binding globulin and insulin-like growth factor (IGF) binding proteins 1 and 2 [28]. These findings reinforce the potential cardioprotective effects of IF, though more studies need to be conducted in humans.
Impact on Aging and cognition
Animal models provide preliminary evidence that CR and IF may delay aging. Evidence includes improved biomarkers, reduced oxidative stress, and preserved memory [42–44]. Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses [45]. Both CR and ADF diets reduce age-related deficits in cognitive and motor function in animal models. The combination of CR and IF has been found to promote longevity and increase resistance to age-related diseases in rodents and monkeys [46]. ADF in mice has been shown to reduce serum glucose and insulin and to increase neuronal resistance to injury, even with isocaloric intake and stable weight [47]. Inbred male mice following an ADF regimen have also shown prolonged lifespan under certain conditions, but strong interactions exist between the genotype, age at initiation, and effect of ADF on body weight and aging [48]. While animal models have provided some promising results, the paucity of human studies prohibit extrapolation of these effects to human models.
In a subset of subjects (n=11), Heilbronn et al. found increased muscle gene expression of SIRT1 post-ADF [34]. SIRT1 is an enzyme that may be implicated in human longevity [49]. Additionally, women had slightly impaired glucose response though this did not change in men. Women had unchanged insulin response, though there was a significant reduction in men. Hence, there may be gender differences in the metabolic response to ADF.
The SIRT1 finding is consistent with another study in which human serum collected pre- and post-intervention was used to culture hepatoma cells. Cells that were cultured in post-intervention sera had increased SIRT1 levels and decreased TG. Additionally, post-intervention cells had decreased proliferation, increased stress resistance, and upregulation of longevity-inducing genes, all of which suggest that IF plays a role in aging and longevity [50]. However, a different three week ADF protocol (25% ER on fast days) was not associated with changes in whole-blood SIRT1 RNA [35].
Most studies assessing IF and aging are conducted on animals. Furthermore, evidence regarding biomarkers of aging and cognition is mixed, and the conclusions of these studies cannot yet be generalized to the larger population.
Psychosocial impact
Because long episodes of fasting may lead to large portions of unhealthy foods at the end of the fast, it is questionable whether weight loss benefits of IF can be maintained. Binge eating disorder (BED), which affects 2.8 million Americans, is especially prevalent among individuals with obesity and those seeking weight loss [51]. BED is larger than normal food consumption in a small time period, often accompanied by a loss of control over eating [52]. Some studies suggest that IF may have implications on depression and BED.
Hoddy et al. found that 8 weeks of ADF in 59 subjects with obesity decreased depression and binge eating (p<0.01) [53]. While the decrease in depression and binge eating was statistically significant, it does not appear clinically significant, as the absolute changes were minimal. Additionally, purgative behavior and fear of fatness remain unchanged, although ADF increased restrictive eating and improved perceived body image [53]. It is important to consider whether the decrease in depression and binge eating indicators is clinically significant enough to risk increased restrictive eating. Further, it is essential to define “restrictive eating” consistently among studies, as Bhutani et al. found that in subjects randomized to ADF or ADF plus exercise, restrained eating increased while uncontrolled eating decreased [25].
Despite the acute psychosocial benefits that Hoddy et al. and Bhutani et al. found, research studies should evaluate the long-term associations between IF, BED, and depression in order to minimize risk of negative psychosocial effects. In the interim, CER seems more appropriate for individuals at risk of any eating disorder, including BED.
Interaction with the Gut Microbiome
The microbiome modulates adiposity and protects against the development of obesity-associated metabolic dysfunction. This recent discovery has sparked interest in modulators of microbial balance. Preliminary animal models suggest that IF may be one of these modulators [54]. ADF in mice, compared to isocaloric ad lib intake, induced white adipose tissue (WAT) beiging, weight loss, and changes in the gut microbiota, including an increase in the Firmicutes:Bacteroides ratio [55]. This was associated with improvements in liver steatosis and metabolic syndrome. However, in microbiota-depleted mice, ADF did not improve obesity or liver steatosis, thus suggesting that the gut microbiota is necessary for ADF to show these benefits. Furthermore, isocaloric ADF in mice modulates the gut microbiota, benefiting adiposity; this has been recently reviewed [56].
Cellular metabolism underlying effects of IF
In addition to its role as an energy carrier, beta-hydroxybutyrate (βOHB) binds to extracellular receptors and inhibits class I histone deacetylases, which may promote resistance to oxidative stress [57,58]. Thus, epigenetic modifications are likely driven by fasting-induced βOHB elevations. Increases in βOHB alter gene expression in nutrient-sensitive pathways that are implicated in longevity. βOHB also has been shown to promote anti-inflammatory effects by blocking activation of the NLRP3 inflammasome [59] and activating a neuroprotective subset of macrophages in the mouse brain [60]. In addition to the association between IF and neuronal resistance, animal studies have found that IF can affect oxidative stress.
Oxidative stress
CR reduces oxidative stress by limiting mitochondrial generation of ROS and increasing endogenous antioxidant activity; this results in reduced oxidative damage to cellular proteins, lipids, and nucleic acids [61,62]. However, IF has mixed effects on oxidative stress in animal models [63]. In theory, IF may induce hormesis, resulting in beneficial adaptive changes that include activation of AMP-activated protein kinase, mitochondrial network and peroxisome remodeling, and increased production of antioxidant enzymes [42,64,65].
In 8 month old mice at high risk of lymphoma, ADF was associated with a significant reduction in lymphoma (0% vs. 33% of controls), decreased spleen mitochondrial ROS generation, and increased antioxidant superoxide dismutase activity [66]. However, Cerqueria reported increased oxidative stress in 8-week-old Sprague-Dawley rats who underwent 32 weeks of ADF [67]. The ADF group had worsened glucose tolerance, lowered adiponectin, and increased insulin receptor nitration and release of ROS in intra-abdominal AT and muscle [67]. Hence unlike long-term CR, long-term ADF may be associated with worsened Si and oxidative stress. Another study further informed these mixed results. One month of ADF in 8-week old Sprague-Dawley rats had complex, tissue-specific effects on ROS balance in rats [68]. For example, biomarkers of oxidative damage were increased in the liver and the brain but were reduced in the heart [68]. Hence, the effects of IF depend on the animal model, age at initiation, and tissue sampled.
Autophagy is a catabolic process of nutrient recycling that is essential for defense against oxidative stress. Nutrient sensing pathways induce autophagy [69]. IF has been shown to restore autophagic function, thereby preserving organelle quality. This restorative function is impaired by insulin resistance [70] and obesity-induced diabetes in mice fed a high fat diet [71]. However, data on the mechanisms of IF in humans are limited. Twenty-three pre-menopausal women (BMI 25–29.9 kg/m2) followed an ICR diet (two days per week of 65% CR) for one menstrual cycle [72]. After the intervention, 196 metabolites increased (including βOHB and acylcarnitine) and 331 metabolites significantly decreased (including succinic acid, alanine, glutamic acid, and tyrosine). This group also compared the effects of their ICR protocol on the metabolome in another group of pre-menopausal women and found many similar trends [73].
Inflammatory effects
Oxidative stress is closely linked to inflammation. Ten subjects with obesity and asthma followed an ADF protocol for 2 months [36]. Body weight decreased a mean 8% and peak expiratory flow and asthma quality of life scores increased [36]. This intervention was associated with significant reductions in inflammatory markers, including TNF-α and ceramides, and markers of oxidative stress, including protein carbonyls and 8-isoprostane.
In mice, IF increases vascular endothelial growth factor (VEGF) in WAT, with associated alternative macrophage activation and WAT beiging [74]. Gene expression of VEGF, alternative macrophage activation, and beige adipocyte-related proteins are also positively correlated in human AT. This suggests that IF may regulate this same pathway.
Fasting is associated with elevations in FFA and ketone bodies, including βOHB, which may have opposing effects on inflammation. Elevated FFA during fasting may activate proinflammatory pathways [75] and reduce Si [76], while elevations in βOHB may activate anti-inflammatory pathways and alter fuel metabolism as reviewed above. Our group is currently conducting a pilot clinical trial on the effects of dietary supplementation of medium chain triglycerides (MCT), which are metabolized into βOHB (NCT02783703). MCT supplementation may activate anti-inflammatory pathways through βOHB without the detriments of elevated FFAs.
FFA released from lipolysis in mast cells may play an important role in eicosanoid release and control of immune activation [77]. Saturated FFA induce an inflammatory response in macrophages while unsaturated FFA do not [78]. Hence, FFA released from lipolysis play an important role in obesity-induced AT inflammation [79], immune regulation [80], and stimulating hepatic VLDL production [81].
Sustained fasting is associated with acute hepatic steatosis and increased insulin resistance [82]. Normal weight subjects who fasted for 72 and 120 hours had increased intramuscular lipids (IMCL) [83,84]. A 60-hour fast in healthy males was associated with elevated IMCL, increased insulin resistance, and a nine-fold elevation in FFA [83]. Prolonged elevations in FFA, combined with metabolic syndrome and insulin resistance, contribute to increasing hepatic IMCL and lipotoxicity, which leads to nonalcoholic steatohepatitis [84]. However, ADF in mice has been shown to induce metabolic changes that protect against steatosis [85]. Increases in ketogenesis during fasting protects against steatohepatitis in mice [86]; thus, IF may have these same protective effects in humans.
The effects of IF on inflammation have been minimally studied in humans. While cellular level mechanisms have been evaluated in animal models, the application in human models is scarce. Cellular analysis and animal models suggest opposing influence of FFA and ketone bodies on inflammation. Despite understanding these cellular mechanisms, it is unclear whether IF has beneficial effects on oxidative stress and inflammation in human.
Clinical Implications
While several rodent studies have demonstrated the statistical significance of IF on weight loss and metabolic biomarkers, it is important to consider the clinical significance of these findings. For example, while LDL cholesterol levels at month 12 were significantly in the ADF group, it is unlikely that an 11 mg/dl difference would have implications on provider recommendations. Additionally, human studies suggest that IF regimens are difficult to sustain due to dietary restrictions [18,47] and implications on hunger and satisfaction [18,67,87].
Research is not robust enough to suggest that healthcare professionals should be recommending IF to patients as standard practice. It is unknown which individuals would most benefit from IF and which form of IF is most effective. It is anticipated that IF would most benefit motivated individuals who are able to avoid overeating following fasting periods. Further, individuals who are highly involved in social events may find it difficult to comply with IF regimens, and skipping social events because they occur during planned fasting periods is unlikely to be beneficial or sustainable. Additionally, the decision to follow an IF regimen depends on the individual’s goals and desired outcomes. For those interested in weight loss methods, CER may be easier and as effective as IF [88]. Those interested in increasing their FFM may benefit more by combining IF with endurance exercise [25]. There may also be potential contraindications to IF, including certain health conditions, medications, psychosocial barriers, and eating practices. Should IF become part of standard practice, a multidisciplinary approach should be used. Collaboration of registered dietitians, physicians, and other essential healthcare providers will ensure the safety of the patient and decrease the possibility of adverse effects such as weight regain, depression, and BED.
Future Research
Considering the American preference for highly palatable, calorically dense foods, it is crucial for researchers and health practitioners to find unique strategies appropriate to this culture. Longer, statistically powered trials in humans are needed to elucidate the current literature. First, the definitions of IF regimens must be clearly defined. For instance, caloric intake on fast days should be consistent across ADF protocols. These studies should account for the types of foods eaten on ad lib days and how these choices influence weight loss and metabolic markers.
Future research should also determine whether outcomes differ by IF regimen. This would enable practitioners to better recommend dietary changes. For instance, individuals seeking weight loss may require a different IF regimen than those pursuing cardioprotective benefits. There are several trials currently underway that aim to determine the effects of IF on numerous outcomes such as cancer, Alzheimer’s, diabetes, and longevity [89]. Given the positive outcomes thus far, IF may prove to be a promising approach to improving health once it is determined which individuals will best benefit and be able to sustain it.
Conclusions
Animal models and human trials suggest that IF may have beneficial effects on weight, body composition, cardiovascular biomarkers, and aging. At the cellular level, IF may also increase resistance against oxidative stress, decrease inflammation, and promote longevity. However, studies vary greatly on their definition of IF, the prescribed protocol, and the duration of IF. Additionally, the studies have been conducted in diverse populations with mixed results.
Due to the increasing prevalence of overweight and obesity, Americans are searching for effective weight loss methods. The paucity of research on IF makes it difficult to prescribe IF as a reliable method for successful long-term weight loss and maintenance. However, IF appears to be a viable weight loss method, though CER may be as effective. It is important to consider desired outcomes when choosing whether an IF is an appropriate diet. Given that CR is a proven method of weight loss, more research is needed to assess whether IF is a sustainable treatment for obesity as well as if the benefits of IF are maintained long-term.
Table 1.
Comparison of different types of intermittent fasting
| Type of IF | Description | Metabolic states involved |
|---|---|---|
| Alternate day fasting | Alternating feast (ad lib intake) and fast days (≤25% of energy needs) | Fed, post-absorptive, fasting (short duration, likely <36 hours between meals) |
| Time- restricted fasting | Eating only during certain time periods (i.e., 8 hours), then fasting for remaining hours of the day | Fed, post-absorptive (maximum duration between meals is usually <16 hours) |
| Periodic fasting | Fasting for up to 24 hours once or twice a week with ad lib intake on the remaining days | Fed, post-absorptive, fasting (up to 48 hours between meals depending on whether fast days are consecutive) |
Acknowledgments
Funding sources: This work was supported in part by the National Institutes of Health [UL1TR001430, P30DK046200, T32DK007201].
Abbreviations
- IF
intermittent fasting
- CR
calorie restriction
- ICR
intermittent calorie restriction
- ER
energy restriction
- TRF
time-restricted feeding
- PF
prolonged fasting
- ADER
alternate day energy restriction
- CER
continuous energy restriction
- RCT
randomized controlled trial
- GIR
glucose infusion rate
- Si
insulin sensitivity
- ROS
reactive oxygen species
- ad lib
ad libitum
Footnotes
Conflict of Interest Disclosures: Mary-Catherine Stockman, Dr. Thomas, and Jacquelyn Burke disclose no conflicts. Dr. Apovian reports grants from National Institutes of Health during the conduct of the study; personal fees from Nutrisystem, personal fees from Zafgen, personal fees from Sanofi-Aventis, grants and personal fees from Orexigen, personal fees from NovoNordisk, grants from Aspire Bariatrics, grants and personal fees from GI Dynamics, grants from Myos, grants and personal fees from Takeda, personal fees from Scientific Intake, grants and personal fees from Gelesis, other from Science-Smart LLC, personal fees from Merck, personal fees from Johnson and Johnson, grants from Vela Foundation, grants from Dr. Robert C. and Veronica Atkins Foundation, grants from Coherence Lab, grants from Energesis, grants from PCORI, and grants from NIH outside the submitted work.
Compliance with Ethics Guidelines
Conflict of Interest
Mary-Catherine Stockman declares that she has no conflict of interest.
Dylan Thomas declares that he has no conflict of interest.
Jacquelyn Burke declares that she has no conflict of interest.
Caroline M. Apovian has received research funding through grants from Orexigen, Aspire Bariatrics, GI Dynamics, MYOS, Takeda, Gelesis, Vela Foundation, Dr. Robert C. and Veronica Atkins Foundation, Coherence Lab, Energesis, Patient-Centered Outcomes Research Institute (PCORI), and the National Institutes of Health (NIH); has received compensation from Nutrisystem, Zafgen, Sanofi-Aventis, Orexigen, Novo Nordisk, GI Dynamics, Takeda, Scientific Intake, Gelesis, Merck, and Johnson & Johnson for service on advisory boards; and owns stock in Science-Smart LLC.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Flegal KMK, Kruszon-Moran D, Carroll MDM, Fryar CDC, Ogden CCL, KMF, et al. JAMA [Internet] Vol. 315. American Medical Association; 2016. Trends in obesity among adults in the united states, 2005 to 2014; pp. 2284–91. Available from: http://dx.doi.org/10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier R. Intermittent fasting: the next big weight loss fad. CMAJ [Internet] 2013;185:E321–E322. doi: 10.1503/cmaj.109-4437. Available from: http://dx.doi.org/10.1503/cmaj.109-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health benefits of fasting and caloric restriction. Curr Diab Rep. 2017;17:123. doi: 10.1007/s11892-017-0951-7. This recent review summarizes some of the cellular mechanisms underlying the benefits of fasting and caloric restriction. [DOI] [PubMed] [Google Scholar]
- 4••.St-Onge M-P, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation [Internet] 2017;135:e96–121. doi: 10.1161/CIR.0000000000000476. Available from: http://circ.ahajournals.org/lookup/doi/10.1161/CIR.0000000000000476This statement provides an up-to-date review of the effects of meal timing on cardiovascular disease risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr [Internet] 2009;90:1138–43. doi: 10.3945/ajcn.2009.28380. Available from: https://doi.org/10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 6.Collier R. CMAJ. Vol. 185. Canadian Medical Association; 2013. Intermittent fasting: The science of going without; pp. E363–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randle PJ, Garland PB, Hales CN, Newsholme EA. Lancet [Internet] Vol. 281. Elsevier; 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus; pp. 785–9. Available from: http://dx.doi.org/10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 8.Hue L, Taegtmeyer H. Am J Physiol Metab [Internet] Vol. 297. Am Physiological Soc; 2009. The Randle cycle revisited: a new head for an old hat; pp. E578–91. Available from: https://doi.org/10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gropper S, Smith J. Advanced nutrition and human metabolism. Biochem Educ. 2013:421–3. [Google Scholar]
- 10.Unger RHH, Roth MGG. Cell Metab [Internet] Vol. 21. Elsevier; 2015. A new biology of diabetes revealed by leptin; pp. 15–20. Available from: http://dx.doi.org/10.1016/j.cmet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Azzout B, Bois-Joyeux B, Chanez M, Peret J. Development of gluconeogenesis from various precursors in isolated rat hepatocytes during starvation or after feeding a high protein, carbohydrate-free diet. J Nutr [Internet] 1987;117:164–9. doi: 10.1093/jn/117.1.164. Available from: http://jn.nutrition.org/content/117/1/164.short. [DOI] [PubMed] [Google Scholar]
- 12.Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr [Internet] 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. Available from: http://www.annualreviews.org/doi/10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman DH. Am J Physiol Metab [Internet] Vol. 296. American Physiological Society; 2009. Four grams of glucose; pp. E11–21. Available from: https://doi.org/10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stannard SR, Thompson MW, Fairbairn K, Huard B, Sachinwalla T, Thompson CH, et al. Am J Physiol Metab [Internet] Vol. 283. American Physiological Society; 2002. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men; pp. E1185–91. Available from: https://doi.org/10.1152/ajpendo.00108.2002. [DOI] [PubMed] [Google Scholar]
- 15•.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2017 doi: 10.1002/oby.22065. This review synthesizes the animal and human data on the metabolic benefits of fasting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science (80- ) [Internet] 2016;354:994–9. doi: 10.1126/science.aah4965. Available from: http://science.sciencemag.org/content/354/6315/994.abstract. [DOI] [PubMed] [Google Scholar]
- 17.Nørrelund H. Growth Horm IGF Res [Internet] Vol. 15. Elsevier; 2005. The metabolic role of growth hormone in humans with particular reference to fasting; pp. 95–122. Available from: http://dx.doi.org/10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr [Internet] 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. Available from: http://ajcn.nutrition.org/content/81/1/69.full. [DOI] [PubMed] [Google Scholar]
- 19•.Byrne NMM, Sainsbury A, King NAA, Hills APP, Wood REE. Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obes [Internet] 2017:1–10. doi: 10.1038/ijo.2017.206. Available from: http://www.nature.com/doifinder/10.1038/ijo.2017.206This recent RCT of ICR and CER in males found that REE decreased to a greater extent in the ICR group, suggesting that IF without CR may lead to weight gain. [DOI] [PMC free article] [PubMed]
- 20.Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12:4. doi: 10.1186/2251-6581-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62:137–43. doi: 10.1016/j.metabol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 22••.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults. JAMA Intern Med [Internet] 2017;177:930. doi: 10.1001/jamainternmed.2017.0936. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2017.0936This study showed minimal between group differences, though dropout rate in the ADF group (38%) was one of the highest observed in this review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, et al. Clin Nutr. Elsevier; 2017. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng NIMF, Shahar S, Manaf ZA, Das SK, Taha CSC, Ngah WZW. Efficacy of fasting calorie restriction on quality of life among aging men. Physiol Behav. 2011;104:1059–64. doi: 10.1016/j.physbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity [Internet] 2013;21:1370–9. doi: 10.1002/oby.20353. Available from: https://doi.org/10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- 26•.Barnosky AR, Kroeger CM, Trepanowski JF, Bhutani S, Hoddy KK, Gabel K, et al. Nutr Heal Aging2. Vol. 4. IOS Press; 2017. Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6-month randomized controlled trial; pp. 255–63. Insulin resistance decreased to a greater extent, independent of a change in lean mass, in the ADF group over the CR group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. 2016;24:1874–83. doi: 10.1002/oby.21581. Changes in fat mass and FFM were more favorable in the ADF group than in the CR group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int J Obes. 2011;35:714–27. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keogh JB, Pedersen E, Petersen KS, Clifton PM. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clin Obes [Internet] 2014;4:150–6. doi: 10.1111/cob.12052. Available from: http://doi.wiley.com/10.1111/cob.12052. [DOI] [PubMed] [Google Scholar]
- 30.Gutch M, Kumar S, Razi S, Gupta K, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab [Internet] 2015;19:160. doi: 10.4103/2230-8210.146874. Available from: http://www.ijem.in/text.asp?2015/19/1/160/146874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. J Appl Physiol [Internet] Vol. 99. American Physiological Society; 2005. Effect of intermittent fasting and refeeding on insulin action in healthy men; pp. 2128–36. Available from: http://www.physiology.org/doi/abs/10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 32.Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans MT, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009;90:1244–51. doi: 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- 33.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr [Internet] 2013;110:1534–47. doi: 10.1017/S0007114513000792. Available from: https://www.cambridge.org/core/article/effect-of-intermittent-energy-and-carbohydrate-restriction-v-daily-energy-restriction-on-weight-loss-and-metabolic-disease-risk-markers-in-overweight-women/BC03063A5D8E9446D5090DB083A4B226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13:574–81. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 35.Wegman MP, Shankar MN, Guo MH, Bennion DM, Chrzanowski SM, Goldberg LA, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015;18:162–72. doi: 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med [Internet] 2007;42:665–74. doi: 10.1016/j.freeradbiomed.2006.12.005. Available from: https://doi.org/10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev [Internet] 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. Available from: http://www.sciencedirect.com/science/article/pii/S1568163716302513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem [Internet] 2010;21:413–7. doi: 10.1016/j.jnutbio.2009.01.020. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2854256&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varady KA, Hudak CS, Hellerstein MK. Metabolism [Internet] Vol. 58. Elsevier Inc; 2009. Modified alternate-day fasting and cardioprotection: relation to adipose tissue dynamics and dietary fat intake; pp. 803–11. Available from: http://dx.doi.org/10.1016/j.metabol.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 40•.Antoni R, Johnston KL, Collins AL, Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br J Nutr. 2016;115:951–9. doi: 10.1017/S0007114515005346. This study suggests that CER could alter cardiometabolic risk independent of weight change. [DOI] [PubMed] [Google Scholar]
- 41.Horne BD, Muhlestein JB, May HT, Carlquist JF, Lappé DL, Bair TL, et al. Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Am J Cardiol [Internet] 2012;109:1558–62. doi: 10.1016/j.amjcard.2012.01.379. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22425331%5Cnhttp://www.sciencedirect.com/science/article/pii/S0002914912005954. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res Rev. 2008:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–53. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betteridge D. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 46.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci [Internet] 2003;100:6216–20. doi: 10.1073/pnas.1035720100. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev [Internet] 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. Available from: http://www.sciencedirect.com/science/article/pii/004763749090107Q. [DOI] [PubMed] [Google Scholar]
- 49.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, et al. Sirtuin 1 and Sirtuin 3: physiological modulators of metabolism. Physiol Rev [Internet] 2012;92:1479–514. doi: 10.1152/physrev.00022.2011. Available from: http://physrev.physiology.org/cgi/doi/10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, et al. PLoS One [Internet] Vol. 3. Public Library of Science; 2008. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets; p. e3211. Available from: https://doi.org/10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pull, Charles B, Pull CB. Binge eating disorder. Curr Opin Psychiatry. 2004;17:43–48. [Google Scholar]
- 52.Vocks S, Tuschen-Caffier B, Pietrowsky R, Rustenbach SJ, Kersting A, Herpertz S. Meta-analysis of the effectiveness of psychological and pharmacological treatments for binge eating disorder. Int J Eat Disord. 2010;43:205–17. doi: 10.1002/eat.20696. [DOI] [PubMed] [Google Scholar]
- 53••.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutr J. 2015;14:44. doi: 10.1186/s12937-015-0029-9. This study found that depression and binge eating scores decreased after 8 weeks of ADF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridaura VK, Faith JJ, Rey FE, Cheng J, Alexis E, Kau AL, et al. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science (80- ) 2013:341. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G, Xie C, Lu S, Nichols RGG, Tian Y, Li L, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab Cell Metab [Internet] 2017;26:672–85. doi: 10.1016/j.cmet.2017.10.007. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5695033/pdf/nihms917439.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen R, Wang B, Giribaldi MG, Ayres J, Thomas JB, Montminy M. Neuronal energy-sensing pathway promotes energy balance by modulating disease tolerance. Proc Natl Acad Sci [Internet] 2016;113:E3307–14. doi: 10.1073/pnas.1606106113. Available from: http://www.pnas.org/content/113/23/E3307.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science (80- ) 2013;339:211–4. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. Nat Med. Vol. 21. Nature Publishing Group, a division of Macmillan Publishers Limited All Rights Reserved; 2015. The ketone metabolite [beta]-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease; pp. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, et al. Nat Commun. Vol. 5. Nature Publishing Group; 2014. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages; p. 3944. [DOI] [PubMed] [Google Scholar]
- 61.Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab [Internet] 2012;16:777–88. doi: 10.1016/j.cmet.2012.11.003. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3544078/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kowaltowski AJ. Redox Rep [Internet] Vol. 16. Taylor & Francis; 2011. Caloric restriction and redox state: does this diet increase or decrease oxidant production? pp. 237–41. Available from: https://doi.org/10.1179/1351000211Y.0000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med [Internet] 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. Available from: http://www.sciencedirect.com/science/article/pii/S0891584913002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mattson MP. Hormesis defined. Ageing Res Rev [Internet] 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2248601/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response [Internet] 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. Available from: https://doi.org/10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Descamps O, Riondel J, Ducros V, Roussel A-M. Mech Ageing Dev. Vol. 126. Elsevier; 2005. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting; pp. 1185–91. [DOI] [PubMed] [Google Scholar]
- 67.Cerqueira FM, Chausse B, Kowaltowski AJ. Intermittent fasting effects on the central nervous system: how hunger modulates brain function. In: Preedy V, Patel VB, editors. Handb Famine, Starvation, Nutr Deprivation [Internet] Cham: Springer; 2017. pp. 1–18. Available from: https://doi.org/10.1007/978-3-319-40007-5_29-1. [Google Scholar]
- 68.Chausse B, Vieira-Lara MA, Sanchez AB, Medeiros MHG, Kowaltowski J, Kowaltowski AJ. PLoS One [Internet] Vol. 10. Public Library of Science; 2015. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state; p. e0120413. Available from: https://doi.org/10.1371/journal.pone.0120413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, et al. Nature [Internet] Vol. 516. Nature Publishing Group, a division of Macmillan Publishers Limited All Rights Reserved; 2014. Nutrient-sensing nuclear receptors coordinate autophagy; p. 112. Available from: http://dx.doi.org/10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H-Y, Han J, Cao SY, Hong T, Zhuo D, Shi J, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FOXO1-dependent expression of key autophagy genes by insulin. J Biol Chem [Internet] 2009;284:31484–92. doi: 10.1074/jbc.M109.033936. Available from: http://www.jbc.org/content/284/45/31484.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, et al. Autophagy [Internet] Vol. 13. Taylor & Francis; 2017. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagylysosome pathway; pp. 1952–68. Available from: https://doi.org/10.1080/15548627.2017.1368596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Harvie MN, Sims AH, Pegington M, Spence K, Mitchell A, Vaughan AA, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res [Internet] 2016;18:57. doi: 10.1186/s13058-016-0714-4. Available from: https://doi.org/10.1186/s13058-016-0714-4This study compared the effects of CER and ICR on serum and urine metabolites as well as breast tissue gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong KR, Sims AH, Harvie M, Chapman M, Dunn WB, Broadhurst D, et al. Biomarkers of dietary energy restriction in women at increased risk of breast cancer. Cancer Prev Res [Internet] 2009;2:720–31. doi: 10.1158/1940-6207.CAPR-09-0008. Available from: http://cancerpreventionresearch.aacrjournals.org/content/2/8/720.abstract. [DOI] [PubMed] [Google Scholar]
- 74••.Kim K-H, Kim YH, Son JE, Lee JH, Kim S, Choe MS, et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res [Internet] The Author(s) 2017;27:1309. doi: 10.1038/cr.2017.126. Available from: http://dx.doi.org/10.1038/cr.2017.126This study demonstrated a new mechanism for IF involving changes in AT inflammation in mice and evaluated correlations between genes involved in this pathway in human AT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 76.Homko CJ, Cheung P, Boden G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes. 2003;52:487–91. doi: 10.2337/diabetes.52.2.487. [DOI] [PubMed] [Google Scholar]
- 77.Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lipid Res [Internet] 2014;55:2471–8. doi: 10.1194/jlr.M048553. Available from: http://www.jlr.org/lookup/doi/10.1194/jlr.M048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas D, Apovian C. Metab - Clin Exp. Vol. 72. Elsevier; 2017. Macrophage functions in lean and obese adipose tissue; pp. 120–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest Am Soc Clin Investig. 2010;120:3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schreiber R, Zechner R. Lipolysis meets inflammation-arachidonic acid mobilization from fat. J Lipid Res ASBMB. 2014;55:2447–9. doi: 10.1194/jlr.C055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic and clinical implications. Hepatology [Internet] 2010;51:679–89. doi: 10.1002/hep.23280. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3575093/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. Hepatology [Internet] Vol. 55. Wiley Subscription Services, Inc, A Wiley Company; 2012. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association; pp. 2005–23. Available from: http://dx.doi.org/10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 83.Hoeks J, van Herpen NA, Mensink M, Moonen-kornips E, van Beurden D, Hesselink MKC, et al. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes [Internet] 2010;59:2117–2125. doi: 10.2337/db10-0519. Available from: http://diabetes.diabetesjournals.org/content/59/9/2117.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larter CZ, Chitturi S, Heydet D, Farrell GC. J Gastroenterol Hepatol [Internet] Vol. 25. Blackwell Publishing Asia; 2010. A fresh look at NASH pathogenesis. Part 1: The metabolic movers; pp. 672–90. Available from: http://dx.doi.org/10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- 85.Li G, Brocker CN, Yan T, Xie C, Krausz KW, Xiang R, et al. Metabolic adaptation to intermittent fasting is independent of peroxisome proliferator-activated receptor alpha. Mol Metab [Internet] 2018;7:80–9. doi: 10.1016/j.molmet.2017.10.011. Available from: http://www.sciencedirect.com/science/article/pii/S2212877817306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, et al. J Clin Invest [Internet] Vol. 124. The American Society for Clinical Investigation; 2014. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia; pp. 5175–90. Available from: https://doi.org/10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–8. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varady KA. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593–601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 89.Intermittent fasting trials [Internet] 2018 [cited 2018 Jan 3]. Available from: clinicaltrials.gov.
- 90.Teng NIMFN, Shahar S, Rajab NFN, Manaf ZA, Johari MHM, Ngah WZWW. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male [Internet] 2013;16:177–83. doi: 10.3109/13685538.2013.832191. Available from: http://www.tandfonline.com/doi/full/10.3109/13685538.2013.832191. [DOI] [PubMed] [Google Scholar]
- 91.Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011;10:119. doi: 10.1186/1476-511X-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hussin NM, Shahar S, Teng NIMF, Ngah WZW, Das SK. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Heal Aging. 2013;17:674–80. doi: 10.1007/s12603-013-0344-9. [DOI] [PubMed] [Google Scholar]
- 93.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harder-Lauridsen NM, Nielsen ST, Mann SP, Lyngbæk MP, Benatti FB, Langkilde AR, et al. The effect of alternate-day caloric restriction on the metabolic consequences of 8 days of bed rest in healthy lean men: a randomized trial. J Appl Physiol [Internet] 2017;122:230–41. doi: 10.1152/japplphysiol.00846.2016. Available from: http://jap.physiology.org/lookup/doi/10.1152/japplphysiol.00846.2016. [DOI] [PubMed] [Google Scholar]