Abstract
Surgery is the first-line treatment for early, localized, or operable breast cancer. Regional anesthesia during mastectomy may offer the prevention of postoperative pain. One potential protocol is the combination of serratus anterior plane block (SAM block) with pectoral nerve block I (PECS I), but the results and potential benefits are limited. Our study compared general anesthesia with or without SAM block + PECS I during radical mastectomy with axillary node dissection and breast reconstruction using evaluations of pain, opioid consumption, side effects and serum levels of interleukin (IL)-1beta, IL-6 and IL-10. This is a prospective, randomized controlled trial. Fifty patients were randomized to general anesthesia only or general anesthesia associated with SAM block + PECS I (25 per group). The association of SAM block + PECS I with general anesthesia reduced intraoperative fentanyl consumption, morphine use and visual analog pain scale scores in the post-anesthetic care unit (PACU) and at 24 h after surgery. In addition, the anesthetic protocol decreased side effects and sedation 24 h after surgery compared to patients who underwent general anesthesia only. IL-6 levels increased after the surgery compared to baseline levels in both groups, and no differences in IL-10 and IL-1 beta levels were observed. Our protocol improved the outcomes of mastectomy, which highlight the importance of improving mastectomy protocols and focusing on the benefits of regional anesthesia.
Introduction
Breast cancer is the most common cancer in females, with 2.4 million incident cases worldwide1. The standard treatment options for early, localized, or operable breast cancer may include breast-conserving surgery and sentinel node biopsy with or without axillary lymph node dissection for positive sentinel lymph nodes or radical mastectomy (removal of the entire breast with axillary dissection of levels I and II) with or without breast reconstruction and sentinel node biopsy with or without axillary lymph node dissection for positive sentinel lymph nodes2.
One concerning problem that affects breast cancer patients after surgery is the pain. The incidence reaches 53% six months after the surgery, which emphasizes the importance of pain management3. One approach for pain management include the use of regional anesthesia3.
The use of regional anesthesia techniques may modulate the immune system, likely via interleukins (IL)4. Deegan reported increased IL-10 levels after propofol/paravertebral anesthesia for breast cancer compared to sevoflurane/opioids5. Additionally, IL-6 and IL-10 are important in the coordination of breast carcinogenesis6,7. The proinflammatory cytokines IL-1 beta and IL-6 are linked to breast cancer progression and have been hypothesized to be targets of anti-inflammatory drugs used to treat breast cancer8,9. IL-10 has prognostic value since its expression is related to recurrence, metastasis and poor survival in breast cancer10–13. As a therapeutic target, a high level of IL-10 has been associated with drug resistance of breast cancer14.
Blanco proposed alternative regional anesthesia techniques, including the serratus anterior plane block (SAM block) and pectoral nerve blocks I and II (PECS I and PECS II)15–18. The effectiveness of PECS for breast surgery was recently investigated and resulted in improved postoperative pain19. However, compared with PECS II, the SAM block is an easier and simpler technique that offers long-lasting regional anesthesia18. The combination of SAM block + PECS I may provide greater levels of analgesia for radical mastectomy with axillary lymph node dissection and breast reconstruction because of the pattern of analgesia, as previously suggested20,21; however, the results and potential benefits of SAM block + PECS I are very limited considering that these authors evaluated the effectiveness of this combination in only two patients, emphasizing the importance of further studies to validate this alternative surgical approach.
The evaluation of general anesthesia associated with SAM block + PECS I may provide important insights for an alternative anesthetic protocol for this type of mastectomy, but the results and potential benefits are limited. Thus, the aim of our study was to compare general anesthesia associated with SAM block + PECS I to general anesthesia only during radical mastectomy with axillary node dissection and breast reconstruction. The primary outcome measure was pain intensity measured before surgery, in the post-anesthetic care unit (PACU) and at 24 h after surgery. Additionally, it was evaluated opioid consumption, side effects and serum levels of IL-1beta, IL-6 and IL-10. Our results improved the outcomes of mastectomy, which highlight the benefits of regional anesthesia.
Results
A total of 182 surgeries for breast cancer were performed from December 2015 to April 2016, and 133 cases were excluded because of different types of surgical procedures (i.e., mastectomy only, quadrantectomy and lumpectomy), as shown in the CONSORT flowchart (Fig. 1). One patient was excluded for meeting the exclusion criteria (i.e., the presence of chronic pain). A total of 49 patients were randomized to the study. The patients were allocated to the general anesthesia associated with SAM + PECS I protocol (n = 25) or the general anesthesia protocol (n = 24). All patients received the allocated intervention during the surgery, and the follow-up included evaluations in the PACU and at 24 h after surgery. Data from all patients were included in the analysis.
Figure 1.
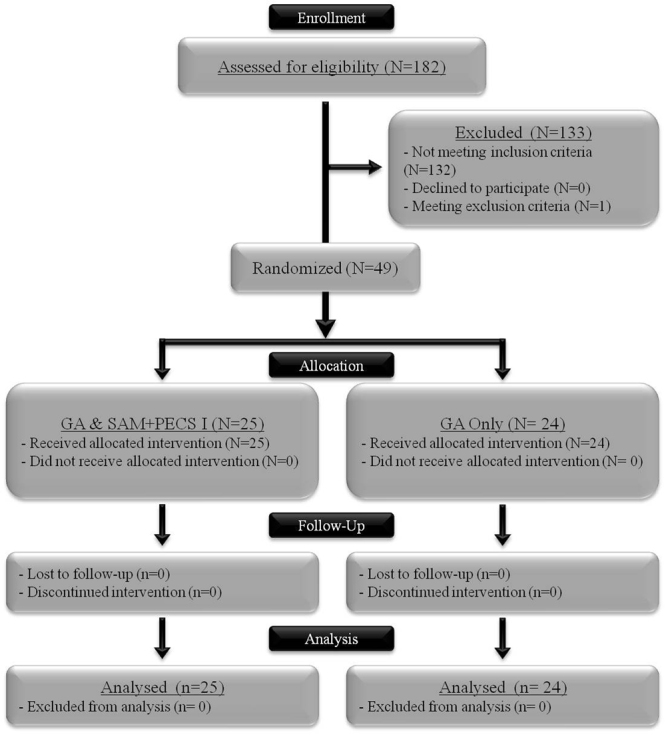
CONSORT flowchart of the surgeries performed during study development. GA: general anesthesia.
Baseline characteristics
The randomized groups were compared, and the baseline characteristics of the patients are shown in Table 1. There were no differences between groups in age (U = 269.5, p = 0.54), weight (U = 298.0, p = 0.97) or body mass index – BMI (U = 246.0, p = 0.28). The women in the general anesthesia with the SAM block + PECS I group were statistically shorter than the women in the general anesthesia only group (U = 169.0, p = 0.04). There were no intraoperative or postoperative complications in the analyzed patient groups. The SF-36 eight scaled scores, including physical functioning, bodily pain, physical role functioning, general health perceptions, vitality, emotional role functioning, social role functioning and mental health, were evaluated before surgery. The Mann-Whitney test revealed no differences in scores between groups (U = 276.5, p = 0.64; U = 300.0; p = 1.00; U = 297.5; p = 0.96; U = 228.0, p = 0.15; U = 261.5, p = 0.44; U = 277.0, p = 0.64; U = 228.5, p = 0.15; and U = 262.5, p = 0.45). Considering comorbidities, there was no difference between groups considering patients taking different classes of medication (anxiolytics (Q(1,24) = 0.14, p = 0.70), antidrepressive (Q(1,24) = 1.80, p = 0.18), thyroids hormone (Q(1,24) = 3.57, p = 0.59), medication to treat dyslipidemias (Q(1,24) = 0.0, p = 1.00), proton pump inhibitors (Q(1,24) = 0.0, p = 1.00), hormone replacement therapy (Q(1,24) = 1.0, p = 0.37), antihypertensives (Q(1,24) = 0.5, p = 0.48), estrogen receptor antagonists (Q(1,24) = 1.0, p = 0.37), bronchodilators (Q(1,24) = 0.0, p = 1.0), oral hypoglycemic drugs (Q(1,24) = 0.2, p = 0.65), anticonvulsants (Q(1,24) = 1.0, p = 0.32), muscle relaxants (Q(1,24) = 1.0, p = 0.32) and corticosteroids (Q(1,24) = 1.0, p = 0.32)).
Table 1.
Demographic data of the population included in the clinical trial.
| Parameters | General Anesthesia Patients’ Data | General Anesthesia & SAM block + PECS I Patients’ Data | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Standard Deviation | Minimum | Maximum | Mean | Standard Deviation | |
| Age (years) | 44 | 74 | 54.83 | 10.49 | 31 | 72 | 56.2 | 11.33 |
| Weight (kg) | 49 | 82 | 66.87 | 7.49 | 49 | 103 | 66.86 | 12.22 |
| Height (cm) | 149 | 172 | 160.58 | 6.69 | 145 | 165 | 156.92 | 5.97 |
| BMI | 20.82 | 31.16 | 25.97 | 2.94 | 19.14 | 37.83 | 27.09 | 4.21 |
| SF-36 Physical functioning | 35 | 100 | 85.33 | 22.97 | 45 | 100 | 83.2 | 21.37 |
| SF-36 Role limitations due to physical health | 0 | 100 | 55.21 | 48.33 | 0 | 100 | 55 | 48.95 |
| SF-36 Pain | 22 | 100 | 81.88 | 26.47 | 0 | 100 | 80.9 | 28.35 |
| SF-36 General health | 20 | 80 | 50.21 | 15.07 | 35 | 90 | 57.6 | 15.88 |
| SF-36 Energy/fatigue | 20 | 100 | 66.88 | 23.02 | 25 | 100 | 62.2 | 19.58 |
| SF-36 Social functioning | 25 | 100 | 92.71 | 18.76 | 0 | 100 | 88 | 26.14 |
| SF-36 Role limitations due to emotional problems | 0 | 100 | 62.54 | 46.44 | 0 | 100 | 82.68 | 34.86 |
| SF-36 Emotional well-being | 20 | 96 | 67.83 | 19.07 | 24 | 100 | 64.48 | 20.24 |
Minimum, maximum, mean and standard deviation data collected from both groups of patients (general anesthesia and general anesthesia with SAM block + PECS I). The parameters measured were as follows: Age (years); Weight (kg); Height (cm); Body mass index (BMI); and Results of the SF-36 survey (divided into eight subtopics: Physical functioning; Role limitations due to physical health; Pain; General health; Energy/fatigue; Social functioning; Role limitations due to emotional problems; and Emotional well-being).
Primary outcome - pain
Significantly lower VAS pain scores were recorded in the PACU and at 24 h after surgery in the general anesthesia associated with SAM + PECS I group compared to those in the general anesthesia only group. The general anesthesia only group exhibited increased pain scores in the PACU and at 24 h after surgery compared to baseline (Factor 1 group F(2,140) = 212.8, p = 0,000001; Factor 2 Time F(4,140) = 8.37, p = 0,000001; Interaction F(4,280) = 4.07, p = 0.003; Fig. 2A).
Figure 2.
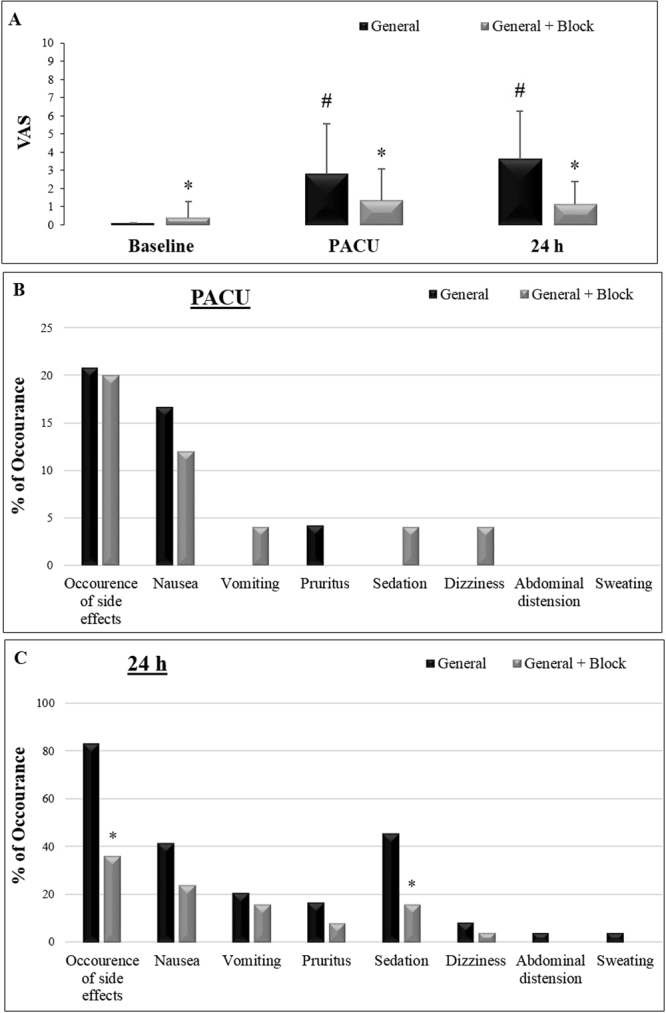
Pain and side effects measured in both groups: general anesthesia (N = 24) and general anesthesia with SAM block + PECS I (N = 25). (A) Pain levels measured using the Visual Analog Scale (VAS) at baseline, in the post-anesthetic care unit (PACU) and 24 h after surgery. (B) Percentages of side effects during the recovery period in the PACU. (C) Percentages of side effects 24 h after the anesthetic procedure. The data are presented as the mean ± standard deviation. *p < 0.05 compared to the general anesthesia only group. #p < 0.05 compared to baseline.
Secondary outcome - side effects
There were no block-related complications reported in the study. No indications of a block-related infection, such as purulent drainage, localized swelling, redness or heat, pain or tenderness at the site of injection, were reported.
In the PACU there were no differences in total side effects (Q(1,24) = 0.0, p = 1.0), nausea (Q(1,24) = 0.33, p = 0.56), vomiting (Q(1,24) = 1.0, p = 0.32), pruritus (Q(1,24) = 1.0, p = 0.32), sedation (Q(1,24) = 1.0, p = 0.32), or dizziness (Q(1,24) = 1.0, p = 0.32) as shown in Fig. 2B. There were no occurrences of abdominal distention or diaphoresis.
Total side effects (Q(1,24) = 9.3, p = 0.002) and sedation (Q(1,24) = 5.4, p = 0.02) decreased 24 h after surgery in the group that received SAM + PECS I association (Fig. 2C). There were no significant differences between groups in nausea (Q(1,24) = 1.33, p = 0.25), vomiting (Q(1,24) = 0.1, p = 0,74), pruritus (Q(1,24) = 0.7, p = 0.41), dizziness (Q(1,24) = 0.3, p = 0.56), abdominal distention (Q(1,24) = 1.0, p = 0.32) or diaphoresis (Q(1,24) = 1.0, p = 0.32).
Secondary outcome - drug consumption
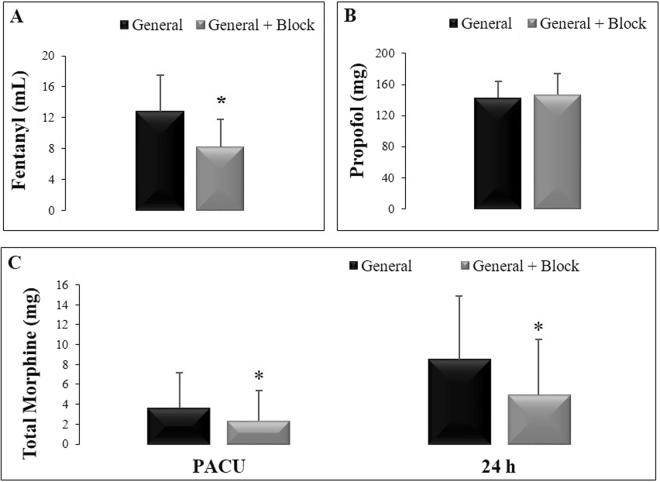
There was a reduction in the intraoperative fentanyl requirement in patients who received general anesthesia associated with SAM + PECS I (U = 119.5, p = 0.0003) compared to patients who received general anesthesia only (Fig. 3A). The intraoperative requirements of propofol (Fig. 3B), cisatracurium and rocuronium were similar in both groups (U = 275.0, p = 0.62; U = 7.5, p = 0.20 and U = 147.0, p = 0.73).
Figure 3.
Amounts of drugs used during the surgical procedures in both groups: general anesthesia only (N = 24) and general anesthesia with SAM block + PECS I (N = 25). (A) Fentanyl consumption (mcg). (B) Propofol consumption (mg). (C) Amount of morphine (mg) consumed by the patients in both groups in the post-anesthetic care unit (PACU) and 24 hours after the anesthetic procedure. The data are presented as the mean ± standard deviation. *p < 0.05 compared to the general anesthesia only group.
Patients who received the SAM + PECS I block required less PCA-morphine in the PACU and at 24 h after surgery compared to patients who received general anesthesia only (Factor 1 group F(2,93) = 139.5, p = 0,000001; Factor 2 Time F(2,93) = 7.23, p = 0.000001; Interaction F(2,93) = 0.68, p = 0.51), as shown in Fig. 3C.
Secondary outcome - cytokines
Figure 4 shows the results for serum IL-6, IL-10 and IL-1 beta. Both groups exhibited an increase in IL-6 levels 24 h after surgery compared to baseline levels (Fig. 4A; Z = 3.9; p = 0.0001 and Z = 4.05; p = 0.0001 for general anesthesia and general anesthesia associated with SAM + PECS 1, respectively). There was no difference in IL-10 levels (Fig. 4B; Z = 1.32, p = 0.18 and Z = 1.34, p = 0.18, respectively) or IL-1 beta levels (Fig. 4C; Z = 1.00, p = 0.32 and Z = 0.45, p = 0.65, respectively).
Figure 4.
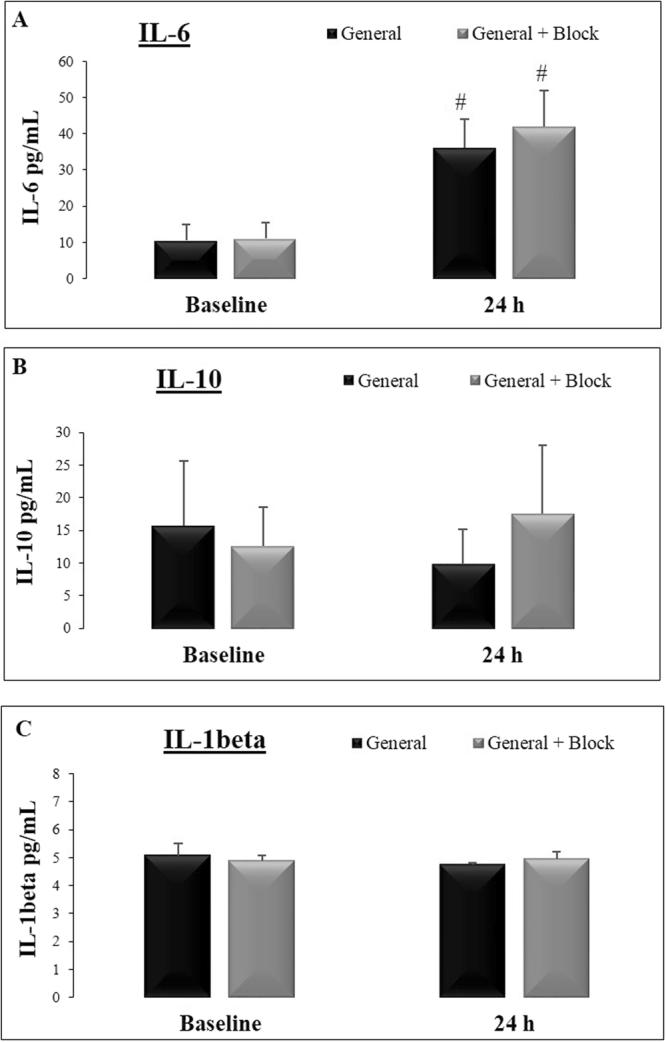
Quantification of serum cytokine levels (pg/mL) in the patients of both groups: general anesthesia only (N = 24) and general anesthesia with SAM block + PECS I (N = 25) at baseline and 24 h after the anesthetic procedure. (A) IL-6. (B) IL-10. (C) IL-1 beta. The data are presented as the means ± standard error. #p < 0.05 compared to baseline.
Additional data
There was no difference in PACU length of stay (U = 250,5; p = 0,32) or the duration of the anesthesia between groups (U = 258; p = 0,41). A Figure illustrating the ultrasound-guided SAM block and PECS I is available in the Supplementary Material (Supplementary Figure S1).
Discussion
To our knowledge, this study is the first prospective, randomized study to report that general anesthesia associated with SAM + PECSI in radical mastectomy with axillary node dissection and breast reconstruction reduced the intraoperative consumption of fentanyl, the delivery of morphine in the PACU and at 24 h after surgery and decreased pain intensity and side effects.
The baseline characteristics of patients were not different in age, weight, BMI, or overall health status. However, there was a statistically significant difference in height between the groups. The risk factors for breast cancer include weight and BMI22,23. A difference in height is not a parameter that would affect the results of regional anesthesia because the most important aspect for calculating the amount of anesthesia is the weight24,25.
No block-related complications occurred during the study. In the literature, the incidence of complications is very low, and ultrasound was reportedly a fundamental tool that provided significant improvements in regional anesthesia in breast surgeries, encouraging its iincorporation into the clinical practice for interfascial chest wall blocks26–29.
The association of SAM + PECS I with general anesthesia reduced morphine consumption in the PACU and at 24 h after the procedure. Previous studies have demonstrated that PECS or SAM block reduced opioid consumption in breast cancer surgery15,17,30,31. Intraoperative high doses of opioids are associated with long-term chronic pain after mastectomy32.
The literature regarding regional anesthesia reports no differences in propofol, cisatracurium and rocuronium consumption33–35, which supports our data.
The use of SAM block + PECS I decreased pain scores in the PACU and at 24 h after the procedure. An effective postoperative pain control is an essential component to avoid chronic pain, which exhibits very high rates in breast cancer patients after surgery3. The role of regional anesthesia in the management of postoperative pain is well known36. Specifically, one case report evaluated PECS block for various types of breast surgeries, and the result revealed an excellent intraoperative and postoperative analgesia37. A cadaver study suggested that PECS I block produced greater analgesia of the axillary region compared to PECS II block38.
Our data demonstrated that the association SAM + PECS I reduced the occurrence of side effects. Consistent with our data, Bashandy and Abbas reported that the use of PECS in mastectomy decreased nausea, vomiting and sedation compared to the use of general anesthesia17. A recent meta-analysis supported that regional anesthesia decreased anesthesia side effects30.
Cytokines exert inhibitory or excitatory effects on tumor growth depending on their concentrations in the tumor microenvironment, and systemic levels of these mediators may correlate with the disease stage and progression39. Furthermore, patients with breast cancer have impaired immune systems40. However, no studies have evaluated inflammatory mediators after the association of SAM block + PECS I during radical mastectomy. Our results revealed higher serum levels of IL-6 24 h after surgery, suggesting that the surgical procedure itself was responsible for the increase. This hypothesis is supported by the fact that the surgical procedure modulates the immune system, especially in patients with malignant diseases41. Therefore, mastectomy increases IL-6 in cancer-associated adipocytes42,43.
There were no differences between groups (general anesthesia versus general anesthesia associated with SAM + PECS I) in IL-6, IL-10 and IL-1 beta levels. Mettler et al. evaluated blood samples of mastectomy or segmental resection patients and demonstrated no difference in the level of IL-1 beta as a consequence of the surgical procedure41. Additionally, data from the literature comparing the effects of general anesthesia and regional spinal/epidural anesthesia in different types of surgeries revealed no difference in IL-1 beta levels compared to anesthesia or the levels before and after the procedure44,45. No difference in the anti-inflammatory IL-10 level was observed between groups or before and after the surgical procedure. Tumor cells secrete IL-1046 especially in breast cancer47,48 and in the context of the surgery T lymphocytes are the main source of IL-10 release49, which may explain the consistent IL-10 levels in our results. One study using paravertebral regional anesthesia reported decreased in IL-1 beta levels and a modest increase in IL-10 levels (10%) without affecting IL-6 compared to the non-blocking group5. One of the main reasons for this discrepancy may be the study design, which compared propofol/paravertebral to sevoflurane/opioid. The type of hypnotic used to maintain anesthesia may also affect the cytokine profile. Specifically, IL-1 beta levels increase when sevoflurane is used compared to propofol50. Our study reported that serum cytokines exerted a minor influence on the possible beneficial effect of the regional anesthesia on clinical outcomes, as previously suggested44.
Surgery is the first choice of treatment, and one of the goals of performing regional anesthesia may be the reduction of post-mastectomy pain syndrome51. Regional anesthesia may be an important tool in the reduction of tumor growth and metastasis recurrence52. It has been implicated that damage to intercostobrachial nerve could be responsible for persistant pain after breast cancer surgery and the local anesthetic block of this region could be an alternative to prevent this type of pain53. Supporting this view, Takimoto demonstrated that SAM block was a treatment modality for chronic neuropathic pain after partial mastectomy54.
One limitation of our trial includes the impossibility of measuring the hospital length of stay after surgery. Since no complications were noted, all mastectomy patients were discharged from the hospital three days after the procedure as part of the standardized protocol of the hospital. In addition, serum levels of ropivacaine were not evaluated, which may represent a bias because there are no studies indicating a possible anti-inflammatory effect of ropivacaine.
In conclusion, our results suggest that SAM + PECS I block in association with general anesthetic provides the most effective analgesia for radical mastectomy, but further studies are required.
Implications
This report is the first prospective, randomized, controlled study to report the benefits of general anesthesia associated with SAM block + PECS I in radical mastectomy with breast reconstruction. Our results highlight the importance of improving anesthetic protocols for radical mastectomy and further examining of the benefits of regional anesthesia and pain reduction on the chronification process.
Methods
Patients
The study was a single-center, prospective, randomized controlled clinical trial. The patients participating in the trial were randomly assigned to the group receiving general anesthesia only (standard treatment) or the group general anesthesia associated with SAM block + PECS I. The block randomization method was designed to randomize subjects into groups. It was generated by the corresponding author and included sealed envelopes, and the patients were blinded to this process. An anesthesiologist recruited participants from our ambulatory care unit. The informed consent was provided to all prospective study subjects. The Ethics in Research Committee of the Hospital Sirio Libanes/Brazil Platform approved the project (CAAE 48721715.0.0000.5461), it is registered at Registro Brasileiro de Ensaios Clinicos (ReBEC), ClinicalTrials.gov, Identifier: NCT02647385, and the trial is registered under the name: ‘Pain Control in Breast Surgery: Analgesia, Opioid Consumption and Inflammatory Response Evaluation’. The principal investigator’s name is Raquel Chacon Ruiz Martinez and the date of registration is 12/28/2015. All methods were performed in accordance with the relevant guidelines and regulations.
The inclusion criteria were female patients aged 18–75 years old who provided written consent, with American Society of Anesthesiology (ASA) physical status I or II, and who were suitable for elective radical mastectomy with axillary node dissection and breast reconstruction. Exclusion criteria were previous allergy to medications used in the study, history of mental disorders and chronic pain. Chronic pain was diagnosed using the Douleur Neuropathique 4 (DN4) questionnaire55. All data were entered into the REDCap (Research Electronic Data Capture) database.
Surgery and postoperative analgesia
Three different surgical teams performed or supervised all surgery. All patients received a standardized postoperative analgesic regimen that consisted of regular metamizole (1 g every 6 h), ketoprofen (100 mg every 12 h) and patient-controlled analgesia (PCA) rescue with intravenous morphine at the end of the surgical procedure using a program of a 2 mg bolus, an interval of 10 min and a maximum dose of 12 mg per hour. The total consumption of opioid was recorded.
Clinical assessments
Demographic data and quality of life parameters were acquired from medical records and an interview with each subject at the time of study enrollment. The short-form health survey (SF-36) was assessed as an indicator of overall health status.
Primary outcome
The primary outcome measure was pain intensity, which was assessed using visual analog pain scale (VAS) scores before surgery, in the post-anesthetic care unit (PACU) and at 24 h after surgery. The VAS scores range from 0–10, and a blinded experimenter evaluated the VAS scores.
Secondary outcomes
Blinded observers evaluated secondary outcomes, which included the consumption of fentanyl and propofol in the intraoperative room, the consumption of PCA-morphine in the PACU and at 24 h after the surgery, the time spent in the PACU, the side effects exhibited in the PACU and at 24 h after the surgery, and serum cytokines IL-1 beta, IL-6 and IL-10 levels before and 24 h after surgery. Any complications were recorded.
General anesthesia
The patients received 7.5 mg of midazolam orally as premedication one h before surgery. Anesthesia was induced with fentanyl (2–3 mcg kg−1), propofol (2–3 mg kg−1) and cisatracurium (0.1 mg kg−1) or rocuronium (0.6 mg kg−1). Anesthesia was maintained with sevoflurane (1.5–2.0%) and 50% oxygen and air with positive pressure ventilation in a circle system. Additional fentanyl boluses were administered if necessary.
SAM Block + PECS I
Three anesthetists who were experienced in performing SAM and PECS I performed these blocks. Prior to the procedure, 2% chlorhexidine solution with 70% isopropyl alcohol and sterile ultrasound conducting gel were applied to the skin. A nonsterile conductive gel was applied to the face of the transducer. The operator wore sterile gloves and applied a sterile cover to the transducer. Before and after each procedure, the ultrasound apparatus was appropriately cleaned and disinfected with disinfectant wipes and allowed to air dry. A 12.5-MHz linear probe was positioned in the midaxillary line at the level of T5, and an in-plane needle was inserted into the fascia between the latissimus dorsi and serratus anterior muscles for ropivacaine (20 mL of 0.375%) injection. The injection for PECS I was performed in the fascia between minor and major pectoral muscles using 10 mL of 0.375% ropivacaine8,11. Needle position was confirmed using visualization of the separation of the layers with dispersion of the injected volume.
ELISA
Blood samples were collected in EDTA-Vacutainer tubes and processed by the Biobanco unit from the Hospital Sirio Libanes. Blood was immediately placed on ice and centrifuged. The plasma was separated and stored at −80 °C until measurement. IL-1beta, IL-6 and IL-10 concentrations in samples were measured in duplicate using specific commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). The ELISA protocol was performed according to the manufacturer’s specifications.
Study design
Consenting patients were interviewed on the day of surgery, and the SF-36, DN4 and VAS scales were used. A blood sample was collected before to surgery. Anesthesia was performed according to group randomization (general anesthesia only or general anesthesia associated with SAM + PECS I), and the consumptions of fentanyl, propofol, cisatracurium and rocuronium were recorded. Patients were admitted to the post-anesthetic care unit (PACU) after surgery, and the following parameters were measured: time spent in PACU, VAS, PCA-morphine consumption, side effects and complications. Blood samples were collected 24 h after surgery, and VAS, PCA-morphine consumption, side effects and complications were assessed. Blood samples were evaluated in the ELISA assay to determinate the cytokine levels.
Duration of the study
The surgeries were performed from December 2015 to June 2016.
Safety Considerations and Follow-up
Patient safety was monitored during the study. All adverse events were monitored throughout the study.
Breaking the codes
At the end of the study, the sponsor of the study had permission to release the code break envelopes/randomization list. These information codes were broken once the trial database was completed.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request in the REDCap database (https://redcap.iephsl.org.br).
Sample size justification and statistical analysis
The sample size was calculated using www.openepi.com based on the results of Wu, who evaluated general anesthesia plus paravertebral versus general anesthesia block and produced an effect size of 1.5 for VAS46. Sixteen patients per group were required to achieve significant results with an alpha of 0.05 and a beta of 90%. There are no previous data on general anesthesia versus SAM + PECS I. Therefore, we estimated that 50 patients divided into two arms would be sufficient to show an effect of regional anesthesia versus general anesthesia.
Data are shown as the means ± standard deviation and Elisa data are shown in mean ± standard error. Demographic data, quality of life, drug consumption and time spent in the PACU were analyzed using the Mann-Whitney test. VAS and opioid consumption were analyzed using two-way ANOVA repeated measures (Factor 1: group, Factor 2: time) followed by Newman-Keuls post-hoc tests. The interleukin data were analyzed using the Wilcoxon test. Medication and side effects were analyzed using Cochran’s Q test. Significance was set at P ≤ 0.05.
Electronic supplementary material
Acknowledgements
The authors thank the research assistants and staff of the Hospital Sirio Libanes (HSL), Centro de Mastologia HSL, Ambulatorio do Projeto de Filantropia, Sao Paulo Servicos Medicos de Anestesia (SMA), Centro de Tratamento da dor do HSL and Biobanco. Grants from PROADI-SUS funded this research. R.C.R.M., F.V.G. and R.L.P. received grants FAPESP (#11/08575-7, 13/20602-5 and 15/26079-8, respectively) from the government of Brazil.
Author Contributions
Study design and data analysis: M.M.; E.F.M.; P.P.K.; C.M.S.; R.L.P. and R.C.R.M. Patient recruitment, data collection and composition of the paper: M.M.; E.F.M.; P.P.K.; F.V.G.; M.A.K.; A.C.S.D.B.; M.M.C.S.; F.E.M.A.; J.V.; E.F.A.; C.M.S.; R.L.P. and R.C.R.M.
Competing Interests
The authors declare no competing interests.
Footnotes
Claudia M. Simões, Rosana L. Pagano and Raquel C. R. Martinez contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26273-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global health estimates (WHO, 2013).
- 2.PDQ Adult Treatment Editorial Board. Breast cancer treatment (PDQ®): patient version, http://www.ncbi.nlm.nih.gov/books/NBK65969/PubMed (2002).
- 3.Smith HS, Wu SX. Persistent pain after breast cancer treatment. Ann. Palliat. Med. 2012;1:182–194. doi: 10.3978/j.issn.2224-5820.2012.10.13. [DOI] [PubMed] [Google Scholar]
- 4.Lee BM, Cata JP. Impact of anesthesia on cancer recurrence. Rev. Esp. Anestesiol. Reanim. 2015;62:570–575. doi: 10.1016/j.redar.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Deegan CA, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg. Anesth. Pain Med. 2010;35:490–495. doi: 10.1097/AAP.0b013e3181ef4d05. [DOI] [PubMed] [Google Scholar]
- 6.Hamidullah CB, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res. Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 7.Carpi A, Nicolini A, Antonelli A, Ferrari P, Rossi G. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr. Cancer Drug Targets. 2009;9:888–903. doi: 10.2174/156800909790192392. [DOI] [PubMed] [Google Scholar]
- 8.Cheung YT, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol. 2015;26:1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr. Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, et al. Relationship between IL-10 expression and prognosis in patients with primary breast cancer. Tumor Biol. 2014;35:11533–11540. doi: 10.1007/s13277-014-2249-6. [DOI] [PubMed] [Google Scholar]
- 11.Lv M, et al. Cytokines as prognstic tool in breast carcinoma. Front. Biosci. 2011;16:2515–2526. doi: 10.2741/3869. [DOI] [PubMed] [Google Scholar]
- 12.Llanes-Fernández L, et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast. 2006;15:482–489. doi: 10.1016/j.breast.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Merendino RA, et al. Interleukin-12 and interleukin-10 production by mononuclear phagocytic cells from breast cancer patients. Immunol. Lett. 1999;68:355–358. doi: 10.1016/S0165-2478(99)00067-X. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med. Oncol. 2015;32:352. doi: 10.1007/s12032-014-0352-6. [DOI] [PubMed] [Google Scholar]
- 15.Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66:847–848. doi: 10.1111/j.1365-2044.2011.06838.x. [DOI] [PubMed] [Google Scholar]
- 16.Blanco R, Fajardo M, Maldonado TP. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev. Esp. Anestesiol. Reanim. 2012;59:470–475. doi: 10.1016/j.redar.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Bashandy GMN, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg. Anesth. Pain Med. 2015;40:68–74. doi: 10.1097/AAP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 18.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:1107–1113. doi: 10.1111/anae.12344. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: a randomised controlled trial. Eur. J. Anaesthesiol. 2018;35:215–223. doi: 10.1097/EJA.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 20.Bouzinac A, Brenier G, Dao M, Delbos A. Bilateral association of pecs I block and serratus plane block for postoperative analgesia after double modified radical mastectomy. Minerva Anestesiol. 2015;81:589–90. [PubMed] [Google Scholar]
- 21.Fusco P, Scimia P, Marinangeli F, Pozone T, Petrucci E. The association between the ultrasound-guided Serratus Plane Block and PECS I Block can represent a valid alternative to conventional anesthesia in breast surgery in a seriously ill patient. Minerva Anestesiol. 2016;82:241–242. [PubMed] [Google Scholar]
- 22.Guo Y, et al. Genetically predicted body mass index and breast cancer risk: mendelian randomization analyses of data from 145,000 women of European descent. PLoS Med. 2016;13:e1002105. doi: 10.1371/journal.pmed.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, et al. The association between body size and breast cancer in han women in northern and eastern China. Oncologist. 2016;21:1362–1368. doi: 10.1634/theoncologist.2016-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mather LE, Copeland SE, Ladd LA. Acute toxicity of local anesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Reg. Anesth. Pain Med. 2005;30:553–566. doi: 10.1016/j.rapm.2005.07.186. [DOI] [PubMed] [Google Scholar]
- 25.Lirk P, Picardi S, Hollmann MW. Local anaesthetics. Eur. J. Anaesthesiol. 2014;31:575–85. doi: 10.1097/EJA.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 26.Greensmith JE, Murray WB. Complications of regional anesthesia. Curr. Opin. Anaesthesiol. 2006;19:531–537. doi: 10.1097/01.aco.0000245280.99786.a3. [DOI] [PubMed] [Google Scholar]
- 27.Hebl JR, Niesen AD. Infectious complications of regional anesthesia. Curr. Opin. Anaesthesiol. 2011;24:573–580. doi: 10.1097/ACO.0b013e32834a9252. [DOI] [PubMed] [Google Scholar]
- 28.Pace MM, et al. Ultrasound-guided thoracic paravertebral blockade: a retrospective study of the incidence of complications. Anesth. Analg. 2016;122:1186–1191. doi: 10.1213/ANE.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 29.Diéguez P, Casas P, López S, Fajardo M. Ultrasound guided nerve block for breast surgery. Rev. Esp. Anestesiol. Reanim. 2016;63:159–167. doi: 10.1016/j.redar.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Kessler J, Marhofer P, Hopkins PM, Hollmann MW. Peripheral regional anaesthesia and outcome: lessons learned from the last 10 years. Br. J. Anaesth. 2015;114:728–745. doi: 10.1093/bja/aeu559. [DOI] [PubMed] [Google Scholar]
- 31.Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: a randomized controlled trial. Br. J. Anaesth. 2016;117:382–386. doi: 10.1093/bja/aew223. [DOI] [PubMed] [Google Scholar]
- 32.Steyaert A, Forget P, Dubois V, Lavand’homme P, De Kock M. Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J. Clin. Anesth. 2016;33:20–25. doi: 10.1016/j.jclinane.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Smith I, Monk TG, White PF, Ding Y. Propofol infusion during regional anesthesia: sedative, amnestic, and anxiolytic properties. Anesth. Analg. 1994;79:313–319. doi: 10.1213/00000539-199408000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Doss NW, et al. Continuous thoracic epidural anesthesia with 0.2% ropivacaine versus general anesthesia for perioperative management of modified radical mastectomy. Anesth. Analg. 2001;92:1552–1557. doi: 10.1097/00000539-200106000-00041. [DOI] [PubMed] [Google Scholar]
- 35.Amin AM, Mohammad MY, Ibrahim MF. Comparative study of neuromuscular blocking and hemodynamic effects of rocuronium and cisatracurium under sevoflurane or total intravenous anesthesia. Middle East J. Anaesthesiol. 2009;20:39–51. [PubMed] [Google Scholar]
- 36.Chou R, et al. Management of postoperative pain: a clinical practice guideline from the american pain society, the american society of regional anesthesia and pain medicine, and the american society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J. Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Khemka R, Chakraborty A, Ahmed R, Datta T, Agarwal S. Ultrasound-guided serratus anterior plane block in breast reconstruction surgery. Case Rep. 2016;6:280–282. doi: 10.1213/XAA.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 38.Kikuchi M, Takaki S, Nomura T, Goto T. Difference in the spread of injectate between ultrasound guided pectoral nerve block I and II. a cadaver study. Masui. 2016;65:314–317. [PubMed] [Google Scholar]
- 39.Rao V, Dyer C, Jameel J, Drew P, Greenman J. Potential prognostic and therapeutic roles for cytokines in breast cancer (Review) Oncol. Rep. 2006;15:179–185. doi: 10.3892/or.15.1.179. [DOI] [PubMed] [Google Scholar]
- 40.Stewart TH. Evidence for immune facilitation of breast cancer growth and for the immune promotion of oncogenesis in breast cancer. Medicina. 1996;56:13–24. [PubMed] [Google Scholar]
- 41.Mettler L, et al. Perioperative levels of interleukin-1beta and interleukin-6 in women with breast cancer. Clin. Exp. Obstet. Gynecol. 2004;31:20–22. [PubMed] [Google Scholar]
- 42.Fujisaki K, et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res. Treat. 2015;150:255–263. doi: 10.1007/s10549-015-3318-2. [DOI] [PubMed] [Google Scholar]
- 43.Iyengar NM, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 2015;22:2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogevold HE, Lyberg T, Kahler H, Haug E, Reikeras O. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total hip replacement surgery in general or regional anaesthesia. Cytokine. 2000;12:1156–1159. doi: 10.1006/cyto.2000.0675. [DOI] [PubMed] [Google Scholar]
- 45.Bravo-Cuellar A, et al. Comparison of two types of anesthesia on plasma levels of inflammatory markers. Cir. Cir. 2007;75:99–105. [PubMed] [Google Scholar]
- 46.Bouhassira D, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Can. J. Anaesth. 2014;62:241–251. doi: 10.1007/s12630-014-0285-8. [DOI] [PubMed] [Google Scholar]
- 48.Venetsanakos E, Beckman I, Bradley J, Skinner JM. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br. J. Cancer. 1997;75:1826–1830. doi: 10.1038/bjc.1997.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joimel U, et al. Stimulation of angiogenesis resulting from cooperation between macrophages and MDA-MB-231 breast cancer cells: proposed molecular mechanism and effect of tetrathiomolybdate. BMC Cancer. 2010;10:375. doi: 10.1186/1471-2407-10-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaewkangsadan V, et al. Crucial contributions by T lymphocytes (effector, regulatory, and checkpoint inhibitor) and cytokines (TH1, TH2, and TH17) to a pathological complete response induced by neoadjuvant chemotherapy in women with breast cancer. J. Immunol. Res. 2016;2016:1–25. doi: 10.1155/2016/4757405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumakura S, et al. Effects of nitrous oxide on the production of cytokines and chemokines by the airway epithelium during anesthesia with sevoflurane and propofol. Mol. Med. Rep. 2013;8:1643–1648. doi: 10.3892/mmr.2013.1745. [DOI] [PubMed] [Google Scholar]
- 52.Couceiro TCDM, Valença MM, Raposo MCF, de Orange FA, Amorim MMR. Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Pain Manag. Nurs. 2014;15:731–737. doi: 10.1016/j.pmn.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wijayasinghe N, Duriaud HM, Kehlet H, Andersen KG. Ultrasound guided intercostobrachial nerve blockade in patients with persistent pain after breast cancer surgery: a pilot study. Pain Physician. 2016;19:E309–E318. [PubMed] [Google Scholar]
- 55.Takimoto K, Nishijima K, Ono M. Serratus plane block for persistent pain after partial mastectomy and axillary node dissection. Pain Physician. 2016;19:E481–E486. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request in the REDCap database (https://redcap.iephsl.org.br).