Abstract
Saccharomyces cerevisiae has the ability to become less sensitive to a broad range of chemically and functionally unrelated cytotoxic compounds. Among multistress resistance mechanisms is the one mediated by plasma membrane efflux pump proteins belonging to the ABC superfamily, questionably proposed to enhance the kinetics of extrusion of all these compounds. This study provides new insights into the biological role and impact in yeast response to acetic acid stress of the multistress resistance determinant Pdr18 proposed to mediate ergosterol incorporation in plasma membrane. The described coordinated activation of the transcription of PDR18 and of several ergosterol biosynthetic genes (ERG2-4, ERG6, ERG24) during the period of adaptation to acetic acid inhibited growth provides further support to the involvement of Pdr18 in yeast response to maintain plasma membrane ergosterol content in stressed cells. Pdr18 role in ergosterol homeostasis helps the cell to counteract acetic acid-induced decrease of plasma membrane lipid order, increase of the non-specific membrane permeability and decrease of transmembrane electrochemical potential. Collectively, our results support the notion that Pdr18-mediated multistress resistance is closely linked to the status of plasma membrane lipid environment related with ergosterol content and the associated plasma membrane properties.
Introduction
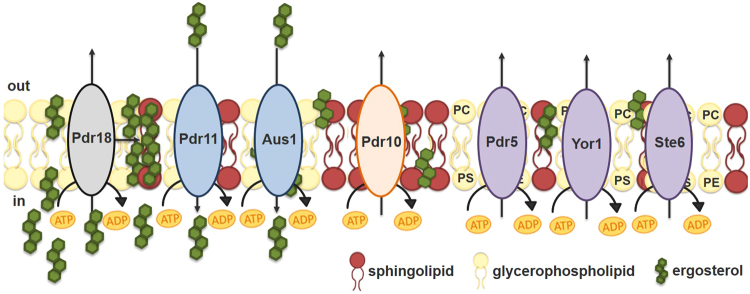
The acquisition of multidrug/multixenobiotic resistance (MDR/MXR) is widespread in nature and has clinical, agricultural and biotechnological implications. MDR/MXR transporters that are presumably able to catalyze the efflux of multiple cytotoxic compounds play a role in this phenomenon1–5. Two of the described MDR/MXR transporter families occur in all classes of organisms and belong to the ATP binding cassette (ABC) superfamily, which uses the hydrolysis of ATP to translocate a variety of solutes across biological membranes2,3, or to the Major Facilitator Superfamily (MFS) which energetically drive their transport utilizing the transmembrane electrochemical gradient1,4. Although those proteins have been traditionally considered drug exporters, their physiological function and the exact mechanism of their involvement in resistance to cytotoxic compounds are still unclear and it is puzzling that a wide range of structurally and functionally unrelated substrates may be exported by a specific transporter. Moreover, numerous ATP-binding cassette (ABC) transporters implicated in MDR/MXR are present in yeast genomes, strongly suggesting that they might have important physiological roles even in the absence of drugs/xenobiotics3. In fact, recent studies support the concept that at least some MDR/MXR transporters exert their effect as the result of a natural physiological role in the cell, rather than through the direct export of cytotoxic compounds1,6–8. In particular, the role of ABC-MDR/MXR transporters in yeast plasma membrane lipid homeostasis is gaining increasing attention9 and can also explain the MDR/MXR phenotype exhibited by cells expressing these transporters. Indeed, the maintenance of plasma membrane integrity is crucial in yeast cell tolerance to stress and lipids play a critical role in determining membrane physical properties and regulating the function of membrane associated proteins10–15. The Saccharomyces cerevisiae ABC transporters demonstrated in the scientific literature as involved in lipid trafficking and membrane lipid homeostasis are shown in Fig. 1. For example, Pdr11 and Aus1 are responsible for the uptake of exogenously supplied ergosterol in anaerobic conditions when ergosterol biosynthesis is impaired16–18. Also, the ABC efflux pumps Pdr5, Yor1 and Ste6 are implicated in the translocation of phospholipids between the two plasma membrane monolayers, thus contributing to plasma membrane asymmetry19,20. Pdr10 is associated with detergent-resistant domains in the plasma membrane and affects lipid distribution and maintenance of membrane micro-environment for the adequate functioning of membrane embedded proteins including the ABC-MDR transporter Pdr1221. Pdr16 and Pdr17 do not localize to the plasma membrane and their expression was proposed to regulate lipid metabolic pathways22–24 but the underlying mechanisms were not detailed.
Figure 1.
Plasma membrane ABC transporters of S. cerevisiae involved in lipid trafficking. Schematic representation of a set of ABC transporters that localize to the plasma membrane and are documented to play physiological roles in lipid trafficking and homeostasis in S. cerevisiae7,8,16–21. Pdr18, Pdr11, Aus1, Pdr10 and Pdr5 belong to the pleiotropic drug resistance (PDR) subfamily, Yor1 belongs to the multidrug resistance-associated protein (MRP) subfamily and Ste6 belongs to the multidrug resistance (MDR) subfamily of ABC transporters. The proposed biological functions are detailed in the Introduction section. Ergosterol transport, from its site of synthesis to the plasma membrane, is considered to occur by equilibration (t1/2 ~ 10–15 min) of endoplasmic reticulum and plasma membrane ergosterol pools via a bidirectional, nonvesicular process66. Abbreviations: phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE).
The present work focuses on the yeast ABC transporter Pdr18, previously described as a MDR/MXR determinant and proposed to mediate ergosterol incorporation in yeast plasma membrane7,8, Pdr18 expression confers increased yeast resistance to the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D), 2-methyl-4-chlorophenoxyaceticacid (MCPA), and barban, the agricultural fungicide mancozeb, the metal cations Zn2+, Mn2+, Cu2+ and Cd2+ 7, and to ethanol8. PDR18 expression was found to contribute to reduce [14C]-2,4-D intracellular accumulation but its action may be indirect since 2,4-D induces the decrease of plasma membrane ergosterol content, this deleterious effect being much stronger in the pdr18Δ background7.
In this article, we describe Pdr18 role in yeast adaptation and tolerance to acetic acid stress. Acetic acid is widely used by the food industry as preservative of acidic food and beverages but there are spoilage yeasts that are able to cope with standard levels causing severe economic losses25. Acetic acid tolerance may however be a highly desirable trait in industrial yeasts, since this weak acid is an industrial fermentation byproduct, its accumulation in the medium contributing to reduced fermentation performance26,27. Moreover, acetic acid is present in lignocellulosic biomass hydrolysates for the production of bioethanol and other bio-based chemicals28,29. Therefore, the understanding of the mechanisms underlying the toxic effects and adaptation and tolerance to acetic acid in the biotechnologically-relevant yeast species S. cerevisiae is essential to guide more efficient strategies either for food spoilage control or for the development of more robust industrial strains30–36.
Yeast adaptation to weak acids is a complex multifactorial process considered to include mechanisms leading to the reduction of the intracellular concentration of the counter-ions through their increased efflux from the cell interior31 and restriction of the diffusional entry of the liposoluble acid form into the cell31,36,37. In particular, incorporation of a higher fraction of sphingolipids was found to improve membrane bilayer thickness and density, leading to the decrease of plasma membrane non-specific permeability in the highly acetic acid resistant species Zygosaccharomyces bailii37. Interestingly, the sphingolipid biosynthetic pathway was found to be up-regulated in response to acetic acid-induced stress38. Also, plasma membrane enrichment in saturated acyl chains arising from glycerophospholipids and complex sphingolipids was also found to occur in response to acetic acid stress and lead to reduced cell permeability and increased membrane order39,40. A chemogenomic study has identified in S. cerevisiae several acetic acid resistance determinant genes encoding ergosterol biosynthetic enzymes30 and the inclusion of a higher content of sterols in yeast plasma membrane demonstrated to counteract the deleterious effects of ethanol41–45 and heat shock46,47. The content of ergosterol is not only crucial for plasma membrane stability and adequate selective permeability barrier to avoid the passive diffusion of toxic compounds into the cell48,49, but is also related with the formation of lipid-raft domains that may modulate the activity of membrane-embedded pumps50,51.
The present study provides new insights into the biological role and impact of Pdr18 in yeast cell response under stress, with a focus on acetic acid stress. The observed coordinated activation of the transcription of PDR18 and of several ergosterol biosynthetic genes during the period of adaptation to acetic acid inhibited growth provides further support to the involvement of Pdr18 in yeast response to maintain ergosterol content in plasma membrane of yeast stressed cells. The observed impact that such biological role has in counteracting acetic acid-induced decrease of plasma membrane lipid order, increase of the non-specific permeability and decrease of the transmembrane electrochemical potential establishes a link between this ABC transporter biological activity and its involvement in multistress resistance.
Results
PDR18 gene expression is required for maximum tolerance to acetic acid
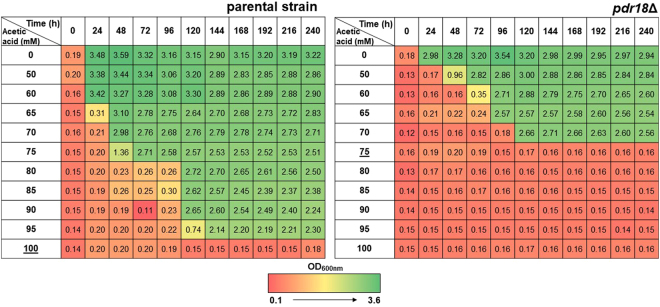
The expression of the ATP-binding cassette plasma membrane transporter encoding gene PDR18 was found to be required to partially overcome the effects of acetic acid induced stress in yeast growth (Figs 2 and 3). Susceptibility towards this weak acid was assessed based on yeast growth in liquid medium by comparing the growth of a parental strain Saccharomyces cerevisiae BY4741 and the derived deletion mutant pdr18Δ exposed to a wide range of acetic acid concentrations and determining the minimum inhibitory concentration (MIC) (Fig. 2). While the parental strain was able to grow in the presence of concentrations equal or below 95 mM, but not in the presence of 100 mM acetic acid (the calculated MIC for this strain), the MIC for the deletion mutant pdr18Δ was 75 mM (Fig. 2). Moreover, the final biomass attained after yeast cultivation under acetic acid stress showed a dose-dependent decrease and was more evident for the pdr18Δ mutant (Fig. 2).
Figure 2.
Inhibitory effect of acetic acid in the parental strain and pdr18Δ mutant growth. Growth was assessed based on culture OD600nm during 240 hours of incubation in liquid MM4 supplemented with increasing concentrations of acetic acid (at pH 4.0). The minimum inhibitory concentration (MIC) found for the parental strain and pdr18Δ mutant (100 or 75 mM acetic acid, respectively) is underlined. Results are representative of three independent growth experiments.
Figure 3.
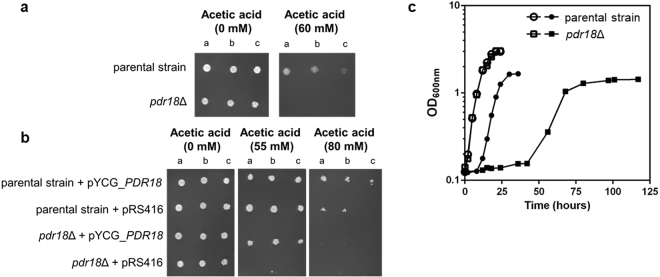
PDR18 gene is a determinant of acetic acid tolerance in yeast. (a) Comparison of growth by spot assays of the parental strain and pdr18Δ mutant cell suspensions plated in solid MM4 media supplemented or not with acetic acid (at pH 4.5). (b) Complementation assays of growth in solid MM4-U media of the parental and pdr18Δ strains, harboring plasmid pRS416 (cloning vector) or the recombinant vector expressing PDR18 from its natural promoter. In (a) and (b) the cell suspensions used to prepare the spots in lanes b) and c) were 1:5 and 1:25 serial dilutions, respectively, of the cell suspensions with an OD600nm = 0.05 ± 0.005 in lane a) prepared from an exponentially growing culture of each strain. (c) Growth curves of the parental (○, ●) and pdr18Δ (□, ■) strains in MM4 liquid medium supplemented (●, ■) or not (○, □) with 60 mM acetic acid at pH 4.0 based on culture OD600nm. Results of all panels of the figure are representative of at least three independent growth experiments.
Spot assays in agar plates of MM4 medium supplemented with 60 mM of acetic acid (at pH 4.5) confirmed the role of Pdr18 as an acetic acid resistance determinant (Fig. 3a). In fact, the susceptibility phenotype of the pdr18Δ mutant under acetic acid was rescued by the insertion of a centromeric plasmid expressing the PDR18 gene under the control of its natural promoter. Moreover, the expression of Pdr18 from the recombinant plasmid in the parental strain improved its resistance to acetic acid (Fig. 3b).
Most of the experiments described in this work were performed using the standardized concentration of acetic acid (60 mM, at pH 4.0). Under these conditions, growth curve of the parental strain in acetic acid supplemented MM4 medium exhibit a lag-phase of approximately 10 hours that was not detectable in the absence of acetic acid stress, while the duration of the acetic acid-induced lag-phase was extended to approximately 40 hours for the pdr18Δ mutant (Fig. 3c; Supplementary Fig. S1). Also, the maximum specific growth rates and the final biomass concentrations attained were more drastically reduced on the mutant compared to the parental strain under acetic acid stress (Fig. 3c).
PDR18 and ERG-genes transcription is up-regulated in response to acetic acid stress contributing to the maintenance of plasma membrane ergosterol content
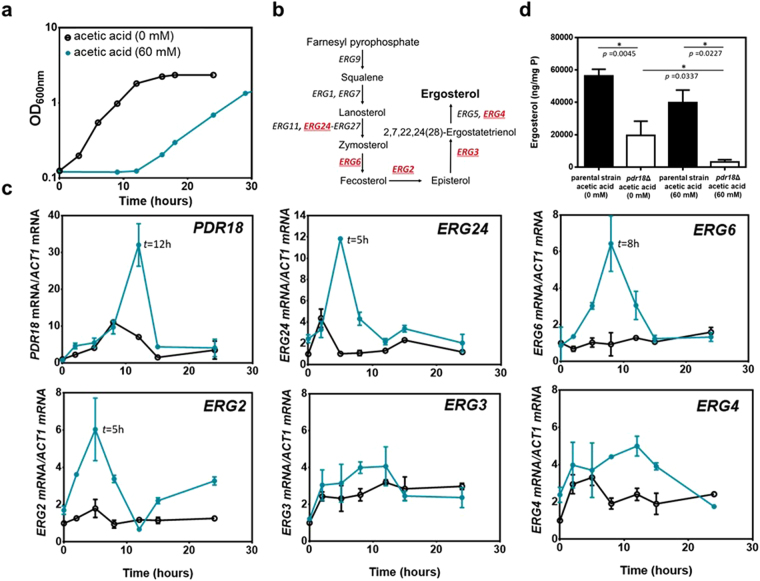
Levels of mRNA from PDR18 and selected ERG-genes in S. cerevisiae BY4741 cells were compared during cultivation of cells not previously adapted to acetic acid in MM4 or MM4 supplemented with 60 mM acetic acid at several time-points (Fig. 4). Results show that PDR18 transcription is activated in response to acetic acid stress. Maximum activation levels occur when the cell population resumes growth after an extended period of adaptation to growth under acetic acid stress and these levels were 3-fold higher than in unstressed cells. However, during exponential growth in the presence of acetic acid, mRNA levels from PDR18 decreased to values close to those in unstressed cells. In the absence of stress, the transcription profile of PDR18 is also dependent on the growth phase, mRNA levels peaking during exponential growth (Fig. 4).
Figure 4.
Levels of mRNA from PDR18 and from several ergosterol biosynthetic pathway genes during cultivation in absence or presence of acetic acid and comparison of ergosterol levels. (a) Growth curve of S. cerevisiae BY4741 in absence (○) or presence ( ) of 60 mM acetic acid (at pH 4.0) based on culture OD600nm. (b) Ergosterol biosynthetic pathway [adapted from KEGG (http://www.genome.jp/kegg/pathway.html)]; the ERG-genes under study are highlighted in red; (c) mRNA levels from the indicated genes by qRT-PCR, using ACT1 as the reference gene. (d) Ergosterol content in cell membranes from exponentially-growing cells of the parental and pdr18Δ strains in the absence or presence of 60 mM acetic acid (60 mM). Error bars represent standard deviation resultant from at least two biological replicates with three technical replicates each.
) of 60 mM acetic acid (at pH 4.0) based on culture OD600nm. (b) Ergosterol biosynthetic pathway [adapted from KEGG (http://www.genome.jp/kegg/pathway.html)]; the ERG-genes under study are highlighted in red; (c) mRNA levels from the indicated genes by qRT-PCR, using ACT1 as the reference gene. (d) Ergosterol content in cell membranes from exponentially-growing cells of the parental and pdr18Δ strains in the absence or presence of 60 mM acetic acid (60 mM). Error bars represent standard deviation resultant from at least two biological replicates with three technical replicates each.
Since several enzymes catalyzing post-squalene steps of the ergosterol biosynthetic pathway have been related with acetic acid stress response30,52, the transcription profiles of ERG2-4, ERG6, and ERG24 genes were obtained. These genes are known determinants of resistance to acetic acid/weak acid stress in yeast30,31,53. The transcription levels from those ERG genes during acetic acid stressed growth show two profiles: ERG24, ERG6 and ERG2 have a peak of activation (6–12 fold) after 5–8 hours, by the mid- to end of the phase of latency, while for ERG3 and ERG4 genes the activation levels are lower (2–5-fold) and were maintained throughout cultivation (Fig. 4). Ergosterol quantification confirms its lower content in membranes of exponentially-growing unstressed cells of the deletion mutant pdr18Δ compared to the parental strain. The ergosterol content in pdr18Δ cells exponentially-growing under acetic acid stress was found to be dramatically reduced (approximately 10-fold), compared to parental strain levels cells growing in the same conditions (Fig. 4). These results confirm the need of Pdr18 expression to maintain ergosterol content in plasma membrane especially under acetic acid stress. Collectively, these results are consistent with the biological function attributed to Pdr18 in ergosterol homeostasis and indicate that under acetic acid stress PDR18 gene expression regulation is part of the yeast response to acetic acid stress together with the activation of ergosterol biosynthesis.
PDR18 expression leads to a more ordered plasma membrane especially under acetic acid stress
As ergosterol content is an important factor affecting yeast plasma membrane order42,54,55, the next step was to assess plasma membrane order in the parental and pdr18Δ cell strains grown either unstressed or in the presence of acetic acid stress to further understand the impact that PDR18 expression has at the level of plasma membrane properties.
Laurdan is a membrane dye whose fluorescence emission spectrum depends on lipid packing. For more ordered membranes, the emission spectrum of Laurdan is shifted to lower wavelengths relative to the spectrum of the same probe in disordered membranes. This spectral shift can be quantified through the calculation of a generalized polarization (GP) factor from the fluorescence intensity of two spectral channels of Laurdan emission spectra56. The GP value of Laurdan is a convenient and quantitative parameter to evaluate membrane packing, with higher values reflecting higher ordering.
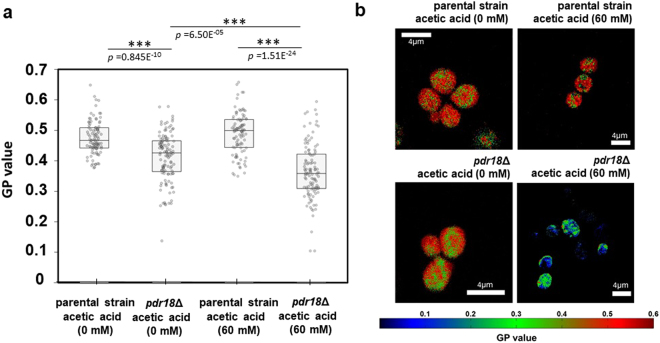
The distribution of Laurdan Generalized Polarization (GP) values for each individual cell is shown in Fig. 5a and illustrative examples of GP images are shown in Fig. 5b. The parental strain cell population harvested in the exponential phase of growth show a significantly higher plasma membrane order than the population of cells lacking PDR18 gene, when both strains were grown either in the absence (p = 0.845E−10) or in presence (p = 1.51E−24) of 60 mM acetic acid at pH 4.0 (Fig. 5a). Parental cells harvested in the exponential phase of growth without acetic acid or adapted to that concentration of acetic acid show no significant difference in plasma membrane order, as assessed by the GP mean value (Fig. 5a). However, Laurdan GP values, reflecting plasma membrane order, of pdr18Δ cells decreased by 12% when cultivated in the presence of acetic acid (Fig. 5a). Also noteworthy is that Laurdan GP values for the pdr18Δ cell population exhibit an interquartile range (Q3-Q1) 53% larger than the parental strain population, both grown in the absence of acetic acid, which indicates a higher cell heterogeneity for plasma membrane order within the pdr18Δ cell population (Fig. 5a). Adapted cells exponentially-growing in the presence of acetic acid that lack the PDR18 gene, exhibit a lower plasma membrane order (25% lower GP mean value) and a more heterogeneous profile for this trait (25% larger interquartile range), compared with the stressed parental strain population (Fig. 5a).
Figure 5.
Plasma membrane order of the parental and pdr18Δ strains growing in absence or presence of acetic acid, as reported by Laurdan Generalized Polarization (GP) values. (a) GP values from individual exponentially-growing cells of the parental and pdr18Δ strains cultivated either in the presence or absence of 60 mM acetic acid at pH 4.0. The boxplots represent the first and third quartiles and median for each cell population; (b) Examples of Laurdan Generalized Polarization images for the parental strain and pdr18Δ cells grown in absence or presence of acetic acid stress. Results arise from the analysis of at least 100 cells obtained from three independent assays. Asterisks represent statistical significant differences, based on t-student tests.
Acetic acid stress induces an increase in plasma membrane permeability when yeast cells lack PDR18
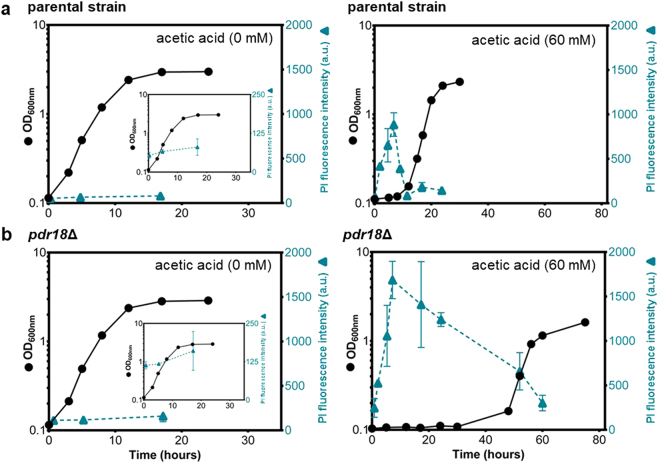
The less ordered plasma membrane lipid environment of the pdr18Δ deletion mutant assessed before was found to have a considerable impact on the physiological function of yeast plasma membrane as a selective barrier in the presence of acetic acid, with plasma membrane permeability being inferred based on propidium iodide (PI) passive uptake (Figs 6 and 7). PI is a cell-impermeant dye with a high affinity for nucleic acids. The dye only penetrates cells with compromised plasma membranes; the subsequent binding to intracellular nucleic acids increases PI fluorescence up to 30-fold57. Therefore, the PI fluorescence intensity parameter can be correlated with the proportion of permeabilized cells.
Figure 6.
Plasma membrane permeability of the parental strain and pdr18Δ cells during the growth curve in the presence or absence of acetic acid. Comparison of plasma membrane permeability ( ; arbitrary units) during cultivation of the parental (a) or pdr18Δ (a) strains in the presence or absence of 60 mM acetic acid at pH 4.0. The estimation of plasma membrane permeability is based on the fluorescence intensity values exhibited by yeast cells upon passive accumulation of propidium iodide. Graphs with a more appropriate fluorescence intensity scale are shown to allow comparison between (a) and (b) in the absence of acetic acid. Error bars represent standard deviation (n = 3).
; arbitrary units) during cultivation of the parental (a) or pdr18Δ (a) strains in the presence or absence of 60 mM acetic acid at pH 4.0. The estimation of plasma membrane permeability is based on the fluorescence intensity values exhibited by yeast cells upon passive accumulation of propidium iodide. Graphs with a more appropriate fluorescence intensity scale are shown to allow comparison between (a) and (b) in the absence of acetic acid. Error bars represent standard deviation (n = 3).
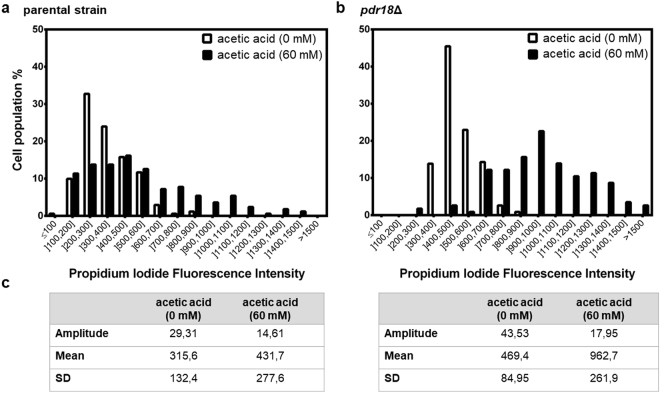
Figure 7.
Cell-by-cell propidium iodide fluorescence in exponentially-growing cell populations of parental or pdr18Δ strain in the presence or absence of acetic acid. Distribution of propidium iodide fluorescence intensity classes of membrane permeability of the parental strain (a) and pdr18Δ (b) cell population harvested in the exponential phase in absence (□) or presence (■) of 60 mM acetic acid; (c) Statistical parameters of a Gaussian curve fit to the respective data shown in (a) and (b).
Even in the absence of any stress, cells deleted for PDR18 are more permeable than cells of the parental strain harvested during the growth curve (25% higher permeability; Fig. 6). Acetic acid supplementation of the growth medium (60 mM at pH 4.0) leads to an increased plasma membrane permeability in parental strain cells during the growth curve, PI uptake values peaking at 7 hours (10-fold the levels in absence of acetic acid) by the time when cell population resumes growth after the adaptation phase (Fig. 6a). Remarkably, during exponential growth under acetic acid stress, yeast cell permeability is reduced, and cells harvested at mid-exponential phase exhibit a permeability value similar (only 1.3-fold higher) to unstressed cells (Fig. 6a). In pdr18Δ mutant cells, plasma membrane permeability peaks during acetic-acid induced lag-phase, reaching values above those registered for the parental strain and much higher (15-fold) than those registered for unstressed cells (Fig. 6b). Even during exponential growth adapted to acetic acid, the permeability of cells deleted for PDR18 gene is above the values for unstressed cells (4-fold higher) and this cell population was unable to recover from acetic acid-induced permeabilization during lag-phase to levels similar to unstressed cells (Fig. 6b).
Analysis of fluorescence levels of individual cells allow the comparison of exponentially-growing cell populations fluorescence distributions (Fig. 7a,b). The distributions fit to a gaussian curve (Fig. 7c) and the acetic acid-adapted parental cell population exhibits a higher average PI fluorescence intensity and more heterogeneous cell-to-cell permeability levels [higher standard deviation (SD) and lower amplitude] than the unstressed cell population (Fig. 7). Deletion of PDR18 gene leads to a fluorescence distribution in the cell population with a higher average value of plasma membrane permeability even for cells cultivated in the absence of acetic acid. Such heterogeneity increases under acetic acid stress (Fig. 7).
PDR18 gene expression is essential to maintain plasma membrane potential in absence or presence of acetic acid stress
The effect of PDR18 expression in plasma membrane electrochemical potential when yeast cells were cultivated in presence or absence of acetic acid was also estimated based on the position of the emission maximum at equilibrium of the diS-C3(3), a dye sensitive to membrane potential. Since this diS-C3(3) assay was used to compare two isogenic strains (parental cells and cells deleted for PDR18), the activity of other MDR-pumps is considered identical and the differences in λmaxeq believed to reflect differences in membrane potential58. Results indicate that even in the absence of stress, parental strain cells exhibit a significantly higher (p = 0.038) plasma membrane potential than pdr18Δ cells (Fig. 8), as previously observed using other methods7. Acetic acid induced a short-term decrease of plasma membrane potential for both the parental strain and pdr18Δ cells after 15 minutes of cultivation in the presence of acetic acid (Fig. 8). However, after five hours of cultivation in the presence of acetic acid, parental strain cells recovered to values that were not significantly different from unstressed cells, and these levels were maintained during exponential growth of adapted cells (Fig. 8). For pdr18Δ acetic acid-adapted cells such recovery was not observed (Fig. 8). The incubation of exponentially-growing parental strain cells for 15 minutes with carbonyl cyanide p-chlorophenylhydrazone (CCCP), used in this assay as a positive control, confirmed the disruption of membrane potential under these conditions (Fig. 8).
Figure 8.
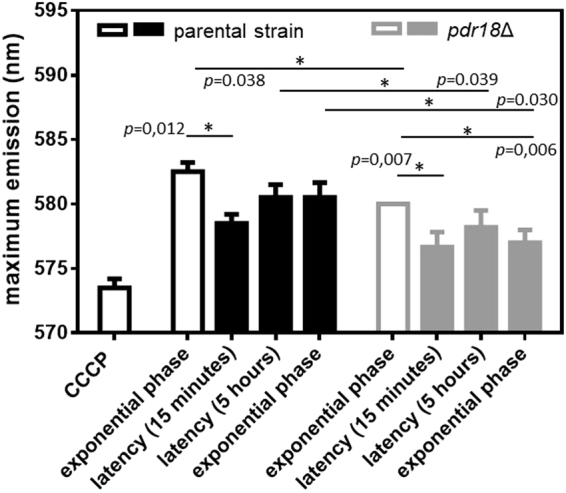
Plasma membrane electrochemical potential of the parental and pdr18Δ strains during cultivation in presence or absence of acetic acid. Comparison of plasma membrane electrochemical potential based on the maximum emission wavelength of the fluorescent probe DiSC3(3) in citrate-phosphate buffer at pH 6.0. Cells of parental (□, ■) and pdr18Δ ( ,
,  ) strains were harvested at the exponential phase of growth in the absence of acetic acid (□,
) strains were harvested at the exponential phase of growth in the absence of acetic acid (□,  ) and at the indicated timepoints of the corresponding growth curves in the presence of 60 mM acetic acid (■,
) and at the indicated timepoints of the corresponding growth curves in the presence of 60 mM acetic acid (■,  ). Error bars represent standard deviation resultant from at least two biological replicates with at least three technical replicates each. Asterisks represent statistical significance in t-student test. CCCP: Carbonyl cyanide p-chlorophenylhydrazone.
). Error bars represent standard deviation resultant from at least two biological replicates with at least three technical replicates each. Asterisks represent statistical significance in t-student test. CCCP: Carbonyl cyanide p-chlorophenylhydrazone.
Discussion
The yeast Saccharomyces cerevisiae has evolved numerous and diverse complex mechanisms that allow cells to grow and thrive in stressful environments. Among them are those related with the remodeling of lipid and protein composition of plasma membrane, an active interface between the cell interior and its environment that shields yeast cells against a potentially harmful environment11,59. The composition of phospholipids, sphingolipids, and sterols in the plasma membrane play a critical role in determining its physical properties, such as permeability and fluidity, and has a strong influence on the activity of the proteins associated to or embedded in the lipid bilayer50,60–62. In this work we demonstrate the importance of the plasma membrane ABC transporter Pdr18 as a determinant of resistance to acetic acid. Pdr18 was previously involved in resistance to multiple stresses presumably by increasing plasma membrane ergosterol content7,8. Ergosterol, the major sterol present in fungal plasma membrane, modulates its thickness, fluidity and permeability and regulates the activity of membrane associated transporters44,45,47–49,54,63. In the present work, PDR18 expression was found to be essential to maintain maximum ergosterol content in yeast plasma membrane and to counteract acetic acid-induced decrease of ergosterol content and plasma membrane order and its non-specific permeability and electrochemical potential levels. This role for Pdr18 in the maintenance of adequate plasma membrane physical properties under acetic acid stress is essential for adequate physiological function of this important cell membrane as a selective barrier thus allowing the efficient import of nutrients and excretion of toxic metabolites, such as the counter-anion acetate, as well as the restriction of the diffusional entry of acetic acid.
The transcriptional activation under acetic acid stress of a number of genes of the ergosterol biosynthetic pathway has been revealed by transcriptomic analysis64. The ERG genes found to be activated under acetic acid stress are also required for tolerance to this weak acid30,31,64, reinforcing the importance of the ergosterol pathway in yeast adaptation and tolerance to acetic acid. The interconnection between the ergosterol pathway and the function of the multistress resistance determinant Pdr18 under acetic acid stress is also suggested by the data obtained in this study, for the transcription analysis of PDR18 and several genes of the ergosterol biosynthetic pathway. Yeast growth under acetic acid stress was found to involve the activation of all these genes with a peak of gene transcripts during advanced acetic acid-induced lag phase although the time-course profiles do not coincide. Remarkably, the peak of the mRNA level from PDR18 was observed later than the peak registered for the first genes of the pathway tested (ERG24, ERG6 and ERG2), consistent with the proposed role for Pdr18 at the end of the ergosterol transport process of the newly synthesized sterols from the endoplasmic reticulum (ER) to the plasma membrane. Moreover, the activation level of the late genes of the ergosterol pathway tested (ERG3 and ERG4) was more moderate and the activation profile less dependent of the growth phase. It would be important to know the sensors that perceive the fluctuations in the membrane environment of acetic acid stressed cells and transduce the signals to PDR18 gene resulting into the compensatory changes in the ergosterol profile and how these processes are controlled.
Yeast cells synthesize ergosterol in the membrane of the endoplasmic reticulum via a cascade of coupled enzymatic reactions65. Ergosterol has then to be transported from the site of its synthesis to the plasma membrane and although this transport is known to be fast, the mechanism by which newly synthesized sterols are transported from the ER to the plasma membrane is not fully understood66,67. An essential role for vesicular transport pathways in transporting ergosterol, from its site of synthesis to the plasma membrane has been ruled out and ergosterol transport was proposed to occur by equilibration (t1/2 ∼ 10–15 min) of endoplasmic reticulum and plasma membrane ergosterol pools via a bidirectional, nonvesicular process66. To reconcile an equilibration process with the high ergosterol concentration present in plasma membrane, and based on the observation that a large fraction of ergosterol is found in the plasma membrane condensed with sphingolipids in membrane rafts that coexist with free sterol, Baumann et al.66 hypothesized that the concentration of free sterol is similar in the plasma membrane and endoplasmic reticulum and that only free (non-raft) sterol molecules have access to a nonvesicular transport pathway that connects the two organelles. Based on the results of the present study, we propose the involvement of the plasma membrane associated ABC transporter Pdr18 in the active ergosterol transport process at the plasma membrane level, allowing the high physiological ergosterol concentration present in this membrane.
Like other organisms, the yeast S. cerevisiae has the ability to acquire resistance to multiple drugs (MDR) or to multiple xenobiotic compounds (MXR), i.e. to become less sensitive to a broad range of chemically and functionally unrelated cytotoxic compounds32,33,68–70. The acquisition of resistance to multiple stressing agents is due to a number of mechanisms, among them the mechanism mediated by efflux pump proteins localized at the plasma membrane and belonging to either the ABC (ATP-binding cassette) superfamily or the MFS (major facilitator superfamily) and proposed to enhance the kinetics of extrusion of these compounds1,2,4,71. The presence of a large number of ABC and MFS transporters homologous to MDR/MXR characterized transporters in the genomes of yeasts and other organism strongly suggests that they may play important physiological roles even in the absence of the cytotoxic compounds. Accumulating evidence has shown that ABC proteins perform endogenous activities extending beyond their accepted role as drug exporters, among them the transport of lipids9,61,72,73 as schematized in Fig. 1. Although PDR18 has a paralogous gene in S. cerevisiae, SNQ274, no demonstration of the eventual involvement of Snq2 in lipid transport and homeostasis was so far reported. However, the affinity of Snq2 transporter for sterols was hypothesized due to its role in alleviating estradiol toxicity in S. cerevisiae75. Moreover, we were not able to identify any phenotype of resistance to acetic acid associated to SNQ2 expression. The coordinate control of plasma membrane ergosterol composition by Pdr18 and the so far well accepted role of Pleiotropic drug resistance (PDR) transporters in drug/xenobiotic efflux activity (in this particular case, the putative role of Pdr18 in the active acetate efflux) is a possible model. However, based on the data gathered in this study, the involvement of Pdr18 in multistress resistance may merely result from its physiological role in ergosterol transport and proper distribution within plasma membrane proved to have a significant impact in plasma membrane properties, especially relevant under acetic acid stress. Apart from serving as substrates of certain ABC transporters, lipid molecules are also crucial molecular determinants that affect the trafficking and functioning of certain ABC transporter proteins18,76–78. Therefore, the role of Pdr18 in ergosterol homeostasis at the plasma membrane level is likely to affect the proper functioning of other efflux pumps of the ABC superfamily or the MFS, already proposed to be required for acetate efflux31, or the essential plasma membrane H+-ATPase activity50,79. Collectively, results support the notion that multistress resistance mediated by Pdr18 is closely linked to the status of plasma membrane lipid environment, specifically related with ergosterol content and the associated plasma membrane properties.
Methods
Strains, plasmids and growth conditions
Saccharomyces cerevisiae parental strain BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) and the derived deletion mutant pdr18Δ were obtained from the EUROSCARF collection (http://web.uni-frankfurt.de/fb15/mikro/euroscarf), as well as plasmid pYCG_PDR18, expressing the PDR18 gene from its natural promoter, and the corresponding cloning vector, pRS416, used for phenotypic complementation assays.
Yeast cells were cultivated at 30 °C with orbital agitation (250 rpm) in liquid minimal growth medium supplemented with the amino acids and the nucleotide to support growth of the auxotrophic strains (MM4). MM4 contained 1.7 g/L yeast nitrogen base without amino acids and ammonium sulphate (Difco, Michigan, USA), 20 g/L glucose (Merck, Darmstadt, Germany), 2.65 g/L (NH4)2SO4 (Panreac AppliChem, Connecticut, USA), 20 mg/L L-methionine, 20 mg/L L-histidine (both from Merck, Darmstadt, Germany), 60 mg/L L-leucine and 20 mg/L L-uracil (both from Sigma, Missouri, USA), adjusted to pH 4.0 with HCl. Solid medium was prepared by the addition of 20 g/L agar (Iberagar, Barreiro, Portugal) and the pH was set to 4.5 with HCl. Cells harboring the cloning vector pRS416 or derived plasmids were grown in the same medium lacking uracil supplementation (MM4-U medium) to maintain selective pressure.
Susceptibility assays
Growth curves were obtained by inoculating a mid-exponential cell suspension in 100 mL flasks containing 50 mL MM4 pH 4.0 supplemented or not with acetic acid pH 4.0 (set with NaOH) at the desired concentration. The starting OD600nm of the cultivation media was standardized at 0.1 ± 0.05. Growth with orbital agitation was followed at 30 °C by measuring OD600nm.
Yeast cell suspensions used for the spot assays were prepared from a mid-exponential cell culture grown in MM4 medium, diluted to an OD600nm of 0.05 (lane a in the figures), followed by two serial dilutions of 1:5 each (lanes b and c). These cell suspensions were plated as 4 µL spots onto the surface of MM4 pH 4.5 solid medium, supplemented or not with acetic acid. Complementation tests were perfomed with yeast cells harboring either the empty vector pRS416 or the recombinant plasmid pYCG_PDR18. For the complementation tests, spot assays were prepared as described before and cell suspensions were spotted onto MM4-U agar plates that were incubated at 30 °C for 72 hours.
Time-course transcription analysis of PDR18 and ERG-genes
For gene transcription assays, BY4741 cells were harvested at adequate time points from liquid medium cultivations performed in the presence and absence of 60 mM acetic acid, as described above. Total RNA was extracted by the hot phenol method80. The real-time Reverse Transcription–PCR protocol used followed the manufacturer’s instructions (Applied Biosystems, California, USA) and the primers used for the amplification of each target cDNA were designed using the Primer Express software (Applied Biosystems, California, USA) (Supplementary Table S1). The RT–PCR reaction was carried out using a thermal cycler block (7500 Real-Time PCR System; Applied Biosystems, California, USA). The ACT1 mRNA level was used as the internal control. The relative value obtained for each target gene at the initial time point (15 minutes of incubation following inoculation) under unstressed conditions was set as 1 and the remaining values are relative to that value.
Ergosterol quantification in yeast cell membranes
For quantification of ergosterol in yeast cell membranes, the parental and pdr18Δ strains were cultivated in MM4 either in the presence or absence of acetic acid stress (60 mM at pH 4.0) as described above. Exponentially-growing cells were harvested by centrifugation (5000 g, 5 minutes, 4 °C), resuspended in homogenization buffer containing 50 mM Tris/HCl (pH 7.5), 2.5 mM EDTA and a protease inhibitor cocktail (1 mM PMSF and 1 μg/mL each of leupeptin, pepstatin A and aprotinin). Cells were broken by vortex-mixing with glass beads (Glaperlon 0.40–0.60 mm). The cell membranes were recovered by centrifugation (1000 g, 10 minutes, 4 °C) to remove unbroken cells and finally the cell membranes were pelleted by ultracentifugation at 25 000 rev./min for 1 hour (rotor type SW41Ti, Beckman Coulter). The pelleted cell membranes were resuspended in a buffer containing 20 mM Tris/HCl (pH 7.5), 150 mM NaCl, 20% glycerol and protease inhibitors at the concentrations mentioned above, stored frozen and freeze-dried until sterol extraction. Sterol extraction involved addition of 250 μL 5-α-cholestane (internal standard, Sigma) in dichloromethane and a lipid extraction using 600 μL methanol (Merck) and 300 μL dichloromethane (Merck). Samples were homogenized for 60 seconds at 2–4 °C in a homogenizer (polytron 10–35 GT, Kinematica) at 10,000 rpm. Afterwards, 300 μL ultra-pure water and 300 μL dichloromethane were added. Samples were centrifuged (3000 g, 10 minutes, 4 °C). The lower phase was filtered through an anhydrous sodium sulfate (Merck) layer and solvent evaporated under N2 at 20 °C. Lipids were saponified with 500 μL of 2.5 N KOH (Merck) at 80 °C for 1 hour. Saponified lipids were extracted with 1 ml of saturated NaCl (Merck) and 5 ml hexane (Merck). Organic phase was evaporated under N2 at 20 °C. Derivatization was done with 100 μL N,O-bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (Sigma) at 80 °C for 12 hours. Derivatized sterols were analyzed using GC-MS (Scion 456 GC-SQ, Bruker) with a DB5 column (30 m × 0.2 mm, 0.20 μm). The carrier gas was helium (flow rate of 1 mL/min). Oven temperature was held at 120 °C for 1 min, increased to 300 °C at a rate of 2 °C/min, and held at 300 °C for 20 min. Injection and detector temperatures were 300 °C. Peak identification was based on retention time and comparison with external standards in the spectral database. Quantification was achieved through the internal standard peak area. Sterol concentration was normalized taking into account phosphate content in the freeze-dried samples.
Assessement of membrane lipid order by Laurdan fluorescence
Membrane lipid order was estimated in exponentially-growing cells of parental and pdr18Δ strains either in the absence or presence of acetic acid, by the normalized ratio of Laurdan fluorescence emission measured in two channels. Growth curves were performed as described above and cells at mid-exponential phase (OD600nm = 0.5) were centrifuged (6000 g, 5 minutes), washed three times and resuspended in 200 µL PBS buffer (137 mM NaCl; 2.7 mM KCl; 10 mM Na2HPO4; 1.8 mM KH2PO4 in 1 L distilled water, pH adjusted to 7.4 with HCl; filtered). These cell suspensions were deposited in a glass 8-wells chamber (Ibidi, Martinsried, Germany) treated with poly-L-lysine (Sigma, Missouri, USA) for better adherence of living yeast cells. The incubation with 5 µM Laurdan (Sigma, Missouri, USA) was performed at room temperature for 1 hour followed by two washing steps with PBS. Images were obtained by two-photon excitation fluorescence microscopy on a Leica TCS SP5 (Leica Microsystems CMS GmbH, Manheim, Germany) inverted confocal microscope (DMI600), using a Ti:sapphire laser (Mai Tai, Spectra-Physics, Darmstadt, Germany) as the excitation source, and an apochromatic water immersion objective (63x, NA 1.2; Zeiss, Jena, Germany). The excitation wavelength was set to 780 nm and the fluorescence emission of Laurdan was collected at 400–460 nm and 470–530 nm. Laurdan exhibits shifts in its fluorescence emission maximum in response to changes in membrane hydration and solvent relaxation and this shift can be estimated through a normalized ratiometric measurement of the fluorescence intensity recorded in the two spectral channels, the generalized polarization (GP) value56,81. GP images were obtained and analysed using homemade software developed in a MATLAB environment (Mathworks, Natick, MA), with the GP value defined as GP = (I400–460 − G·I470–530)/(I400–460 + G·I470–530). Both channel intensities are corrected for background contributions, and the calibration factor G was obtained from imaging Laurdan in DMSO using the same experimental conditions as those set for the samples under study82.
Permeability and plasma membrane potential estimation
Differences in the plasma membrane potential of the parental strain BY4741 and the derived deletion mutant pdr18Δ grown in the presence or absence of acetic acid were estimated based on the shift in the maximum emission wavelength of the fluorescent probe DiS-C3(3) (3-3′-Dipropylthiacarbocyanine iodide), as described before83. For the timepoints tested, yeast cells were harvested as described above for other assessments and resuspended in citrate-phosphate (CP) buffer to a final OD600nm of 0.5. 5-mL aliquots of cell suspensions were labelled with DiS-C3(3) (Sigma-Aldrich) at a final concentration of 10−7 M, for 30 minutes at room temperature with orbital agitation of 70 rpm. Carbonyl cyanide p-chlorophenylhydrazone (CCCP) was added to an exponentially-growing culture of parental strain cells, to a final concentration of 5 µM, and this condition was used as a positive control for full membrane depolarization84. Fluorescence emission spectra of the cell suspensions were collected from 555 to 790 nm (at wavelength excitation of 531 nm) in 0.5 cm × 0.5 cm quartz cuvettes using a SLM-Aminco 8110 Series 2 spectrofluorometer (Rochester, New York, USA).
Plasma membrane permeability was assessed by the passive uptake of propidium iodide (PI; 20 mM in DMSO, Invitrogen), from cells harvested at adequate time points of the cultivation of parental strain and pdr18Δ cells either in the presence of absence of 60 mM acetic acid. PI was added to 1 mL of 4 × 107 cells/mL to a final concentration of 20 µM, cell suspensions and incubated in the dark with orbital agitation (15 minutes, 250 rpm). Cells exposed to PI were centrifuged (17,500 × g for 5 minutes), washed twice and ressuspended in PBS buffer to a final 107 cells/mL aliquots. PI-fluorescence emission spectrum (collected in the range 563–700 nm, at an excitation wavelength of 535 nm) were obtained using a SLM-Aminco 8100 series 2 spectrofluorometer with double excitation and emission monochromators MC400 (Spectronic Instruments, Inc., Rochester, NY), in a right-angle geometry. The light source was a 450 W Xe arc lamp and the reference a Rhodamine B quantum counter solution. All measurements were performed in 0.5 cm × 0.5 cm quartz cuvettes and under a constant temperature of 30 °C by using a thermostated sample holder with a Julabo circulating water bath (Houston, Texas, USA). Gain adjustment of the apparatus was performed using a positive control for full permeabilization (exponentially-growing parental strain cells exposed during 30 minutes to 50% ethanol in PBS). The spectra obtained were integrated and the area under the curve was calculated.
To evaluate the population heterogeneity for the cell permeability trait, at adequate time points the cell population strained with PI was observed using an Axioplan microscope equipped with adequate epifluorescence interface filters (BP450-490 and LP520; Zeiss). Fluorescence images were obtained with a cooled charge-coupled device camera (Cool SNAPFX; Roper Scientific Photometrics), and the images were analyzed with MetaMorph, version 3.5. Cell-to-cell fluorescence intensity was defined as the average of pixel by pixel intensity in the selected region of interest and a minimum of 50 cells/experiment were used. The fluorescence images were background corrected by using dark-current images.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This work was supported by ‘Fundação para a Ciência e a Tecnologia’ (FCT) (project contract ERA-IB-2/0003/2015 and PhD and postdoctoral fellowships to CPG (SFRH/BD/92252/2013) and SNP (SFRH/BPD/92409/2013) and an FCT researcher grant to FF (IF/00386/2015)). Funding received by iBB from FCT (UID/BIO/04565/2013) and from Programa Operacional Regional de Lisboa 2020 (Project N. 007317), and by CQFM from FCT and FEDER (FAPESP/20107/2014 and POCI-01-0145-FEDER-022122) is also acknowledged.
Author Contributions
C.P.G. performed most of the experimental work with the collaboration of C.S.P. (fig 2 results), C.C. and N.B. (ergosterol quantification) and with S.N.P. and F.F. guidance for the experiments involving fluorescent probes. I.S.C. designed and coordinated the study and with the collaboration of C.P.G. prepared the manuscript. All authors revised and agreed on the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26128-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.dos Santos SC, Teixeira MC, Dias PJ, Sá-Correia I. MFS transporters required for multidrug/multixenobiotic (MD/MX) resistance in the model yeast: Understanding their physiological function through post-genomic approaches. Front. Physiol. 2014;5:1–15. doi: 10.3389/fphys.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jungwirth H, Kuchler K. Yeast ABC transporters - A tale of sex, stress, drugs and aging. FEBS Lett. 2006;580:1131–1138. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Piecuch A, Obłak E. Yeast ABC proteins involved in multidrug resistance. Cell. Mol. Biol. Lett. 2014;19:1–22. doi: 10.2478/s11658-013-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sá-Correia I, dos Santos SC, Teixeira MC, Cabrito TR, Mira NP. Drug:H+ antiporters in chemical stress response in yeast. Trends Microbiol. 2009;17:22–31. doi: 10.1016/j.tim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Monk BC, Goffeau A. Outwitting multidrug resistance to antifungals. Science. 2008;321:367–369. doi: 10.1126/science.1159746. [DOI] [PubMed] [Google Scholar]
- 6.Prasad R, Panwar S. Physiological functions of multidrug transporters in yeast. Current Science. 2004;86:62–73. [Google Scholar]
- 7.Cabrito TR, Teixeira MC, Singh A, Prasad R, Sá-Correia I. The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance. Biochem. J. 2011;440:195–202. doi: 10.1042/BJ20110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira MC, Godinho CP, Cabrito TR, Mira NP, Sá-Correia I. Increased expression of the yeast multidrug resistance ABC transporter Pdr18 leads to increased ethanol tolerance and ethanol production in high gravity alcoholic fermentation. Microb. Cell Fact. 2012;11:98. doi: 10.1186/1475-2859-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad R, Khandelwal NK, Banerjee A. Yeast ABC transporters in lipid trafficking. Fungal Genet. Biol. 2016;93:25–34. doi: 10.1016/j.fgb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Singh P. Budding Yeast: An ideal backdrop for in vivo lipid biochemistry. Front. Cell Dev. Biol. 2017;4:1–8. doi: 10.3389/fcell.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caspeta, L., Castillo, T. & Nielsen, J. Modifying Yeast Tolerance to Inhibitory Conditions of Ethanol Production Processes. Front. Bioeng. Biotechnol. 3 (2015). [DOI] [PMC free article] [PubMed]
- 12.Henderson CM, Block DE. Examining the role of membrane lipid composition in determining the ethanol tolerance of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014;80:2966–2972. doi: 10.1128/AEM.04151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont S, Beney L, Ferreira T, Gervais P. Nature of sterols affects plasma membrane behavior and yeast survival during dehydration. Biochim. Biophys. Acta - Biomembr. 2011;1808:1520–1528. doi: 10.1016/j.bbamem.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Beney L, Gervais P. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl. Microbiol. Biotechnol. 2001;57:34–42. doi: 10.1007/s002530100754. [DOI] [PubMed] [Google Scholar]
- 15.Ziółkowska NE, Christiano R, Walther TC. Organized living: Formation mechanisms and functions of plasma membrane domains in yeast. Trends Cell Biol. 2012;22:151–158. doi: 10.1016/j.tcb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox LJ, et al. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 2002;277:32466–32472. doi: 10.1074/jbc.M204707200. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Prinz WA. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J. Biol. Chem. 2004;279:45226–45234. doi: 10.1074/jbc.M407600200. [DOI] [PubMed] [Google Scholar]
- 18.Gulati S, et al. ATP-binding cassette transporters and sterol O-acyltransferases interact at membrane microdomains to modulate sterol uptake and esterification. FASEB J. 2015;29:4682–4694. doi: 10.1096/fj.14-264796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decottignies A, et al. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- 20.Ruetz S, Brault M, Dalton W, Gros P. Functional interactions between synthetic alkyl phospholipds and the ABC transporters P-glycoprotein, Ste-6, MRP, and Pgh-1. Biochemistry. 1997;36:8180–8188. doi: 10.1021/bi970564o. [DOI] [PubMed] [Google Scholar]
- 21.Rockwell NC, Wolfger H, Kuchler K, Thorner J. ABC transporter Pdr10 regulates the membrane microenvironment of Pdr12 in Saccharomyces cerevisiae. J. Membr. Biol. 2009;229:27–52. doi: 10.1007/s00232-009-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, et al. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec. 14p-independent cell growth. Mol. Biol. Cell. 2000;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Den Hazel HB, et al. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J. Biol. Chem. 1999;274:1934–1941. doi: 10.1074/jbc.274.4.1934. [DOI] [PubMed] [Google Scholar]
- 24.Holič R, et al. Phosphatidylinositol binding of Saccharomyces cerevisiae Pdr16p represents an essential feature of this lipid transfer protein to provide protection against azole antifungals. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2014;1841:1483–1490. doi: 10.1016/j.bbalip.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleet GH. Yeasts in foods and beverages: impact on product quality and safety. Curr. Opin. Biotechnol. 2007;18:170–175. doi: 10.1016/j.copbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Gibson, B. R., Lawrence, S. J., Leclaire, J. P. R., Powell, C. D. & Smart, K. A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiology Reviews31 (2007). [DOI] [PubMed]
- 27.Lu Y, Cheng YF, He XP, Guo XN, Zhang BR. Improvement of robustness and ethanol production of ethanologenic Saccharomyces cerevisiae under co-stress of heat and inhibitors. J. Ind. Microbiol. Biotechnol. 2012;39:73–80. doi: 10.1007/s10295-011-1001-0. [DOI] [PubMed] [Google Scholar]
- 28.Almeida JRM, et al. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007;82:340–349. doi: 10.1002/jctb.1676. [DOI] [Google Scholar]
- 29.Koppram R, Tomás-Pejó E, Xiros C, Olsson L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014;32:46–53. doi: 10.1016/j.tibtech.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Mira NP, Palma M, Guerreiro JF, Sá-Correia I. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb. Cell Fact. 2010;9:79. doi: 10.1186/1475-2859-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mira NP, Teixeira MC, Sá-Correia I. Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: A genome-wide view. Omics. 2010;14:525–40. doi: 10.1089/omi.2010.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dos Santos SC, Sá-Correia I. Yeast toxicogenomics: Lessons from a eukaryotic cell model and cell factory. Curr. Opin. Biotechnol. 2015;33:183–191. doi: 10.1016/j.copbio.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 33.dos Santos SC, Teixeira MC, Cabrito TR, Sá-Correia I. Yeast toxicogenomics: Genome-wide responses to chemical stresses with impact in environmental health, pharmacology, and biotechnology. Front. Genet. 2012;3:1–17. doi: 10.3389/fgene.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng, P., Zhang, L. & Shi, G. Y. Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 33 (2017). [DOI] [PubMed]
- 35.Mollapour M, Shepherd A, Piper PW. Novel stress responses facilitate Saccharomyces cerevisiae growth in the monocarboxylate preservatives. Yeast. 2008;25:169–177. doi: 10.1002/yea.1576. [DOI] [PubMed] [Google Scholar]
- 36.Palma, M., Guerreiro, J. F. & Sá-Correia, I. Adaptive Response and Tolerance to Acetic Acid in Saccharomyces cerevisiae and Zygosaccharomyces bailii: A Physiological Genomics Perspective. Frontiers in Microbiology. 9, 274, 10.3389/fmicb.2018.00274 (2018). [DOI] [PMC free article] [PubMed]
- 37.Lindahl L, Genheden S, Eriksson LA, Olsson L, Bettiga M. Sphingolipids contribute to acetic acid resistance in Zygosaccharomyces bailii. Biotechnol. Bioeng. 2016;113:744–753. doi: 10.1002/bit.25845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerreiro JF, Muir A, Ramachandran S, Thorner J, Sá-Correia I. Sphingolipid biosynthesis upregulation by TOR complex 2-Ypk1 signaling during yeast adaptive response to acetic acid stress. Biochem. J. 2016;473:4311–4325. doi: 10.1042/BCJ20160565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindberg L, Santos AXS, Riezman H, Olsson L, Bettiga M. Lipidomic Profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii Reveals Critical Changes in Lipid Composition in Response to Acetic Acid Stress. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0073936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:1–17. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi Z, Arneborg N. Relationship between lipid composition, frequency of ethanol-induced respiratory deficient mutants, and ethanol tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 1999;86:1047–1052. doi: 10.1046/j.1365-2672.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- 42.Vanegas JM, Contreras MF, Faller R, Longo ML. Role of unsaturated lipid and ergosterol in ethanol tolerance of model yeast biomembranes. Biophys. J. 2012;102:507–516. doi: 10.1016/j.bpj.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanegas JM, Faller R, Longo ML. Influence of ethanol on lipid/sterol membranes: Phase diagram construction from AFM imaging. Langmuir. 2010;26:10415–10418. doi: 10.1021/la1012268. [DOI] [PubMed] [Google Scholar]
- 44.del Castillo Agudo L. Lipid content of Saccharomyces cerevisiae strains with different degrees of ethanol tolerance. Appl. Microbiol. Biotechnol. 1992;37:647–651. doi: 10.1007/BF00240742. [DOI] [Google Scholar]
- 45.Aguilera F, Peinado RA, Millán C, Ortega JM, Mauricio JC. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int. J. Food Microbiol. 2006;110:34–42. doi: 10.1016/j.ijfoodmicro.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Swan TM, Watson K. Stress tolerance in a yeast sterol auxotroph: Role of ergosterol, heat shock proteins and trehalose. FEMS Microbiol. Lett. 1998;169:191–197. doi: 10.1111/j.1574-6968.1998.tb13317.x. [DOI] [PubMed] [Google Scholar]
- 47.Caspeta L, et al. Altered sterol composition renders yeast thermotolerant. Science. 2014;346:75–78. doi: 10.1126/science.1258137. [DOI] [PubMed] [Google Scholar]
- 48.Parks LW, Casey WM. Physiological Implications of Sterol Biosynthesis in Yeast. Annu. Rev. Microbiol. 1995;49:95–116. doi: 10.1146/annurev.mi.49.100195.000523. [DOI] [PubMed] [Google Scholar]
- 49.Eisenkolb M, Zenzmaier C, Leitner E, Schneiter R. A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast. Mol. Biol. Cell. 2002;13:4414–28. doi: 10.1091/mbc.e02-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagnat M, Chang A, Simons K. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell. 2001;12:4129–38. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasrija R, Panwar SL, Prasad R. Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: Both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts. Antimicrob. Agents Chemother. 2008;52:694–704. doi: 10.1128/AAC.00861-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawahata M, Masaki K, Fujii T, Iefuji H. Yeast genes involved in response to lactic acid and acetic acid: Acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res. 2006;6:924–936. doi: 10.1111/j.1567-1364.2006.00089.x. [DOI] [PubMed] [Google Scholar]
- 53.Schüller C, et al. Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:706–20. doi: 10.1091/mbc.e03-05-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe F, Hiraki T. Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae. Biochim. Biophys. Acta - Biomembr. 2009;1788:743–752. doi: 10.1016/j.bbamem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Nes WR, Sekula BC, Nes WD, Adler JH. The functional importance of structural features of ergosterol in yeast. J. Biol. Chem. 1978;253:6218–6225. [PubMed] [Google Scholar]
- 56.Parasassi T, Krasnowska EK. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998;8:365–373. doi: 10.1023/A:1020528716621. [DOI] [Google Scholar]
- 57.Stiefel, P., Schmidt-Emrich, S., Maniura-Weber, K. & Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 15 (2015). [DOI] [PMC free article] [PubMed]
- 58.Maresova L, Urbankova E, Gaskova D, Sychrova H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006;6:1039–1046. doi: 10.1111/j.1567-1364.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 59.Thibault G, et al. The Membrane Stress Response Buffers Lethal Effects of Lipid Disequilibrium by Reprogramming the Protein Homeostasis Network. Mol. Cell. 2012;48:16–27. doi: 10.1016/j.molcel.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opekarová M, Tanner W. Specific lipid requirements of membrane proteins - A putative bottleneck in heterologous expression. Biochim. Biophys. Acta - Biomembr. 2003;1610:11–22. doi: 10.1016/S0005-2736(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 61.Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rattray JBM, Schibeci A, Kidby DK. Lipids of Yeasts. Bacteriol. Rev. 1975;39:197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kodedová M, Sychrová H. Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mira NP, Becker JD, Sá-Correia I. Genomic expression program involving the Haa1p-regulon in Saccharomyces cerevisiae response to acetic acid. Omics. 2010;14:587–601. doi: 10.1089/omi.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klug L, Daum G. Yeast lipid metabolism at a glance. FEMS Yeast Research. 2014;14:369–388. doi: 10.1111/1567-1364.12141. [DOI] [PubMed] [Google Scholar]
- 66.Baumann NA, et al. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 67.Schnabl M, Daum G, Pichler H. Multiple lipid transport pathways to the plasma membrane in yeast. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2005;1687:130–140. doi: 10.1016/j.bbalip.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Balzi E, Goffeau A. Yeast multidrug resistance: The PDR network. J. Bioenerg. Biomembr. 1995;27:71–76. doi: 10.1007/BF02110333. [DOI] [PubMed] [Google Scholar]
- 69.Paul S, Moye-Rowley WS. Multidrug resistance in fungi: Regulation of transporter-encoding gene expression. Front. Physiol. 2014;5:1–14. doi: 10.3389/fphys.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryot. Cell. 2007;6:1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004;1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 72.Pomorski T, et al. Tracking down lipid flippases and their biological functions. J. Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 73.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/S1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 74.Seret ML, Diffels JF, Goffeau A, Baret PV. Combined phylogeny and neighborhood analysis of the evolution of the ABC transporters conferring multiple drug resistance in hemiascomycete yeasts. BMC Genomics. 2009;10:459. doi: 10.1186/1471-2164-10-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahe Y, et al. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: a novel target for the transcription factors Pdr1 and Pdr3. 1996;20:109–117. doi: 10.1111/j.1365-2958.1996.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 76.Krishnamurthy SS, Prasad R. Membrane fluidity affects functions of Cdr1p, a multidrug ABC transporter of Candida albicans. FEMS Microbiol. Lett. 1999;173:475–481. doi: 10.1111/j.1574-6968.1999.tb13541.x. [DOI] [PubMed] [Google Scholar]
- 77.Aye ILMH, Singh AT, Keelan JA. Transport of lipids by ABC proteins: Interactions and implications for cellular toxicity, viability and function. Chem. Biol. Interact. 2009;180:327–339. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 78.Rice AJ, Alvarez FJD, Davidson AL, Pinkett HW. Effects of lipid environment on the conformational changes of an ABC importer. Channels. 2014;8:327–333. doi: 10.4161/chan.29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alexandre H, Mathieu B, Charpentier C. Alteration in membrane fluidity and lipid composition, and modulation of H+-ATPase activity in Saccharomyces cerevisiae caused by decanoic acid. Microbiology. 1996;142:469–475. doi: 10.1099/13500872-142-3-469. [DOI] [PubMed] [Google Scholar]
- 80.Collart, M. A. & Oliviero, S. In Current Protocols in Molecular Biology 13.12.1-13.12.5 (John Wiley & Sons, Inc., 1993).
- 81.Sýkora J, Kapusta P, Fidler V, Hof M. On what time scale does solvent relaxation in phospholipid bilayers happen? Langmuir. 2002;18:571–574. doi: 10.1021/la011337x. [DOI] [Google Scholar]
- 82.Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2012;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- 83.Gaskova D, et al. Fluorescent probing of membrane potential in walled cells: diS-C3(3) assay in Saccharomyces cerevisiae. Yeast. 1998;14:1189–1197. doi: 10.1002/(SICI)1097-0061(19980930)14:13<1189::AID-YEA320>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 84.Höfer M, Novacky A. Measurement of plasma membrane potentials of yeast cells with glass microelectrodes. Biochim. Biophys. Acta - Biomembr. 1986;862:372–378. doi: 10.1016/0005-2736(86)90240-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).