Abstract
Seven years after she had a total abdominal hysterectomy for benign leiomyomas, a 46-year-old woman presented with a pelvic mass and multiple pulmonary nodules. She underwent resection of the mass and core needle biopsy of a pulmonary lesion. Histopathologic analysis revealed that both the pelvic and the pulmonary lesions were consistent with benign leiomyomas. Benign metastasizing leiomyoma should be considered if a woman of reproductive age and with a history of leiomyomas presents with extrauterine nodules without evidence of malignancy. The final diagnosis should be based on histopathological examination. Treatment depends on tumor size, location, receptor positivity, and disease progression.
Keywords: Leiomyoma, Fibroids, Benign, Metastatic, Pulmonary nodules
Highlights
-
•
Reproductive aged woman with prior leiomyomas who present with extrauterine nodules may have BML.
-
•
Histopathological examination is required for definitive diagnosis of BML.
-
•
Optimal management depends on tumor size, location, receptor status, and progression over time.
1. Introduction
Benign metastasizing leiomyoma (BML) is a rare disorder in which histologically benign smooth muscle tumor is found in extrauterine sites. The condition was first described in 1939 by Steiner in a report of a patient who died from the effects of extensive pulmonary metastases of benign-appearing leiomyomas that were histologically identical to multiple leiomyomas concurrently present in the uterus [1].
The condition typically affects women of late reproductive age with a history of leiomyomas, the majority of whom have undergone surgical management with myomectomy and/or hysterectomy [2]. The mean age of women at the time of primary surgery and BML diagnosis is 38.5 and 47.3 years, respectively. The most frequent site of metastasis is the lungs (80%), with other organs including the heart, liver, esophagus, abdominal lymph nodes, skeletal muscle, skin, and central nervous system occasionally reported [2]. We present the case of a woman who had undergone a distant total hysterectomy for benign leiomyomas who subsequently developed a local pelvic recurrence and was simultaneously found to have numerous benign pulmonary metastases.
2. Case Report
A 46-year-old Hispanic woman presented to her gynecologist for her annual exam. She had no significant past medical history and took no medications. Her past surgical history was notable only for an uncomplicated total abdominal hysterectomy without salpingo-oophorectomy for benign leiomyomas at the age of 39.
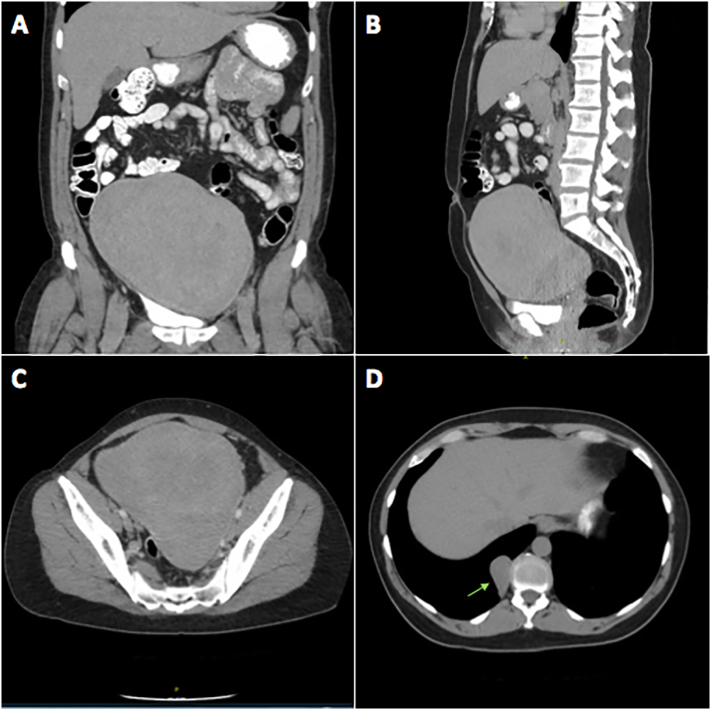
Physical examination was concerning for a large central pelvic mass, which was subsequently confirmed on pelvic ultrasound (Fig. 1). Computed tomography (CT) of the abdomen and pelvis was ordered to better characterize the mass and revealed a large heterogeneous solid mass measuring 16.4 cm × 13.0 cm × 18.2 cm arising centrally from the mid and upper pelvis. The mass appeared separate from the ovaries and bowel with well-defined margins and no associated fat or calcification (Fig. 2A–C). The CT also identified multiple bibasilar non-calcified pulmonary nodules measuring up to 4.2 cm × 2.3 cm noted to be highly suspicious for metastatic disease (Fig. 2D).
Fig. 1.
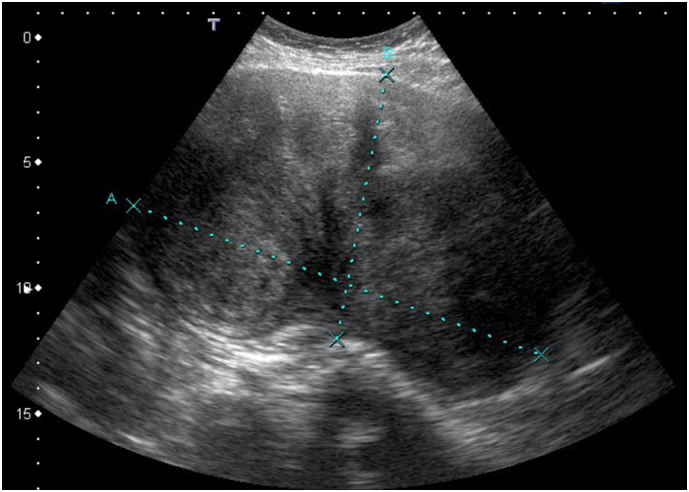
Pelvic ultrasound with sagittal view of pelvic mass measuring 17.4 cm × 10.7 cm.
Fig. 2.
CT abdomen and pelvis at the time of initial diagnosis. Large heterogeneous solid mass measuring 16.4 cm × 13 cm × 18.2 cm arising centrally from the mid and upper pelvis as seen in the coronal, sagittal, and axial planes, respectively (A–C). Largest of the incidentally noted bibasilar non-calcified pulmonary nodules at the right posterior medial lung base measuring 2.3 × 4.2 cm marked with the arrow (D).
In order to establish a diagnosis, CT-guided needle core biopsy of the pelvic mass was performed. On microscopy, the specimen was notable for the proliferation of bland spindle cells without evidence of necrosis, cytologic atypia, or increased mitotic activity.
Immunohistochemistry revealed diffuse positivity for desmin and smooth muscle actin, confirming smooth muscle differentiation. Negative staining for the cell surface marker CD117 and the chloride channel protein DOG-1 excluded the diagnosis of gastrointestinal stromal tumor (GIST), another spindle cell neoplasm in the histomorphological differential. Taken together, the findings were consistent with a smooth muscle neoplasm without definitive features of malignancy.
The patient underwent an exploratory laparotomy and was found to have a 15 cm × 11 cm × 11 cm right para-adnexal mass densely adherent to the right tube and ovary. She underwent bilateral salpingo-oophorectomy with resection of the mass as well as an opportunistic appendectomy. Final pathology confirmed the findings of the needle biopsy and supported the diagnosis of a benign leiomyoma. Given low concern for malignancy, the patient was started on transdermal estradiol 0.025 mg every 24 h as hormone replacement therapy (HRT) in the setting of surgical menopause following her bilateral oophorectomy.
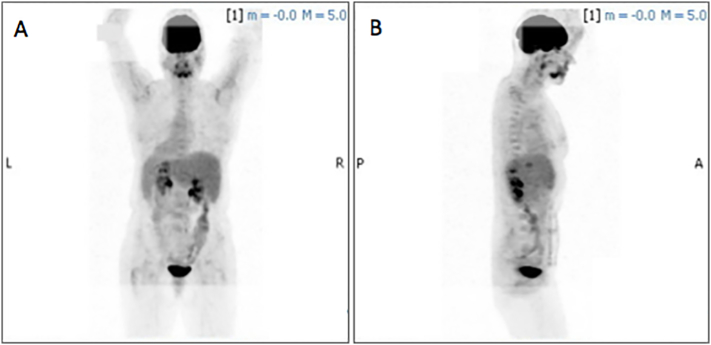
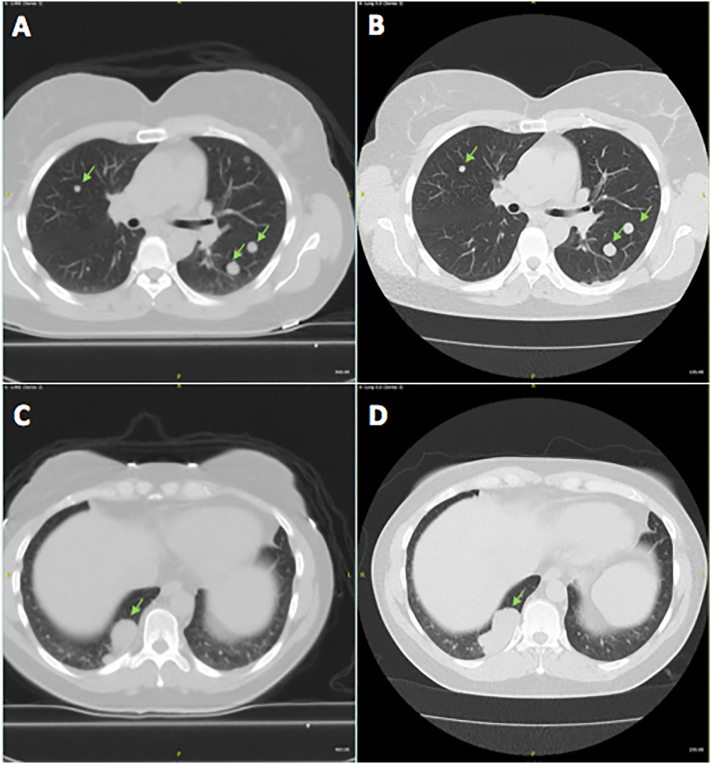
Following her abdominal surgery, the patient was referred to a pulmonologist for evaluation of the concerning CT findings. On evaluation, the patient denied any symptoms of chest pain, hemoptysis, cough, or shortness of breath. She denied recent weight loss, and reported no history of smoking or recent travel. A positron emission tomography CT (PET-CT) was ordered to better characterize the pulmonary nodules which were found to have no increased 18F–fluorodeoxyglucose (18F–FDG) avidity or evidence of hypermetabolic activity (Fig. 3). The scan identified over 30 pulmonary nodules; however, given the low likelihood of primary pulmonary or metastatic cancer, the decision was made to observe the patient off HRT with repeat imaging in six months. The patient was unable to tolerate HRT cessation secondary to menopausal symptoms and was restarted on estrogen supplementation shortly thereafter. Repeat CT six months later revealed largely stable pulmonary nodules with no new lesions but with interval enlargement of the most prominent nodule at the right posterior medial lung base from 4.2 cm × 2.3 cm to 5.2 cm × 2.3 cm (Fig. 4) prompting a CT-guided percutaneous biopsy of the lesion. Final pathology confirmed the diagnosis of benign leiomyoma. Pathology was reviewed with that of the excised pelvic mass and felt to be cytologically similar (Fig. 5, Fig. 6). Of note, the tumor was strongly estrogen and progesterone receptor positive (>95%). The risks and benefits of continuing HRT were discussed and the patient opted to discontinue estrogen supplementation.
Fig. 3.
PET CT negative for increased fluorodeoxyglucose (FDG)-avidity in the lung fields bilaterally, anterior/posterior and lateral views (A, B).
Fig. 4.
CT chest showing interval growth of bibasilar pulmonary nodules, marked with arrows. At time of diagnosis (A,C), largest nodule measured 4.2 cm × 2.3 cm. Six months later (B,D), the largest nodule measured 5.2 cm × 2.5 cm.
Fig. 5.
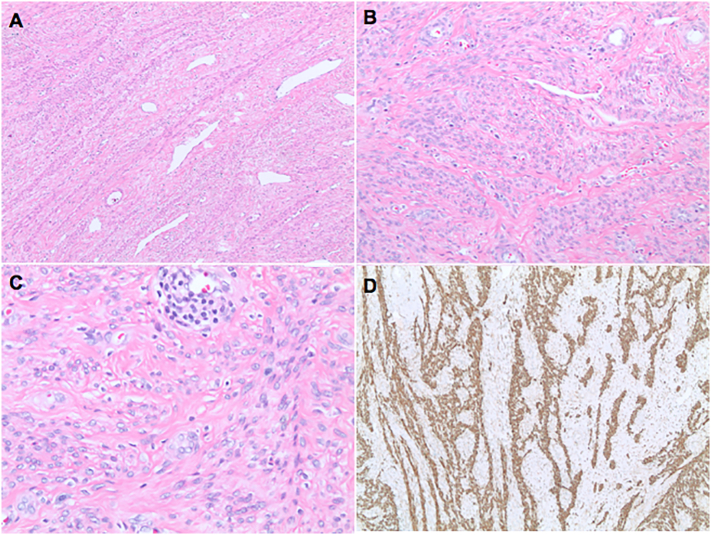
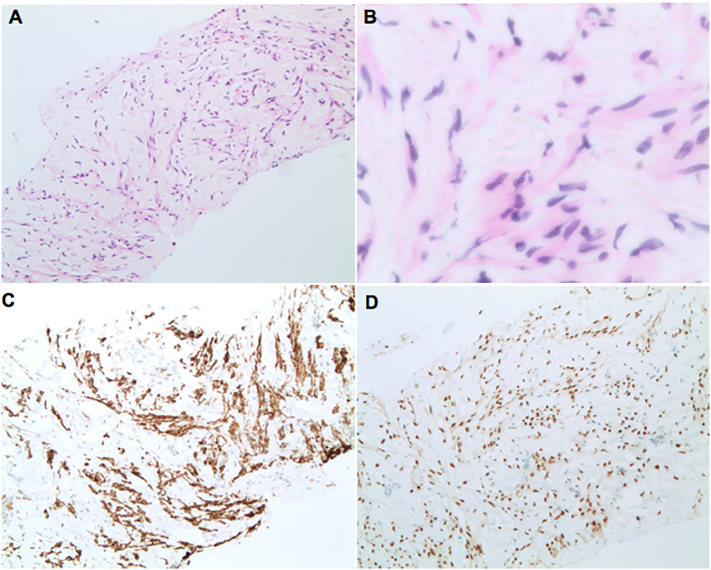
The original pelvic mass demonstrating the typical morphology of a leiomyoma with bland, smooth muscle cells arranged in a fascicular pattern. Atypia and mitotic activity were absent. 40× (A), 100× (B), and 200× (C). Desmin was positive, confirming muscular differentiation (D).
Fig. 6.
Lung core biopsy consisting of a proliferation of benign-appearing spindled cells with eosinophilic fibrillary cytoplasm within a loose, edematous stromal background. Atypia and mitotic activity were absent. 100× (A), 400× (B). Immunohistochemistry for desmin (C) and estrogen receptor (D) were positive.
The patient has continued to undergo CT surveillance every six months. Twelve months after her initial diagnosis, a scan again revealed a slight increase in the size of her pulmonary BML prompting referral to a gynecologic oncologist who started her on letrozole, an aromatase inhibitor, to further restrict her endogenous estrogen production. Two years later, surveillance imaging has confirmed stability of her pulmonary BML and the patient remains asymptomatic.
3. Discussion
Benign metastasizing leiomyoma (BML) is a rare disorder in which patients with a history of histologically benign uterine leiomyomas present years later with multiple extrauterine nodules of benign smooth muscle origin. The extrauterine leiomyoma localize primarily in the lungs, with rare reports of localization in extrapulmonary sites. We report the case of a 46-year-old Hispanic woman with a history of a total hysterectomy for symptomatic benign leiomyomas found to have pulmonary and pelvic BML seven years after her primary surgery.
The pathological origin of BML remains somewhat controversial, with hypotheses including (i) derivation from a multifocal but independent smooth muscle proliferation, (ii) derivation from a low-grade leiomyosarcoma, and (iii) mechanical dissemination or intravascular spread of existing smooth muscle cells from the uterus to distant locations. Recent molecular and genetic studies give credence to this hypothesis with evidence of a clonal relationship among synchronous BML suggesting derivation from a common pre-existing leiomyoma [3,4]. Furthermore, nearly all known case reports in the literature involve women with a history of uterine surgery, suggesting that surgery may predispose to the development of BML by means of facilitating dissemination of the primary tumor [2].
The diagnosis of BML remains challenging as patients are often asymptomatic. According to a recent systematic review of the literature, the mean interval between initial surgery and diagnosis of BML is approximately 9 years [2], though cases of BML have been identified as early as the time of initial surgery [[5], [6], [7]] and as late as 31 years post-surgically [8]. As such, identification of new masses or lesions at any time in women with a history of leiomyomas warrants consideration of BML on the differential. Given the genetic etiology and preponderance of fibroids within families and specific ethnic groups, a thorough family history may identify those individuals most at risk for BML, although this has yet to be explored. Characteristic X-ray findings include solitary or multiple nodular opacities in the lungs. On CT, pulmonary lesions often present as soft tissue densities with well-defined borders and without enhancement with intravenous contrast. Radiologic findings of extrapulmonary nodules are rarer and less well-characterized in the literature. However, for both pulmonary and extrapulmonary nodules, PET-CT with 18F–FDG is helpful to assess for significant metabolic activity, which would be unexpected in BML and would raise concern for malignant disease [9].
Definitive diagnosis of BML requires histopathologic evaluation. Pathologically, BML consist of a proliferation of smooth muscle cells identical to those seen in primary leiomyomas at other sites. Malignancies such as leiomyosarcoma should be excluded by the absence of significant cellular atypia, necrosis, or increased mitotic figures [10]. The immunohistochemical profile of BML is indistinguishable to that of primary uterine leiomyoma, with positive staining for actin, desmin, and smooth muscle actin and a low Ki-67 expression (<5%) [11]. Furthermore, BML are typically estrogen and progesterone receptor positive, with estrogen known to stimulate and progesterone known to inhibit tumor growth [12]. These effects correlate clinically with the observation that BML tend to subside when patients become pregnant or postmenopausal [13].
Given the rarity of the condition, there is no standardized treatment for BML. Several strategies have been published, including careful observation, surgical resection, progesterone therapy, oophorectomy, and medical castration using aromatase inhibitors and GnRH agonists [[14], [15], [16]]. The appropriate therapy depends on the particular presentation of each patient, as there can be important genetic variation under the broad diagnosis of BML. For example, patients presenting with cutaneous lesions found to be consistent with benign leiomyomas may warrant additional work-up for a possible diagnosis of hereditary leiomyomatosis and renal cell carcinoma (HLRCC), an autosomal dominant disorder resulting from a mutation in the gene (FH) encoding fumarate hydratase, an enzyme important in cellular metabolism. A mutation in FH leads not only to disseminated cutaneous benign leiomyomas, but also to aggressive renal cell carcinoma [17,18]. Early detection of such a variant could be life-saving for both the patient and her family members and would have important implications for patient surveillance and monitoring.
In patients with surgically resectable disease, there are a number of considerations that should guide the appropriate surgical approach. It is important to select an approach that will allow for en bloc removal of lesions and completeness of resection while limiting dissemination of resected tissue and seeding of extrapelvic sites. When considering a laparoscopic approach, it is important to be mindful that morcellation could lead to tissue dissemination even with use of a tissue containment bag, should leakage occur. While such considerations are important for all patients presenting with symptomatic uterine leiomyoma, women with BML may be at greater risk of recurrence and dissemination given their history of disease.
Our patient had a large pelvic mass that was amenable to complete resection with bilateral oophorectomy through an open approach. She had ER/PR positive pulmonary nodules that were managed with cessation of HRT, initiation of an aromatase inhibitor, and careful observation. Regardless of the approach to treatment, BML warrants surveillance imaging to monitor disease progression and response to therapy.
4. Conclusion
We report a case of pelvic and pulmonary BML arising after total abdominal hysterectomy for benign leiomyomas with surgical resection of the pelvic mass and bilateral salpingo-oophorectomy with subsequent surveillance of pulmonary nodules which remain stable to date.
BML is a rare entity; however, it should be considered among late reproductive age women with a history of leiomyoma who present with extrauterine nodules with no other evidence of malignancy. Final diagnosis requires histopathological examination to exclude primary or metastatic malignancy.
There is no standardized treatment for BML, with the choice of therapy ultimately depending on tumor size, location, receptor positivity, and progression over time.
Contributors
Jennifer B. Bakkensen and Pietro Bortoletto analyzed and interpreted the patient data and drafted the manuscript.
Wesley Samore performed the histological examination and was a major contributor in drafting the manuscript.
Cynthia C. Morton assisted with the interpretation of the data and provided critically important insights in revising the manuscript.
Raymond M. Anchan was involved in the conception of the study, the acquisition of data, and the revision of the manuscript.
All authors read and approved the final manuscript.
Conflict of Interest
Raymond M. Anchan is supported by the Agency for Healthcare Research and Quality (AHRQ), and Patient-Centered Outcomes Research Institute (PCORI) through COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids).
Funding
No funding was sought or secured in relation to this case report.
Consent
The patient in question granted the authors consent to include her history, exam, images, and evaluation in this manuscript.
Provenance and peer review
This case report was peer reviewed.
References
- 1.Steiner P.E. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am. J. Pathol. 1939 Jan;15(1):89. [PMC free article] [PubMed] [Google Scholar]
- 2.Barnaś E., Książek M., Raś R., Skręt A., Skręt-Magierło J., Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: a review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017 Apr 20;12(4) doi: 10.1371/journal.pone.0175875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H.J., Choi J., Kim K.R. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int. J. Gynecol. Pathol. 2008 Jul 1;27(3):340–345. doi: 10.1097/PGP.0b013e3181656dab. [DOI] [PubMed] [Google Scholar]
- 4.Wu R.C., Chao A.S., Lee L.Y., Lin G., Chen S.J., Lu Y.J., Huang H.J., Yen C.F., Han C.M., Lee Y.S., Wang T.H. Massively parallel sequencing and genome-wide copy number analysis revealed a clonal relationship in benign metastasizing leiomyoma. Oncotarget. 2017;8(29):47547–47554. doi: 10.18632/oncotarget.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rege A.S., Snyder J.A., Scott W.J. Benign metastasizing leiomyoma: a rare cause of multiple pulmonary nodules. Ann. Thorac. Surg. 2012 Jun 1;93(6):e149–51. doi: 10.1016/j.athoracsur.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Ki E.Y., Hwang S.J., Lee K.H., Park J.S., Hur S.Y. Benign metastasizing leiomyoma of the lung. World J. Surg. Oncol. 2013 Dec;11(1):279. doi: 10.1186/1477-7819-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon H.W., Choi S.H., Sung S.W., Park J.K. Pulmonary benign metastasizing leiomyoma: report of three cases. World J. Surg. Oncol. 2013 Dec;11(1):281. doi: 10.1186/1477-7819-11-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berti A.F., Santillan A., Velasquez L.A. Benign metastasizing leiomyoma of the cervical spine 31 years after uterine leiomyoma resection. J. Clin. Neurosci. 2015 Sep 1;22(9):1491–1492. doi: 10.1016/j.jocn.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Chen S., Liu R.M., Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J. Thorac. Dis. 2014 Jun;6(6) doi: 10.3978/j.issn.2072-1439.2014.04.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban J.M., Allen W.M., Schaerf R.H. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch. Pathol. Lab. Med. 1999 Oct;123(10):960–962. doi: 10.5858/1999-123-0960-BMLOTU. [DOI] [PubMed] [Google Scholar]
- 11.Kayser K., Zink S., Schneider T., Dienemann H., André S., Kaltner H., Schüring M.P., Zick Y., Gabius H.J. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000 Sep 7;437(3):284–292. doi: 10.1007/s004280000207. [DOI] [PubMed] [Google Scholar]
- 12.Jautzke G., Müller-Ruchholtz E., Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas): a report on 5 cases. Pathol. Res. Pract. 1996 Jan 1;192(3):215–223. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- 13.Horstmann J.P., Pietra G.G., Harman J.A., Cole N.G., Grinspan S. Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer. 1977 Jan 1;39(1):314–321. doi: 10.1002/1097-0142(197701)39:1<314::aid-cncr2820390148>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Banner A.S., Carrington C.B., Emory W.B., Kittle F., Leonard G., Ringus J., Taylor P., Addington W.W. Efficacy of oophorectomy in lymphangioleiomyomatosis and benign metastasizing leiomyoma. Obstet. Gynecol. Surv. 1981 Nov 1;36(11):653–655. doi: 10.1056/NEJM198107233050406. [DOI] [PubMed] [Google Scholar]
- 15.Hague W.M., Abdulwahid N.A., Jacobs H.S., Craft I. Use of LHRH analogue to obtain reversible castration in a patient with benign metastasizing leiomyoma. BJOG Int. J. Obstet. Gynaecol. 1986 May 1;93(5):455–460. [PubMed] [Google Scholar]
- 16.Nasu K., Tsuno A., Takai N., Narahara H. A case of benign metastasizing leiomyoma treated by surgical castration followed by an aromatase inhibitor, anastrozole. Arch. Gynecol. Obstet. 2009 Feb 1;279(2):255–257. doi: 10.1007/s00404-008-0698-0. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson I.P., Alam N.A., Rowan A.J., Barclay E., Jaeger E.E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., Roylance R.R. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002 Apr 1;30(4):406. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 18.Costa B., Dettori D., Lorenzato A., Bardella C., Coltella N., Martino C., Cammarata C., Carmeliet P., Olivero M., Di Renzo M.F. Fumarase tumor suppressor gene and MET oncogene cooperate in upholding transformation and tumorigenesis. FASEB J. 2010 Aug 1;24(8):2680–2688. doi: 10.1096/fj.09-146928. [DOI] [PubMed] [Google Scholar]