Abstract
Introduction
Ospemifene, an oral selective estrogen receptor modulator approved for the treatment of mild to moderate dyspareunia from menopause, has been shown to moderate sexual pain and vaginal epithelial cell characteristics. However, no prospective vulvoscopic studies have been performed.
Aim
To examine, in menopausal women taking ospemifene 60 mg daily, changes to the vulva, vestibule, urethral meatus, and vaginal region over 20 weeks using vulvoscopy in a prospective open-label pilot study.
Methods
Vulvoscopic photographs taken at screening and the end of therapy assessed for changes in the appearance of the vulva, vestibule, urethral meatus, and vagina rated by a single reviewer using a 10-parameter Likert rating scale, the Vulvoscopic Genital Tissue Appearance Scale (VGTA). In addition, the cotton-tipped swab test and subject diary scores were assessed over the 20-week treatment period and compared before and after the intervention using Wilcoxon signed-rank test.
Main Outcome Measure
Changes in VGTA score from baseline to end of study.
Results
8 subjects (age = 59 ± 4.7 years) completed all visits and were included in the analysis of vulvoscopic photographs (n = 258). There were significant changes during the study period for urethral meatal prominence, introital stenosis, vestibular pallor, vestibular erythema, mucosal moisture, vaginal rugation, and anterior wall prominence (P < .05). Total pain score during cotton-tipped swab testing decreased from 11 (interquartile range = 10–16) before the intervention to 1 (interquartile range = 0–3) at the end of the study. Quantitative diary analysis indicated an increase in the number of sexual events, decrease in rates of pain during foreplay and intercourse, and decrease in use of lubricant at study completion (P < .05).
Conclusions
Ospemifene 60 mg daily for 20 weeks showed improvement in physical examination findings in this prospective study of menopausal women with dyspareunia, as documented on vulvoscopic photography. These changes were consistent with improvements in subject-reported pain and sexual function.
Goldstein SW, Winter AG, Goldstein I. Improvements to the Vulva, Vestibule, Urethral Meatus, and Vagina in Women Treated With Ospemifene for Moderate to Severe Dyspareunia: A Prospective Vulvoscopic Pilot Study. Sex Med 2018;6:154–161.
Key Words: Vulva, Vestibule, Urethral meatus, Ospemifene, Vulvoscopy, Dyspareunia
Introduction
Ospemifene is an oral estrogen agonist and antagonist and a selective estrogen receptor modulator (SERM).1 It is indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, from menopause.1
It is estimated that within 1 to 6 years after menopause 64% to 85% of women will experience vulvar and vaginal atrophy,2 currently called genitourinary syndrome of menopause (GSM).3 GSM represents signs and symptoms associated with decreased hormone levels in menopause that can adversely affect such tissues as the labia majora, labia minora, vestibule, clitoris, vagina, urethra, urethral meatus, bladder, pelvic floor muscles, and periurethral anterior vaginal wall prostate tissue.3 With an average age of onset of menopause of 51 years,4 most women born from 1946 to 1964, the Baby Boomer generation, are menopausal. Because the life expectancy of women in the United States is approximately 80 years, women can expect to spend approximately 1/3 of their lives in menopause.5
A common bothersome symptom of women with GSM is painful intercourse, or dyspareunia, experienced by 2/3 of women in the 1st year of menopause.6, 7 Dyspareunia is chronic and progressive and unlikely to resolve spontaneously.3, 8 Adverse changes to the vagina in GSM that play a role in dyspareunia include shortening and narrowing of the vaginal lumen, loss of rugae in the vaginal epithelium, pallor and dryness of the vaginal mucosa, a decrease in vaginal epithelial superficial cells, an increase in vaginal epithelial parabasal cells, and an increase in vaginal pH.9 Ospemifene is considered safe and efficacious for the treatment of dyspareunia from menopause.10, 11, 12 In double-blinded, placebo-controlled studies, ospemifene at week 12 significantly increased superficial cells (10.8% vs 2.7% for placebo), significantly decreased parabasal cells (−34.4% vs +5.84% for placebo), significantly decreased vaginal pH (−0.97% vs −0.002% for placebo), and significantly lowered the dyspareunia severity score compared with placebo (P = 0.0012).12 There also are non-vaginal genitourinary tissue adverse changes associated with dyspareunia including erythema of minor vestibular glands; fissures at the posterior fourchette; labia minora resorption; protrusion, tenderness, and prolapse of the urethral meatus; and atrophy of the clitoris.3
A publication of 2 case studies of long-term use of ospemifene in a clinical setting showed on presentation continued disease of the vestibule.13 Another publication of a 60-day open-label clinical trial to examine changes in vestibular innervation used photography and a visual analog pain scale that showed that the vestibule was effectively treated with ospemifene, which also improved genitourinary tissue health in the region.14 To better understand the role of ospemifene as an effective oral agent in the broader construct of vaginally and non-vaginally based genitourinary tissue changes, we conducted a pilot study prospectively assessing changes in vulvoscopic appearance of multiple tissues of the genitourinary system before and after 20 weeks of treatment with ospemifene.
Methods
This was an open-label pilot study conducted at a single research center under approval of an independent review board. The primary purpose of the study was to evaluate visible vulvoscopic changes by comparing baseline with the end of the study at 20 weeks in the vulva, vestibule, clitoris, urethral meatus, and vaginal and periurethral anterior vaginal wall regions in women with moderate to severe dyspareunia treated with oral ospemifene 60 mg daily. There was no placebo arm in this pilot study. The secondary objectives were to assess for changes in pain noted with the cotton-tipped swab (Q-tip [Unilever NV, Rotterdam, The Netherlands] testing in the vestibule) and changes in sexual function as recorded in the subject diary. After signing consent, subjects who met the inclusion and exclusion criteria (Table 1) were enrolled.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Written informed consent | Hypersensitivity to ingredients |
| Female | Previously used ospemifene |
| Age 21–80 y | Suspected breast cancer, history of heart attack or stroke |
| BMI < 37 kg/m2 | Clinically significant findings at physical examination |
| Menopausal | Uncontrolled hypertension |
| Has moderate to severe dyspareunia | Chronic medical condition or psychologic disorder |
| Has moderate to severe pain at cotton-tipped swab testing | Using local or systemic androgen therapy |
| Agrees to comply with study procedures | Using local or systemic estrogen therapy |
| Using a SERM | |
| Using itraconazole, ketoconazole, digitalis, alkaloids heparin, strong cytochrome P450 3A4 inhibitors | |
| History of substance abuse | |
| Received investigational drug within 30 d | |
| Behavior indicating unlikely to be compliant |
BMI = body mass index; SERM = selective estrogen receptor modulator.
Vulvoscopy is a procedure that allows a health care provider to perform a detailed examination of the vulva, vestibule, clitoris, urethral meatus, vagina, and periurethral anterior wall prostate using a vulvoscope to magnify the area 4 to 40 times and to take multiple photographs.15 For each subject, a standardized method of optically magnified vulvoscopic examination with photography performed from lateral to medial and from external to internal was used to maximize diagnostic information.15, 16 The vulvoscope was a Wallach ZoomScope vulvoscope (Wallach Surgical Devices, Trumbull, CT, USA) with an attached foot-pedal–controlled Cannon EOS XSi Digital SLR camera (Cannon, Tokyo, Japan) that was linked to an LED monitor so the subject and the health care provider could observe the vulvoscopic findings in real time. At the end of the procedure, the photographic images were transferred to a file on an encrypted computer. The photographs were labeled by date taken and subject number.
Each subject’s baseline and end-of-study vulvoscopic photographs were subsequently assessed using a 4-point rating scale, the Vulvoscopic Genital Tissue Appearance Scale (VGTA). The VGTA has not been validated. This scale assesses 10 different parameters of the appearance of the genital tissue including loss of labia majora, loss of labia minora, decreased glans clitoris, prominence of the urethral meatus, stenosis of the introitus, vestibular pallor, vestibular erythema, loss of vestibular moisture, loss of vaginal rugae, and loss of prominence of the anterior vaginal wall (Table 2). Each subject could score 0 to 30 total points on the VGTA at each visit. A higher VGTA score was considered consistent with a worse vulvoscopic genital tissue appearance.
Table 2.
Vulvoscopic Genital Tissue Appearance Scale
| Component | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Labia majora | Normal | Mild loss | Moderate loss | Severe to total loss |
| Labia minora | Normal | Mild loss | Moderate loss | Severe to total loss |
| Clitoris | Normal | Mild decrease in size | Moderate decrease in size | Severe decrease in size or phimosis |
| Urethral meatus | Normal | Mild prominence | Moderate prominence | Severe prominence or large relative to introitus |
| Introitus | Normal | Mild stenosis | Moderate stenosis | Severe stenosis |
| Color | Normal | Mild pallor | Moderate pallor | Severe pallor |
| Erythema | Normal | Mild | Moderate | Severe |
| Moisture | Normal | Mild loss | Moderate loss | None |
| Vaginal rugae | Normal | Mild loss | Moderate loss | Severe loss |
| Anterior vaginal wall prominence | Normal | Mild loss | Moderate loss | Severe loss |
Baseline physical examination was performed as part of the screening. The physical examination included performance of cotton-tipped swab testing in which the examiner applied gentle pressure at each location of the vestibule: 1:00, 3:00, 5:00, 6:00, 7:00, 9:00, and 11:00 o’clock. The subject was asked to rate her pain on a scale from 0 to 3. Aggregate pain scores at baseline and study end were compared using the Wilcoxon signed-rank test.
Baseline sexual function diary data concerning dryness and sexual pain were collected. The questions of the sexual function diary have not been validated. For 4 weeks before each study visit, subjects recorded yes or no answers to the following questions for each sexual encounter: (i) did the subject experience dryness before and was she less dry now, (ii) did the subject use lubricant during this sexual activity, (iii) did the subject experience pain or discomfort during foreplay, (iv) did the subject experience pain during masturbation, (v) did the subject experience pain during oral sex, (vi) did the subject experience pain during intercourse, and (vii) did the subject stop early because of discomfort. Diaries were collected and new diaries were distributed at each subsequent visit. The number of total sexual events and the percentage of total sexual events with pain or lubrication difficulty were compared for diaries collected at week 0 and week 20 using the Wilcoxon signed-rank test.
At completion of the study, the photographs were sorted by subject number and date of examination and assessed by the principal investigator, a sexual medicine physician, who had not examined any of the women in person. He assigned scores to the photographs using predetermined Likert scales for the 10 parameters of the VGTA (Table 2).
Differences in pre- and post-treatment scores were compared using Wilcoxon signed-rank test for non-parametric matched pairs. All tests of significance were 2-tailed with a P value less than .05 considered significant. All analyses were performed in STATA 13 (StataCorp, College Station, TX, USA).
Results
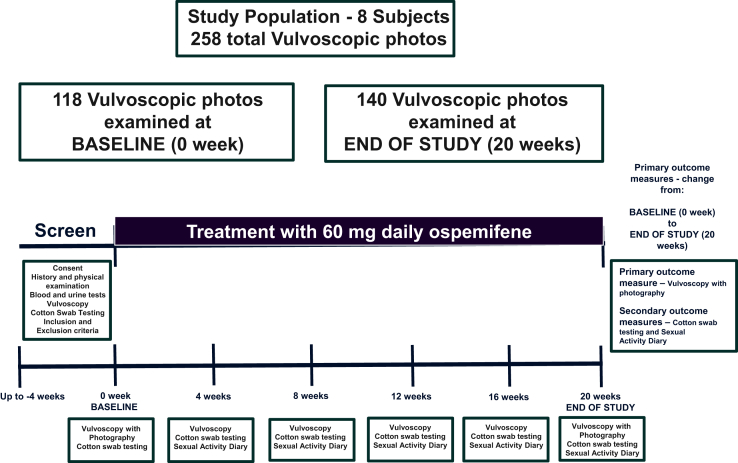
23 subjects were screened. 8 subjects met the inclusion and exclusion criteria and completed the study, forming the study population (Figure 1). Baseline characteristics of the study population are presented in Table 3. One subject withdrew consent at visit 4, citing lack of efficacy of the study medication. Data from this subject were not evaluated.
Figure 1.
Schematic showing study protocol, including duration of treatment with ospemifene and number of vulvoscopic photographs examined.
Table 3.
Baseline patient characteristics
| Age (y), mean (SD) | 59 (4.7) |
| BMI (kg/m2), mean (SD) | 26.8 (5.37) |
| Race, n (%) | |
| White | 6 (75) |
| Black | 2 (25) |
| Years since menopause, mean (SD) | 10.8 (6.5) |
| Estradiol (pg/mL), mean (SD) | 18.9 (13.2) |
| FSH (mIU/mL), mean (SD) | 79 (26.9) |
| LH (mIU/mL), mean (SD) | 33.2 (14.4) |
BMI = body mass index; FSH = follicle-stimulating hormone; LH = luteinizing hormone.
Results for the primary objective are presented in Table 4 and Figure 1. The primary outcome measure was based on the assessment of 118 photographs at baseline and 140 at the end of the study. The 8 subjects evaluated by the VGTA had a median score of 17.5 points (interquartile range = 16–18.5) at screening and 9.5 points (interquartile range = 8–11.5) at the end of the study (P = .0115), with an average improvement of 8 points. Individual VGTA scores for the parameters loss of labia majora, loss of labia minora, and decreased glans clitoris were not different before and after treatment. At 20 weeks there were significant changes: average urethral meatus appearance improved from moderate to mild prominence (P = .025), average introitus improved from moderate to mild stenosis (P = .0102), average vestibular pallor decreased from moderate to mild (P = .0067), average vestibular erythema decreased from moderate to mild (P = .0083), average moisture improved from moderate to mild loss (P = .0124), average rugation loss improved from moderate to mild (P = .047), and average anterior vaginal wall atrophy improved from moderate to mild (P = .027; Figures 2 and 3).
Table 4.
Vulvoscopic photo scoring of vulva, vestibule, and vagina in women before and at completion of 20 weeks therapy with ospemifene 60 mg/d
| Parameter | Week 0, median (IQR) | Week 20, median (IQR) | P value |
|---|---|---|---|
| Loss of labia majora | 0 (0–0) | 0 (0–0) | .32 |
| Loss of labia minora | 1 (1–1.5) | 1 (1–1) | .16 |
| Decreased size of glans clitoris | 1 (1–2) | 1 (1–1.5) | .32 |
| Prominence of urethral meatus | 2 (2–2) | 1 (1–1.5) | .025 |
| Stenosis of introitus | 2 (2–2.5) | 1 (1–1.5) | .0102 |
| Vestibular pallor | 2 (2–2) | 1 (1–1) | .0067 |
| Vestibular erythema | 2 (2–2) | 1 (0.5–1) | .0083 |
| Loss of vestibular moisture | 2.5 (2–3) | 1 (1–1) | .0124 |
| Loss of vaginal rugae | 2 (1.5–2) | 1 (1–1.5) | .047 |
| Loss of prominence of the anterior vaginal wall | 2 (1.5–2) | 1 (1–1) | .027 |
| Total score | 17.5 (16–18.5) | 9.5 (8–11.5) | .0115 |
IQR = interquartile range.
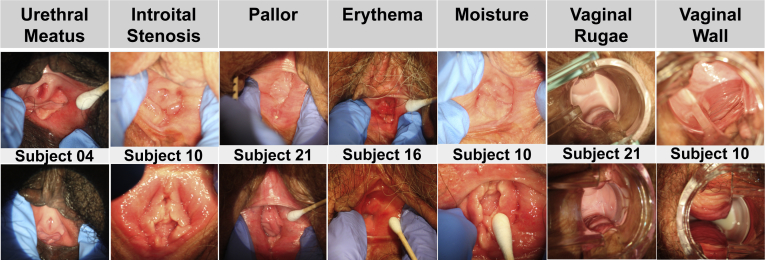
Figure 2.
Vulvoscopic photographs representing parameters in the Vulvoscopic Genital Tissue Appearance Scale that showed significant improvement from baseline (top row) to end of study (bottom row). Photographs from various subjects are representative of the study population. Note significant changes in some tissues, such as the urethral meatus, vestibule, and anterior vaginal wall, that are androgenic.
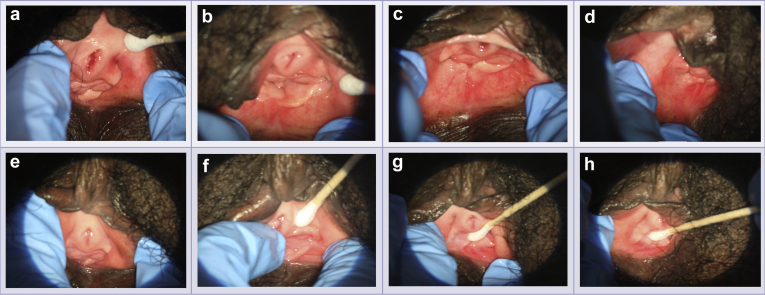
Figure 3.
Vulvoscopic photographs from subject 004. Vulvoscopic photographs were taken in order. Panels a to d show baseline evaluations at 1:00, 3:00, 6:00, and 9:00 o’clock, respectively. Panels e to h show end-of-study evaluations at 1:00, 3:00, 6:00, and 9:00 o’clock, respectively. These images show improvement in the Vulvoscopic Genital Tissue Appearance Scale for urethral meatal prominence, introital stenosis, vestibular pallor, vestibular erythema, and vestibular moisture.
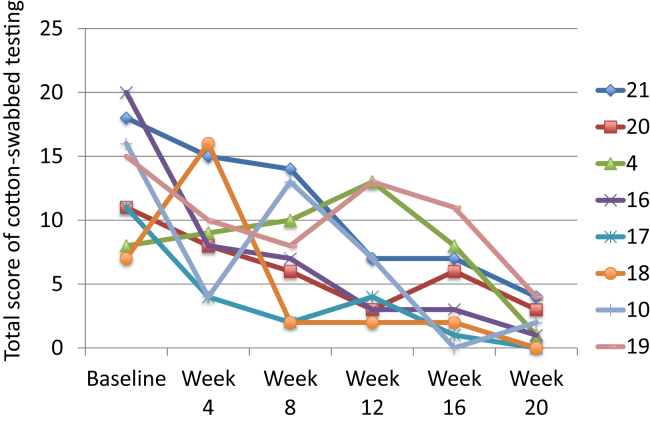
Total pain score during cotton-tipped swab testing decreased from 12.9 ± 3.6 before the intervention to 1.9 ± 1.6 at study end (P = .011; Figure 4).
Figure 4.
Graph shows significant improvement in total score of cotton-tipped swab (Q-tip) testing over time for each subject in the study.
Quantitative analysis of sexual function diaries is presented in Table 5. The number of sexual events was not significantly different before and after the intervention (4.5 and 7, respectively). The percentage of sexual events showing improvement from baseline to the end of the study was significant for 3 of 7 questions. There was an increase from 20% to 100% (P = .019) for the question asking whether the subject felt less dry than previously, a decrease from 41.5% to 0% (P = .0293) for the question regarding pain during foreplay, and a decrease from 80% to 0% (P = .0186) for the question regarding pain during intercourse. There was a decrease from 85% to 50% (P = .095) for the question asking about sexual events requiring lubricant, but this was not significant.
Table 5.
Subject diary responses showing changes in sexual function, lubrication, and pain in women undergoing 20 weeks of therapy with ospemifene 60 mg/d
| Diary metric | Week 0 |
Week 20 |
P value | ||
|---|---|---|---|---|---|
| Diary events, n | Median (IQR) | Diary events, n | Median (IQR) | ||
| Sexual events (n) | 8 | 4.5 (2.5–7.5) | 8 | 7 (5–10) | .075 |
| Events less dry than before (%) | 8 | 20 (0–82) | 8 | 100 (75–100) | .019 |
| Events using lubricant (%) | 8 | 85 (50–100) | 8 | 50 (16.5–91.5) | .095 |
| Events pain with foreplay (%) | 8 | 41.5 (0–70) | 8 | 0 (0–0) | .0293 |
| Events pain during masturbation (%) | 1 | 0 (0–0) | 2 | 0 (0–0) | — |
| Events with pain during oral sex (%) | 5 | 0 (0–0) | 6 | 0 (0–0) | .32 |
| Events with pain during intercourse (%) | 8 | 80 (20–100) | 8 | 0 (0–0) | .0186 |
| Events stopped early due to pain (%) | 8 | 0 (0–17.5) | 8 | 0 (0–0) | .085 |
IQR = interquartile range.
There were no serious adverse events. Adverse events experienced by subjects included microscopic hematuria, influenza, sinus infection, diarrhea, cough, acute coryza, and tooth extraction, none of which were considered related to the medication, which has known side effects of hot flush, vaginal discharge, muscle spasms, genital discharge, and hyperhidrosis.
Discussion
This pilot study was a prospective, open-label, single-center, 20-week vulvoscopic assessment of women with moderate to severe dyspareunia from menopause. The primary goal was to use vulvoscopy data obtained at baseline and study end to better understand the role of oral ospemifene 60 mg daily as a safe and effective estrogen agonist and antagonist SERM agent in the treatment of menopause-associated dyspareunia. The secondary goal was to use other measures of efficacy, such as the results of cotton-tipped swab testing on the ostia of the minor and major vestibular glands and the answers to questions on sexual function concerning dryness and sexual pain.
Our study population consisted of 8 subjects at an age consistent with menopause (mean age = 59 years), with increased gonadotropin values, on average 11 years in menopause (Table 3). In this population, the average sex steroid estradiol value was low (18 pg/mL), consistent with a limited ability to synthesize estradiol-dependent structural and functional proteins. We did not obtain testosterone, SHBG, or dihydrotestosterone blood test values and thus did not assess levels of androgens in this population. All subjects were excluded from using exogenous testosterone and estrogen treatment. It can be predicted that the testosterone values would be low; Chakravarti et al17 reported that the mean testosterone blood test value 10 years after the onset of menopause is 40 ng/dL.
The data supporting the action of ospemifene in effectively treating the dyspareunia of menopause vs placebo have been exclusively based on the drug’s estrogenic agonism of vaginal tissues. In double-blinded, placebo-controlled studies, the vaginal estrogenic agonism of ospemifene resulted in significantly increased superficial cells, significantly decreased parabasal cells, and significantly lower vaginal pH.10, 11, 12
However, menopausal-associated dyspareunia, a symptom of GSM, involves more genitourinary tissues than the vagina. Specifically, the tissue changes from GSM in the vestibule are strongly associated with dyspareunia, because the vestibule is an endodermal tissue at the vaginal entrance associated with a high concentration of nerve endings.14 Additional non-vaginal tissues include the labia majora, labia minora, clitoris, urethra, urethral meatus, bladder, pelvic floor muscles, and periurethral anterior vaginal wall prostate. Many of these tissues contain androgen and estrogen receptors. Sex steroid hormones, androgens and estrogens, induce the synthesis of critical androgen- and estrogen-dependent proteins such as enzymes, growth factors, and structural proteins that act on such cells as the epithelium, smooth muscle, nerves, and blood vessels to induce genital structure and function changes that can alleviate painful intercourse or dyspareunia symptoms.3, 9, 18, 19, 20, 21, 22 In menopausal women testosterone and estradiol values decrease to relatively constant levels, typically 40 ng/dL and 12 pg/mL, respectively, 10 years after the start of menopause.17 These sex steroid levels can result in multiple adverse changes to the genitourinary tract system that could play a role in the bothersome dyspareunia experienced by menopausal women.9, 23
In our pilot study, we used several outcome measures to better understand the role of ospemifene in efficaciously managing dyspareunia in menopausal women with GSM. In the pivotal ospemifene trials, the efficacy of management of dyspareunia was assessed by changes on the Bothersome Symptom Scale, a 4-point scale in which 0 was no pain, 1 was mild pain, 2 was moderate pain, and 3 was severe pain.10, 11, 12
Our primary data measure was based on using a 4-point rating scale of 10 individual parameters, the VGTA. Using this scale, we noted significant changes (P < .05) from week 0 to week 20 in decreased prominence of the urethral meatus, decreased stenosis of the introitus, decreased vestibular pallor, decreased vestibular erythema, increased vestibular moisture, increased vaginal rugae, and increased prominence of the anterior vaginal wall (Figures 2 and 3). These tissues are not exclusively based on estrogen. This implies that ospemifene, a SERM, could have the ability to synthesize structural and functional proteins that are dependent on androgen from crosstalk between the androgen and estrogen receptors, but more research is needed.24, 25, 26
Concerning the secondary outcome measures, we recorded the results of the cotton-tipped swab testing in which the subject was asked to rate her pain on a scale from 0 to 3 at multiple locations of the vestibule. Aggregate pain scores at baseline and study end showed that the total pain score during cotton-tipped swab testing decreased significantly by 11 points from before the intervention to study end. The vestibule is embryologically endodermal in origin. The vestibule is well recognized to have androgen receptors and the condition of hormonally mediated provoked vestibulodynia is well documented to respond to local testosterone therapy.27 This further implies that ospemifene might have the ability to synthesize structural and functional proteins that are dependent on androgen, but more research is needed.
Concerning the secondary outcome measure of quantitative analysis of sexual function diaries, we found a significant increase in the percentage of sexual events with less dryness and significant decreases in pain during foreplay and intercourse, consistent with the significant changes in genitourinary tissue health displayed at vulvoscopy.
A SERM is a pharmaceutical agent with a chemical structure similar to estrogen that can interact with the estrogen receptor and modulate agonist or antagonist responses in various target tissues. The clinical profile of an ideal SERM as it relates to sexual health would have estrogen receptor agonist activity in tissues where mimicking the action of estrogens is desirable, such as the vagina and bone, and estrogen receptor neutral or antagonist activity in tissues where mimicking the action of estrogens is not desirable, such as the uterus and breast. Our pilot study suggests that ospemifene might have agonist activity in endodermal tissues, such as the vestibule, urethral meatus, and prostate, that have androgen receptors. Such findings are consistent with ospemifene being a SERM sex steroid that might have crosstalk between the estrogen and androgen receptors and therefore the ability to synthesize structural and functional proteins that revitalize and remodel genitourinary tissues not exclusively estrogenic to address the bothersome GSM symptom of dyspareunia.24, 25, 26
Although ospemifene showed significant improvement in vulvoscopy, cotton-tipped swab testing, and sexual function questionnaires in women in menopause with moderate to severe dyspareunia, it should be recognized that the management of women with painful sexual activity is complex and often requires an interdisciplinary approach. It is common to address more factors than the hormonal deficiency state as the prime individual driver of a woman’s sexual pain experience. Other factors contributing to painful intercourse for women in menopause include non-hormonal biological factors (eg, neurologic), musculoskeletal concerns (eg, high tone pelvic floor), and psychological, social, and/or partner factors. For example, the dyspareunia state can affect such issues as relationship satisfaction, genital self-image, interoceptive awareness, depression, and anxiety. Sex therapy strategies such as mindfulness can be very helpful for women with genital pain.28 Musculoskeletal factors can be contributory to the sexual pain condition. Pelvic floor physical therapists trained in pelvic health conditions, including genitourinary tract changes during menopause, can apply various pelvic floor muscle relaxation and manual-based therapy techniques to improve the outcome of a pelvic pain condition.29, 30 Non-hormonal strategies, such as CO2 fractional lasers, can be useful in women in menopause who have moderate to severe dyspareunia.31
Limitations of the study include the small pilot study size and the fact that the study was open label without placebo. To minimize vulvoscopic reader bias, the individual chosen to read the photographs, who had more than 10 years of experience performing and assessing vulvoscopy, was not involved in any part of data collection or subject visits, such as results of cotton-tipped swab testing. This individual was supplied the photographs by dates, so the reader was aware of whether the photos were from baseline or study end.
Despite the limitations, this was the 1st prospective study to show the value of vulvoscopy in assessing dyspareunia in GSM. Based on the results of this pilot study, we strongly recommend that vulvoscopy be included as a measure of genitourinary tissue health in future studies assessing the safety and efficacy of treatment of dyspareunia in menopausal women.
Conclusion
Daily use of ospemifene, prescribed for the treatment of moderate to severe dyspareunia, improves the quality of the genitourinary tissue in the vulva, vestibule, urethral meatus, and vagina. More research is needed to understand the mechanism of action of this SERM in improving the quality of androgenic genitourinary tract tissues.
Statement of authorship
Category 1
-
(a)Conception and Design
- Sue W. Goldstein; Irwin Goldstein
-
(b)Acquisition of Data
- Sue W. Goldstein; Irwin Goldstein
-
(c)Analysis and Interpretation of Data
- Sue W. Goldstein; Ashley G. Winter; Irwin Goldstein
Category 2
-
(a)Drafting the Article
- Sue W. Goldstein; Ashley G. Winter; Irwin Goldstein
-
(b)Revising It for Intellectual Content
- Sue W. Goldstein; Ashley G. Winter; Irwin Goldstein
Category 3
-
(a)Final Approval of the Completed Article
- Sue W. Goldstein; Ashley G. Winter; Irwin Goldstein
Footnotes
Conflicts of Interest: Dr Irwin Goldstein is a consultant for Shionogi. The other authors declare no conflicts of interest.
Funding: Investigator-Initiated Grant from Shionogi.
References
- 1.FDA Highlights of prescribing information 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203505s000lbl.pdf Available at:
- 2.Nappi R.E., Biglia N., Cagnacci A. Diagnosis and management of symptoms associated with vulvovaginal atrophy: expert opinion on behalf of the Italian VVA study group. Gynecol Endocrinol. 2016;32:602–606. doi: 10.1080/09513590.2016.1183627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portman D.J., Gass M.L., Vulvovaginal Atrophy Terminology Consensus Conference Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063–1068. doi: 10.1097/GME.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 4.North American Menopause Society Perimenopause & premature menopause FAQS. http://www.menopause.org/for-women/expert-answers-to-frequently-asked-questions-about-menopause/perimenopause-premature-menopause-faqs Available at: Published 2017.
- 5.Manton K.G., Vaupel J.W. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. N Engl J Med. 1995;333:1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- 6.Kao A., Binik Y.M., Kapuscinski A. Dyspareunia in postmenopausal women: a critical review. Pain Res Manag. 2008;13:243–254. [Google Scholar]
- 7.Cagnacci A., Carbone M.M., Palma F. Prevalence and association between objective signs and subjective symptoms of vaginal atrophy: the AGATA study. Menopause. 2016;23:1139–1145. doi: 10.1097/GME.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 8.Faubion S.S., Sood R., Kapoor E. Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin Proc. 2017;92:1842–1849. doi: 10.1016/j.mayocp.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Nappi R.E., Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17:3–9. doi: 10.3109/13697137.2013.871696. [DOI] [PubMed] [Google Scholar]
- 10.Portman D., Palacios S., Nappi R.E. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas. 2014;78:91–98. doi: 10.1016/j.maturitas.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Nappi R.E., Panay N., Bruyniks N. The clinical relevance of the effect of ospemifene on symptoms of vulvar and vaginal atrophy. Climacteric. 2015;18:233–240. doi: 10.3109/13697137.2014.975199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmann G.A., Komi J.O., Ospemifene Study Group Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480–486. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein A.T., King M.A. Ospemifene may not treat vulvar atrophy: a report of two cases. Sex Med. 2016;4:e217–e220. doi: 10.1016/j.esxm.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murina F, Di Francesco S, Oneda S. Vulvar vestibular effects of ospemifene: a pilot study. Gynecol Endocrinolhttps://doi.org/10.1080/09513590.2018.1427717. E-pub ahead of print. [DOI] [PubMed]
- 15.Kottmel A., Goldstein I. Vulvoscopy. J Sex Med. 2012;9:2990–2993. [Google Scholar]
- 16.Winter A., Rubin R.S., Gagnon C. Standardized step-wise approach to vulvoscopy to maximize diagnostic yield. J Sex Med. 2017;14:e361–e362. [Google Scholar]
- 17.Chakravarti S., Collins W.P., Forecast J.D. Hormonal profiles after the menopause. Br Med J. 1976;2:784–787. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich W., Susani M., Stifter L. The human female prostate-immunohistochemical study with prostate-specific antigen, prostate-specific alkaline phosphatase, and androgen receptor and 3-D remodeling. J Sex Med. 2011;8:2816–2821. doi: 10.1111/j.1743-6109.2011.02408.x. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes T., Costa-Paiva L.H., Pinto-Neto A.M. Efficacy of vaginally applied estrogen, testosterone, or polyacrylic acid on sexual function in postmenopausal women: a randomized controlled trial. J Sex Med. 2014;11:1262–1270. doi: 10.1111/jsm.12473. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein A.T., Belkin Z.R., Krapf J.M. Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. J Sex Med. 2014;11:2764–2771. doi: 10.1111/jsm.12668. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein A., Burrows L., Goldstein I. Can oral contraceptives cause vestibulodynia? J Sex Med. 2010;7:1585–1587. doi: 10.1111/j.1743-6109.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- 22.Panzer C., Wise S., Fantini G. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: a retrospective study in women with sexual dysfunction. J Sex Med. 2006;3:104–113. doi: 10.1111/j.1743-6109.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 23.Bell RJ, Rizvi F, Islam RM, Davis SR. A systematic review of intravaginal testosterone for the treatment of vulvovaginal atrophy. Menopausehttps://doi.org/10.1097/GME.0000000000001052. E-pub ahead of print. [DOI] [PubMed]
- 24.Basile D., Cinausero M., Iacono D. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat Rev. 2017;61:15–22. doi: 10.1016/j.ctrv.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Kono M., Fujii T., Lim B. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3:1266–1273. doi: 10.1001/jamaoncol.2016.4975. [DOI] [PubMed] [Google Scholar]
- 26.Need E.F., Selth L.A., Harris T.J. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor alpha in luminal breast cancer cells. Mol Endocrinol. 2012;26:1941–1952. doi: 10.1210/me.2011-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrows L.J., Goldstein A.T. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med. 2013;1:30–33. doi: 10.1002/sm2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora N., Brotto L.A. How does paying attention improve sexual functioning in women? A review of mechanisms. Sex Med Rev. 2017;5:266–274. doi: 10.1016/j.sxmr.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Vandyken C., Hilton S. Physical therapy in the treatment of central pain mechanisms for female sexual pain. Sex Med Rev. 2017;5:20–30. doi: 10.1016/j.sxmr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Morin M., Carroll M.S., Bergeron S. Systematic review of the effectiveness of physical therapy modalities in women with provoked vestibulodynia. Sex Med Rev. 2017;5:295–322. doi: 10.1016/j.sxmr.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Salvatore S., Nappi R.E., Parma M. Sexual function after fractional microablative CO(2) laser in women with vulvovaginal atrophy. Climacteric. 2015;18:219–225. doi: 10.3109/13697137.2014.975197. [DOI] [PubMed] [Google Scholar]