Abstract
Background:
Sheep industry has taken steps toward transforming itself into a more efficient and competitive field. There are many varieties of sheep breeds in the world that each of them serves a useful purpose in the economies of different civilizations. Ghezel sheep is one of the Iranian important breeds that are raised for meat, milk and wool. Field of spermatogonial cell technologies provides tools for genetic improvement of sheep herd and multiple opportunities for research. Spermatogonial cells are the only stem cells capable of transmitting genetic information to future generations.
Methods:
This study was designed to extend the technique of isolation and in vitro proliferation of spermatogonial cells in Ghezel sheep.
Results:
Isolated cells were characterized further by using specific markers for type A spermatogonia, including PLZF. Also, sertoli cells were characterized by vimentin which is a specific marker for sertoli cells. After 10 days of co-culture, viability rates of the cells was above 94.7%, but after the freezing process the viability rates were 74 percent.
Conclusion:
In this study, a standard method for isolation and in vitro proliferation of spermatogonial stem cells in Ghezel sheep was developed.
Keywords: Ghezel sheep, Isolation, Spermatogonia
Introduction
Ghezel sheep are raised in north-west of Iran and north east of Turkey. This breed is native, fat-tailed and large-sized. Birth weight, weaning weight, weight at six months, average daily gain from birth to weaning and average daily gain from weaning to six months were 4.47, 20.88, 33.14 Kg, 0.184 and 0.134 gr, respectively1.
The economic importance of sheep industry has led to the progress of new technologies such as cell culture, In Vitro Fertilization (IVF) and Intracytoplasmic Sperm Injection (ICSI) technique. Spermatogonial Stem Cell (SSC) technology in sheep has been more limited. SSCs have the ability to transmit genetic information to future generation, so these cells could be used in the generation of transgenic animals2. Advances in genomic editing, mainly the CRISPR-Cas9 system, are likely to permit genomic alteration of SSCs3. Also, SSC technology is a new tool for studying spermatogenesis4.
SSCs can be isolated, cultured and cryopreserved in mouse5,6–9, bovine10,11, goat12, pig13 and sheep14. But the ability to manipulate them in different species is unique. Therefore, this study was designed to establish the technique of spermatogonial isolation, proliferation and freezing protocol in sheep.
For creation of transgenic animals, establishment of this technology in specific breeds of sheep is important. The purpose of this study was to adapt spermatogonial cell isolation, co-culture them with sertoli cells and proliferate them in Ghezel breed sheep.
Materials and Methods
Animal
In this survey, two months old male lambs (Ghezel breed) were used. The research was conducted in accordance with the National Research Council guidelines. Lambs were sedated with 0.1 mg/kg IM administration of xylazine. Also, each lamb received a 3-ml local injection of 2% lidocaine 20 min before surgery. Respiration rate, heart rate, and rectal temperature were monitored before and after recovery from analgesia. Lambs were given flunixin meglumine (2.2 mg/kg of body weight, IM) for 3 days after surgery.
Germ cell collection
A testis biopsy was taken from the right testis of all lambs. Testicular germ cell collection was done according to Qasemi-Panahi et al’s instructions15. Briefly, testis pieces were floated in DMEM containing 1 mg/ml collagenase type IV, 1 mg/ml trypsin, 1 mg/ml hyaluronidase type II and then incubated at 37°C for 40 min. All the enzymes and culture medium were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). After washing in DMEM, seminiferous tubules were entered in to secondary digestion process, with the same enzymes for 20 min at 37°C. To achieve favorite cell population, cellular suspension was centrifuged at 30 g for 2 min. The cells were then filtered through a nylon mesh with 60 mm pore size. The cells were pelleted and subjected to co-culture of spermatogonial and sertoli cells for 10 days.
Cells were cultured in DMEM supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Gaithersburg, MD, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin for 10 days when the cells reached to about 80% confluency. Colonization began from day 4 after co-culture. The size and number of colonies were measured during the culture for 2 weeks but not mentioned in this paper.
SSCs and sertoli cell identification
For identification of SSCs and sertoli cells in sheep, immunocytochemistry staining (ICC) was used. According to Bahadorani et al, as a specific marker of SSCs, anti-PLZF was used in this study16. Eight-well glass slides (Marienfeld, Germany) were used in which 1–2×104 cells were grown in each well. The cells were incubated overnight in a humidified incubator followed by washing with pre-warmed medium. After being dried for 15 min, cells were fixed and permeabilized in acetone for 2 min at −20°C and kept at 4°C for 30 min until slides were completely dried. Slides were then washed three times (3×3 min) with Tris-buffered saline, pH=7.4 containing 5% Bovine Serum Albumin (TBS/BSA).
The obtained colonies of SSCs were immunocytochemically stained with anti-PLZF. Briefly, goat anti-PLZF (1:100, Santa Cruz Biotechnology, USA) was applied for 60 min at room temperature. After being washed, mouse anti-goat IgG (1:50, PLZF TexasRed) was added and incubation was further continued for 45 min at room temperature. After washing with TBS/BSA, the nuclei were counterstained by 4′,6-diamidi-no-2-phenylindole dihydrochloride (DAPI; Calbiochem, Nottingham, UK) at 0.1 μg/ml for 5 min, then the slides were washed, mounted in PBS-glycerol 90%, and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
The obtained colonies of SSCs were immunocytochemically stained with anti Oct-4. Briefly, anti Oct-4 (Abcam) diluted in TBS/BSA was applied over slides. After 60 min, washing of slide was done. FITC conjugated donkey polyclonal secondary antibody to Goat IgG was added and incubation was further continued for 45 min at room temperature. After washing, it was mounted in PBS-glycerol 90%, and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Vimentin was detected in sertoli cells by the procedure which was described by Tajik et al17. Briefly, anti-vimentin (Abcam, Cambridge, UK) optimally diluted in TBS/BSA (2 μg/ml) was applied over slides for 60 min at room temperature. After being washed, Fluorescein Isothiocyanate (FITC)-conjugated sheep anti-mouse Ig was diluted in TBS/BSA at a ratio of 1:50 and incubation was further continued for 45 min at room temperature. Following the first washing with TBS/BSA, slide was exposed to 7-Amino-actinomycin D (7AAD) 5 μg/ml for 5 min. It was then washed and mounted in PBS-glycerol 90% and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Cryopreservation
After 10 days of co-culture, spermatogonial and sertoli cells were cryopreserved according to Izadyar et al’s instructions with some modification18. The freezing media were based on DMEM supplemented with 90% (v/v) FBS, 1.4 M DMSO and 0.07 M sucrose. For freezing, cryovials (Nunc, Denmark) were placed in a polystyrene container at −80°C for at least 1 day and then plunged into liquid nitrogen. Thawing process was done at 38°C water bath for 2 min. A sample was taken for viability assessment.
Results
Testicular cells were isolated from testis samples of two months old lambs and co-cultured with sertoli cells. The sertoli cells which proliferated and created a monolayer of feeder cells and spermatogonia cells were colonized. Type A spermatogonia were round cells (Figure 1) and small colonies of these cells appeared four days after co-culture (Figure 2). Vimentin was detected in this monolayer cell (Figure 3). PLZF which is a molecular marker for SSCs was detected in the obtained round cells (Figure 4). Also, Oct-4 was detected in sheep SSCs (Figure 5) that is a specific marker for SSCs. The viability rate of the cells before and after freezing were 90.3 and 71%, respectively.
Figure 1.
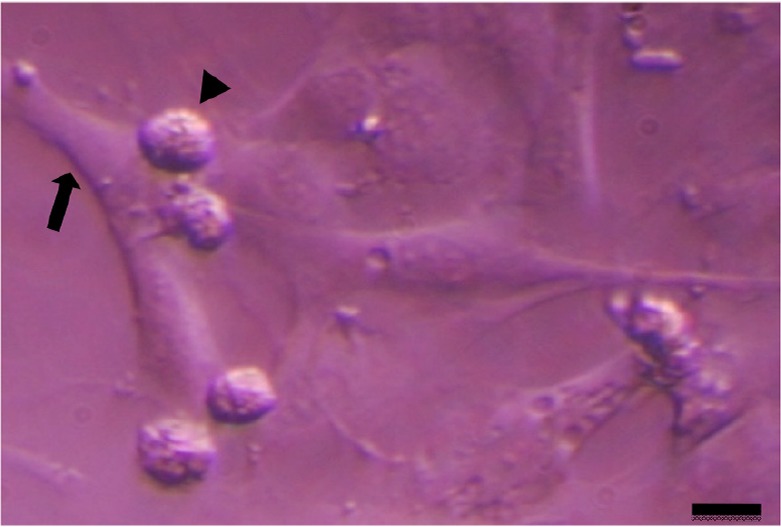
Sertoli cell (arrow) and spermatogonia (arrow head) of sheep. Scale bars represent 15 μm.
Figure 2.
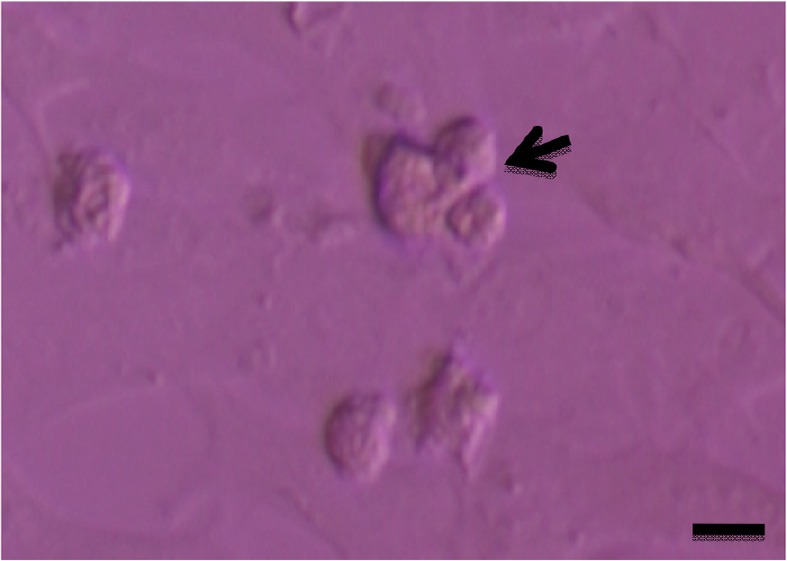
Cell clumps of sheep spermatogonia (arrow). Scale bars represent 15 μm.
Figure 3.
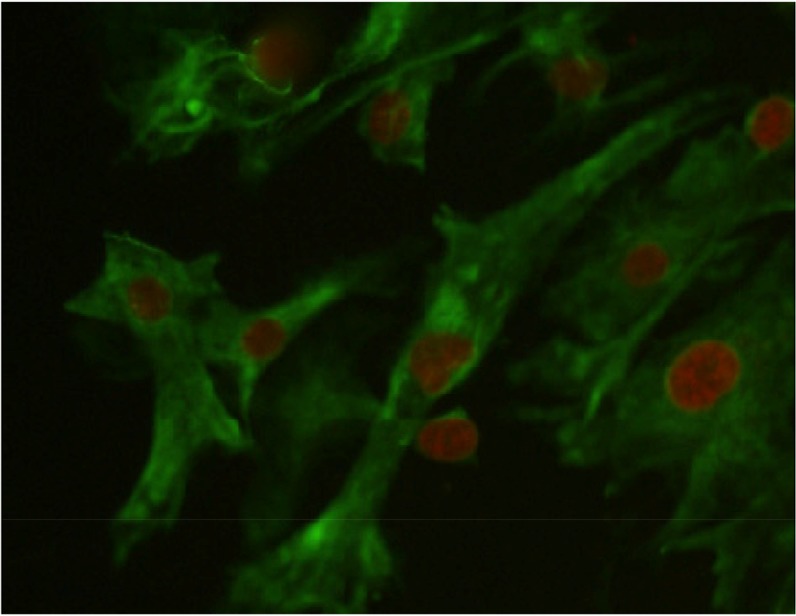
Vimentin was detected in the feeder monolayer cells (Vimentin-specific monoclonal antibodies). Nuclei were counterstained with 7AAD, (×40).
Figure 4.
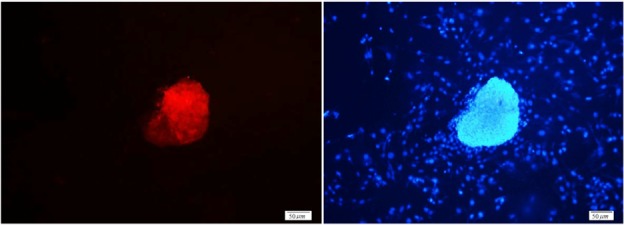
PLZF immunocytochemical staining of sheep spermatogonial stem cells. Nuclei were counterstained with DAPI (×20).
Figure 5.
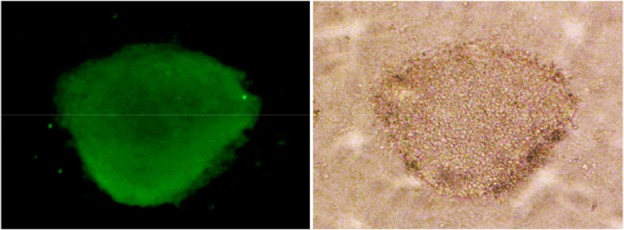
Oct-4 immunocytochemical staining of sheep spermatogonial stem cells (×40).
Discussion
Ghezel sheep originated in northwestern Iran and northeastern Turkey. This region in Iran is known as Azarbayjan and is typified by dry, cold mountain weather1,19. Because of economic importance, the new biotechnological methods are applied in this industry. SSC technology in sheep has been more limited. These cells are the only cells in adults capable of transmitting genetic information to future generations20. Also, SSC technology with genetic engineering might be used to impede paternal transmission of genomic diseases3.
The number of SSCs in seminiferous tubules is 0.3%21. For cell handling, the culture and proliferation of these cells are necessary. There are many researches about the isolation and culture of spermatogonial cells in rodent, bovine, ovine and other species4,17,21. But, there are many differences among species of animals and isolation, culture and freezing methods for different species are needed to be set up. In this study, after one week of co-culture, the colonies of spermatogonial cells were appeared. Medium was changed every 3 days and colony diameter was growing slowly. This finding is in agreement with the reports by Koruji et al in rodents25. PLZF was detected in ovine spermatogonial cells which was in line with Costoya et al’s22, Phillips et al’s23 and Ketkar et al’s24 findings. In the testis, PLZF expression is restricted to As, Apr and Aal undifferentiated spermatogonia, including SSCs25. PLZF’s role in spermatogonia may be the maintenance of an undifferentiated state26, similar to the role suggested for PLZF in haematopoietic precursor cells27.
In other researches, Oct-4 was used as a specific marker for identification of bovine spermatogonial stem cells15,28. Similarly, in the present study, sheep spermatogonial stem cells were positive for anti Oct-4 immunoflourscent staining.
In addition, the presence of vimentin was analyzed in sertoli cells of lamb testes, using specific antibodies and immunocytochemistry staining. Vimentin was prominently present in sertoli cells in all cases investigated. These results are in line with Vogl et al’s29, Aumuller et al’s30 and Rogatsch et al’s31 findings. According to Wayne Vogl et al, unlike most other epithelia where the intermediate filaments are of keratin type, intermediate filaments in mature sertoli cells are of the vimentin type32. Vimentin filaments play an important role in the adaptation of sertoli cells to the varying configurations of neighboring cells during spermatogenesis as well as under pathological conditions30.
Due to diminishing cell recovery following the freeze/thaw procedure, the viability rate of frozen/thawed cells was lower than freshly isolated cells. Reduced cell recovery following the freeze/thaw procedure was also reported by others6.
SSCs’s colony number declined during the 2nd and 3rd weeks of culturing, probably due to apoptosis or detachment of differentiating germ cells11. So, for long term studies, establishment of a freezing method for long term preservation of these cells is needed. It seems that freezing medium containing 90% FBS and 10% DMSO is suitable for freezing procedure.
Conclusion
The field of SSC technologies provides the tools for genetic improvement of sheep herds and multiple opportunities for research. Despite all the challenges that the development of SSC technologies raise, still many questions are open as how sheep work in a biotechnological setting. Progress in the field of sheep reproduction biotechnology will probably derive new applications not only for use in sheep but also in other ruminants, which are of great importance to the food production chains of many developing countries. In this study, a standard method for isolation and in vitro proliferation of SSCs in Ghezel sheep was developed.
Acknowledgement
This work was supported by Iran National Science Foundation (INSF).
References
- 1.Baneh H, Hafezian SH, Rashidi A, Gholizadeh M, Rahimi GH. Estimation of genetic parameters of body weight traits in ghezel sheep. Asian Aust J Anim Sci 2010;23 (2):149–153. [Google Scholar]
- 2.Aponte PM, de Rooij DG. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod 2008;5(1/2):16–22. [Google Scholar]
- 3.Mulder CL, Zheng Y, Jan SZ, Struijk RB, Repping S, Hamer G, et al. Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update 2016;22(5):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology 2006;66(9):2091–2103. [DOI] [PubMed] [Google Scholar]
- 5.Koruji M, Movahedin M, Mowla SJ, Gourabi H. Colony formation ability of frozen thawed spermatogonial stem cell from adult mouse. Int J Reprod BioMed 2007;5(3): 109–115. [Google Scholar]
- 6.Avarbock MR, Brinster CJ, Brinster RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med 1996;2(6):693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagano M, Brinster RL. Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS 1998;106(1):47–55. [DOI] [PubMed] [Google Scholar]
- 8.Nagano M, Avarbock MR, Leonida EB, Brinster CJ, Brinster RL. Culture of mouse spermatogonial stem cells. Tissue Cell 1998;30(4):389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA 2001;98:13090–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction 2002; 124(1):85–94. [PubMed] [Google Scholar]
- 11.Aponte PM, Soda T, van de Kant HJ, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology 2006;65(9):1828–1847. [DOI] [PubMed] [Google Scholar]
- 12.Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev 2003;64(4):422–428. [DOI] [PubMed] [Google Scholar]
- 13.Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod 2002;66(1):21–28. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Luo F, Zhang Y, Wu S, Liu L, Batu B, et al. Purification and Culture of Sheep Spermatogonial Stem Cells. Biol Reprod 2011;85(1):785. [Google Scholar]
- 15.Qasemi-Panahi B, Tajik P, Movahedin M, Moghaddam GH, Geranmayeh MH. Study of insulin-like growth factor 1 effects on bovine type A spermatogonia proliferation and viability. Turk J Vet Anim Sci 2014;38:693–698. [Google Scholar]
- 16.Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari tafti Z, et al. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol Histol 2011;2(6):126. [Google Scholar]
- 17.Tajik P, Barin A, Movahedin M, Zarnani AH, Hadavi R, Moghaddam G, et al. Nestin, a neuroectodermal stem cell marker, is expressed by bovine sertoli cells. Comp Clin Pathol 2012;21:395–399. [Google Scholar]
- 18.Izadyar F, Matthijs-Rijsenbilt JJ, Den Ouden K, Creemers LB, Woelders H, de Rooij DG. Development of a cryopreservation protocol for type A spermatogonia. J Androl 2002;3(4):537–545. [PubMed] [Google Scholar]
- 19.Najafi Gh, Cedden F, Mojtahedi S, Aliverdinasab Ramin. Estrus synchronization and twinning rate of ghezel ewes treated with CIDR and PMSG during the breeding season. Online J Anim Feed Res 2014;4(6):144–149. [Google Scholar]
- 20.Dobrinski I. Transplantation of germ cells and testis tissue to study mammalian spermatogenesis. Anim Reprod 2006;3(2):135–145. [Google Scholar]
- 21.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000;21 (6):776–798. [PubMed] [Google Scholar]
- 22.Costoya JA, Hobbs RH, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004;36 (6):653–659. [DOI] [PubMed] [Google Scholar]
- 23.Phillips BT, Gassei K, Orwing KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010;365(1546):1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketkar AA, Reddy KVR. Expression pattern of OCT-4 and PLZF transcription factors during the early events of spermatogenesis in mice. J Cell Sci Ther 2012;3:120–126. [Google Scholar]
- 25.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36(6):647–652. [DOI] [PubMed] [Google Scholar]
- 26.Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol 2007;27(19):6770–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, et al. Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood 1995;86(12):4544–4552. [PubMed] [Google Scholar]
- 28.Qasemi-Panahi B, Tajik P, Movahedin M, Moghaddam G, Barzgar Y, Heidari-Vala H. Differentiation of bovine spermatogonial stem cells into osteoblasts. Avicenna J Med Biotech 2011;3(3):149–153. [PMC free article] [PubMed] [Google Scholar]
- 29.Vogl AW, Vaid KS, Guttman JA. The sertoli cell cytoskeleton. Adv Exp Med Biol 2008;636:186–211. [DOI] [PubMed] [Google Scholar]
- 30.Aumüller G, Steinbrück M, Krause W, Wagner HJ. Distribution of vimentin-type intermediate filaments in Sertoli cells of the human testis, normal and pathologic. Anat Embryol (Berl) 1988;178(2):129–136. [DOI] [PubMed] [Google Scholar]
- 31.Rogatsch H, Jezek D, Hittmair A, Mikuz G, Feichtinger H. Expression of vimentin, cytokeratin, and desmin in Sertoli cells of human fetal, cryptorchid, and tumour-adjacent testicular tissue. Virchows Arch 1996;427(5): 497–502. [DOI] [PubMed] [Google Scholar]
- 32.Wayne VA, Kuljeet SV, Julian AG. The sertoli cell cytoskeleton: molecular mechanisms in spermatogenesis. In: Cheng CY, editor. Advances in experimental medicine and biology. New York: Springer; 2008. p. 186–211. [DOI] [PubMed] [Google Scholar]


