Abstract
Background
A recent study reported that patients with higher cortisol levels on the 1st postoperative morning after cardiac surgery exhibited an increased risk of early postoperative cognitive decline (POCD). Therefore, we conducted the current study to gain further insight into the stress response to a surgical procedure as a potential risk factor for early POCD after cardiac surgery.
Material/Methods
This prospective cohort study enrolled 125 patients undergoing elective cardiac surgery with or without cardiopulmonary bypass (CPB). Patient serum cortisol levels were determined 1 day before surgery (at 08: 00) and on the 1st (at 08: 00, 16: 00 and 24: 00), 3rd (at 08: 00), and 5th (at 08: 00) postoperative days. A battery of 9 neuropsychological tests were used to assess the participants 2 days before the surgical procedure and on the 6th postoperative day. POCD was defined as a decrease in performance of 1 SD or greater between the postoperative and preoperative z scores on at least 1 neuropsychological test. A mixed-design ANOVA was used to determine the correlations of the perioperative cortisol levels with the occurrence of POCD and with the surgical technique performed.
Results
Mixed-design ANOVA showed no statistically significant differences in the cortisol levels between non-POCD and POCD patients (F=0.52, P=0.690) or between patients with and without CPB (F=2.02, P=0.103) at the 6 perioperative time points.
Conclusions
The occurrence of early POCD and the use of CPB were not associated with significantly higher cortisol levels in the repeated measurement design.
MeSH Keywords: Cardiac Surgical Procedures, Cardiopulmonary Bypass, Cognition Disorders
Background
Unlike other complications following cardiac surgery, the reported incidence of postoperative cognitive decline (POCD) has been consistent over the years, affecting up to 60% of patients in the early postoperative period; therefore, this incidence of POCD has become an intriguing issue [1–3]. A POCD diagnosis includes memory difficulties as well as a general slowing of information processing; thus, a specific sensitive neuropsychological test battery that examines different cognitive domains must be used for cognitive evaluations. Because there is no gold standard established for the diagnosis of POCD, defining it becomes a controversial matter; as a result, the reported POCD incidence varies extensively among studies [1,4]. Although the importance of POCD was underestimated in the beginning, POCD has been shown to be associated with markedly adverse outcomes, including increased mortality. In addition to interfering with a patient’s ability to perform daily functions and leading to an early retirement, POCD also represents a noticeable burden on the healthcare system [5,6].
Until recently, POCD following cardiac surgery was attributed to physiological disturbances associated with the cardiopulmonary bypass (CPB) technique [6]. However, several randomized studies have been unable to identify any beneficial cognitive effect associated with avoiding CPB [5–9]. The surgical procedure stimulates a series of hormonal and inflammatory changes that constitute the stress response, in which cortisol plays a key role [10]. Chernow et al. demonstrated that the cortisol response reflects the degree of surgical trauma [11]. Cardiac surgery significantly stimulates the endogenous release of cortisol [12]. Cognition is impaired by the sustained elevation of glucocorticoid levels [13]; therefore, the prolonged and pronounced stress response in cardiac surgical patients may play an important role in the development of POCD. Mu et al. reported that patients with higher cortisol levels on the 1st postoperative morning had an increased risk of early POCD after coronary artery bypass graft surgery (CABG) [14].
To gather a deeper understanding of the potential involvement of the stress response to a surgical procedure in the pathogenesis of POCD, we measured the perioperative cortisol levels at multiple time points. We hypothesized that higher postoperative cortisol levels are related to the occurrence of early POCD after cardiac surgery.
Material and Methods
Study design and participants
The current prospective cohort study was performed at the University Hospital of Split, Croatia, between March 2015 and June 2016. Ethical approval for this study (Ethical Committee No. 2181-147-01/06/J.B.-16-2) was provided by the Ethics Committee of the University Hospital of Split on March 10, 2015. All the patients provided written informed consent.
The study enrolled patients aged between 41 and 84 years who were scheduled for an elective CABG, heart valve surgery, or combined surgery (CABG and valve surgery) with or without CPB. Exclusion criteria were any cerebrovascular incident in the last 3 years; visual, hearing or motor impairment interfering with cognitive assessment; mental illness; previous cardiac or carotid surgery; left ventricular ejection fraction of less than 35%; adrenal gland disease; steroid treatment for longer than 7 days in the past year; alcohol (>20 g per day or >150 g per week) or controlled substance abuse; a preoperative Mini Mental State Examination (MMSE) score less than 26 points; a preoperative Beck Depression Inventory-Second Edition (BDI-II) score more than 19 points; perioperative stroke; and steroid treatment throughout the study period.
Surgery and anesthesia
For all the patients, the procedure began at 08: 00. Anesthesia was induced and subsequently maintained with fentanyl, midazolam, vecuronium, and sevoflurane. The depth of anesthesia was titrated to achieve a bispectral index between 40 and 55. Access to the heart was achieved via a median sternotomy. Heparin (1.5 mg/kg) was administered prior to cannulation to achieve an activated clotting time greater than 280 s, and the effect of heparin was reversed with an equivalent dose of protamine sulfate at the time of decannulation. CPB was performed using a non-pulsatile roller pump (Terumo Europe N.V., Eschborn, Germany) equipped with microporous membrane oxygenators containing integrated 40-μM arterial line filters and heparin-coated circuits (Carmeda; Medtronic Inc., Minneapolis, MN, USA). A perfusion flow rate of 2.2 to 2.4 L/min/m2 was applied. Myocardial protection was achieved with intermittent antegrade and occasionally retrograde blood cardioplegia. During CPB, the pH-stat technique was used, along with maintenance of normothermia or spontaneous hypothermia (as low as 32°C). Noradrenaline was used to maintain mean arterial pressure within the range of 60 to 80 mmHg during CPB or above 70 mmHg during beating-heart surgery. As required, dobutamine was used for inotropic support. In patients undergoing surgery without CPB, distal anastomoses were performed with the help of an Octopus tissue stabilizer (Medtronic Inc.). Proximal anastomoses were fashioned onto the aorta by means of a single side-clamp. During beating-heart surgery, the same principles of heparinization and neutralization were applied, while the core temperature was maintained near normothermia (36ºC to 37ºC).
Neuropsychological assessment
According to the Statement of Consensus on the Assessment of Neurobehavioral Outcomes after Cardiac Surgery [15], we used a validated battery of 6 neuropsychological tests, including 9 main variables to assess global cognitive status, short-term and intermediate-term memory, attention, concentration, and psychomotor skills. The evaluation was based on the following tests: MMSE, Rey Auditory Verbal Learning Test (RAVLT, immediate and delayed recall), the Wechsler Memory Scale and its 3 subscales (Visual Memory Span, Digit Span Forward, Digit Span Backward), Symbol Digit Modalities Test (SDMT), Trail Making Test A (TMT A) [16] and computerized PsychE (simple reaction time) test [17]. Validated Croatian editions of the neuropsychological tests were used, as appropriate. Alternate forms of all the applied tests, except the TMT A, were used to reduce learning effects across sessions (refer to Supplementary Table 1, Supporting Information, for an explanation of the different tests and the cognitive domains examined). As in our previous study [18], 2 days before the surgical procedure, neuropsychological tests and the BDI-II were conducted to assess the participants. Neuropsychological tests were repeated on the 6th postoperative day when the patients were free of sedative and pain medications and chest drains and were able to walk and sit in a chair. A battery of tests of approximately 40 min duration was administered by a neuropsychologist from our hospital in a standardized fashion at the same time of the day (10: 00) and in the same quiet room of the cardiac surgery ward.
Cortisol measurements
Cortisol levels in the patients were determined 1 day before surgery (at 08: 00) and the 1st (at 08: 00, 16: 00, and 24: 00), 3rd (at 08: 00), and 5th (at 08: 00) postoperative days. For each sample, 5 mL of venous blood was collected in a biochemistry tube with gel to determine the serum cortisol concentration using a sandwich electro-chemiluminescence immunoassay (ECLIA) method (Cobas e601; Roche Diagnostics GmbH, Mannheim, Germany). The serum was prepared via centrifugation (Z 400; Hermle Labortechnik GmbH, Wehingen, Germany) at 3500 rpm for 15 min. The intra-assay and inter-assay coefficients of variation were less than 2.8% and 1.7%, respectively. In the hospital laboratory in which the measurement was performed, the normal cortisol ranges between 07: 00 and 10: 00, between 16: 00 and 18: 00, and at 24: 00 are 171 to 536 nmol/L, 64 to 327 nmol/L, and 41 to 69 nmol/L, respectively [19].
Primary outcome measure
The primary aim of the current study was to assess differences in changes of perioperative cortisol levels between patients who developed POCD on the 6th day after cardiac surgery and those who did not. In contrast to a previous study that only measured cortisol levels at 1 time point after cardiac surgery [14], we used a repeated measurement design with cortisol levels at baseline and 5 postoperative time points.
Given the varied definitions used to evaluate POCD [1,4,6], we selected a definition similar to that used by Knipp et al. [3]. Changes in cognitive performance or within-patient changes were expressed as differences between 2 testing points (baseline and the 6th postoperative day). First, preoperative and postoperative cognitive test results based on the mean and standard deviation (SD) of the scores on 9 neuropsychological tests were transformed into z scores. For timed tests (TMT A, simple reaction time), the sign of the z score was reversed such that improved performance resulted in a higher score in all tests. Then, we calculated the difference in z scores between those 2 measurements (the later score minus the earlier score). POCD was defined as a decrease in performance of 1 SD or greater in postoperative z scores compared to preoperative z scores in 1 or more neuropsychological tests. Furthermore, to better understand the methodological impact of the different applied definitions on the POCD incidence, we performed 2 additional analyses using the strict POCD definition (i.e., as a decrease in performance of 1 SD or greater between 2 testing points in at least 2 and 3 tests, respectively) [6].
Secondary outcome measure
The secondary aim of this study was to assess differences in changes of perioperative cortisol levels between patients with and without CPB.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics, version 24.0 (IBM Corp., Armonk, NY, USA) and RStudio (RStudio Inc., Boston, MA, USA). Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as the mean and SD or median and interquartile range (IQR). The independent samples t-test, the Mann-Whitney U test and the chi-square test were used as appropriate to estimate significant differences in demographic, clinical, surgical, and postoperative characteristics between non-POCD and POCD patients. Variables that were significantly associated with POCD in univariate analyses were included in a multivariate logistic regression analysis (Enter method) to determine the risk-adjusted predictors of POCD. A general linear model (GLM) mixed-design (between- and within-subjects) analysis of variance (ANOVA) was used to test the primary and secondary outcomes, i.e., to determine any differences in the cortisol levels and neuropsychological test results between non-POCD and POCD patients and, likewise, in the cortisol levels between patients with and without CPB, in repeated experimental conditions. Levene’s test for checking the variance equality of the cortisol levels and neuropsychological test results across samples was not significant. Because our data included some legitimate, mostly mild cortisol outliers at the top end of the distribution, we also conducted a sensitive mixed ANOVA analysis without outliers to check for any interference from the outlying observations on the primary and secondary outcomes. Bonferroni correction was applied for the post hoc pairwise comparisons included in the mixed ANOVA. Statistical values were considered significant at 95% (2-sided P<0.05).
According to previous studies [1–3], we expected POCD to occur in 50% of the patients and assumed an equal number of patients in each group. Furthermore, we assumed moderate differences in the perioperative cortisol level changes between non-POCD and POCD patients based on the calculated effect size of 0.23 from the study of Mu et al. [14]. To test this association between POCD and cortisol levels using a 2×6 mixed between- and within-subjects, ANOVA at a statistical significance level of 0.05 and 90% power, we required a total sample size of 118 patients. Considering an estimated attrition rate of less than 5% at the time of hospital discharge [2,14,18], the final sample size was increased to a total of 125 patients. G*Power 3.1.9.2 (Heinrich-Heine-Universität Düsseldorf, Germany) was used to calculate the required sample size.
Results
Study population
During the study period, 163 patients were screened, and 145 of these patients matched the selection criteria. Among the eligible patients, 125 provided written informed consent, passed the MMSE exclusion criteria and were enrolled in the study. Three patients died in the early postoperative period, resulting in an overall in-hospital mortality rate of 2.4%. Two (1.6%) patients experienced a stroke during the perioperative period. Ultimately, we analyzed the data on 120 patients. The flow of patients through the study is shown in Figure 1, and the baseline demographic, clinical, surgical, and postoperative characteristics of the patients are presented in Table 1.
Figure 1.
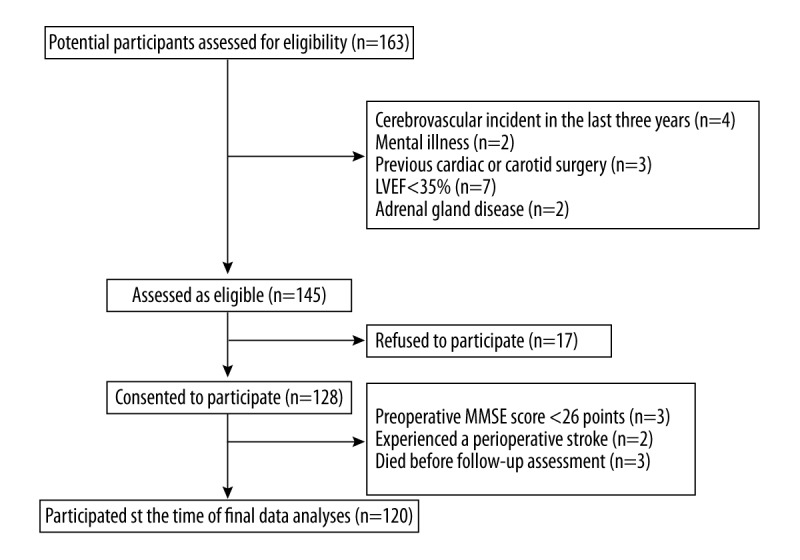
Patient recruitment flow chart. LVEF – left ventricular ejection fraction; MMSE – Mini Mental State Examination.
Table 1.
Demographic, clinical, surgical and postoperative characteristics.
| Non-POCD patients (n=54) | POCD patients (n=66) | p Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age; years | 62.1±8.4 | 65.6±9.9 | 0.040 |
| Male sex | 45 (83.3%) | 51 (77.3%) | 0.409 |
| Weight; kg | 85.0±13.6 | 85.8±11.4 | 0.743 |
| Height; cm | 176.9±8.0 | 175.5±8.2 | 0.343 |
| Elementary education | 9 (16.7%) | 13 (19.7%) | 0.667 |
| Secondary education | 38 (70.3%) | 39 (59.1%) | 0.201 |
| Higher education | 7 (13.0%) | 14 (21.2%) | 0.238 |
| Active smoking | 19 (35.2%) | 16 (24.2%) | 0.190 |
| Clinical characteristics | |||
| General anesthesia | 14 (25.9%) | 14 (21.2%) | 0.542 |
| Hypertension | 38 (70.4%) | 48 (72.7%) | 0.776 |
| Insulin-dependent diabetes mellitus | 4 (7.4%) | 3 (4.5%) | 0.503 |
| Non-insulin-dependent diabetes mellitus | 16 (29.6%) | 15 (22.7%) | 0.390 |
| Hyperlipidemia | 42 (77.8%) | 47 (71.2%) | 0.414 |
| Carotid artery disease | 33 (61.1%) | 37 (56.1%) | 0.575 |
| Peripheral vascular disease | 7 (13.0%) | 5 (7.6%) | 0.328 |
| Atrial fibrillation | 9 (16.7%) | 9 (13.6%) | 0.644 |
| Myocardial infarction | 25 (46.3%) | 26 (39.4%) | 0.447 |
| LVEF; % | 62.5±11.8 | 61.6±10.2 | 0.651 |
| EuroSCORE | 2.0 (1.2–3.0) | 2.2 (1.2–3.9) | 0.562 |
| Surgical characteristics | |||
| CABG | 41 (75.9%) | 38 (57.6%) | 0.035 |
| Heart valve surgery | 8 (14.8%) | 18 (27.3%) | 0.099 |
| CABG and valve surgery | 5 (9.3%) | 10 (15.1%) | 0.332 |
| Surgery with CPB | 20 (37.0%) | 36 (54.5%) | 0.056 |
| CPB duration; min | 99.1±32.6 | 102.1±34.8 | 0.755 |
| Cross-clamp duration; min | 62.4±20.2 | 66.9±28.1 | 0.564 |
| Lowest BIS | 30.3±7.8 | 31.2±6.4 | 0.515 |
| Lowest temperature; ºC | 34.5±2.5 | 33.9±2.4 | 0.167 |
| Lowest MAP; mmHg | 57.2±8.8 | 55.2±9.2 | 0.225 |
| Lowest hematocrit; % | 30.0±6.3 | 27.0±5.9 | 0.007 |
| Highest glucose level; mmol/l | 9.6±3.6 | 9.4±2.8 | 0.813 |
| Insulin administered | 2 (3.7%) | 4 (6.1%) | 0.556 |
| Vasopressor administered | 25 (46.3%) | 45 (68.2%) | 0.016 |
| Inotropic agent administered | 21 (38.9%) | 39 (59.1%) | 0.028 |
| Blood transfusion; ml | 401.2±519.4 | 654.7±604.4 | 0.016 |
| SVRi; dyne·sec/cm5/m2 | 1496.8±432.9 | 1531.3±579.7 | 0.719 |
| Surgery duration; min | 212.9±53.9 | 232.3±67.3 | 0.089 |
| Postoperative characteristics | |||
| Drainage in the first 12 hours; ml | 501.5±654.3 | 538.7±495.7 | 0.724 |
| Duration of m.v. in the ICU; h | 17.3±8.5 | 21.1±13.2 | 0.073 |
| Failed weaning from m.v. in the ICU | 0 (0.0%) | 2 (3.0%) | 0.197 |
| Time to extubation; h | 18.4±9.1 | 22.3±13.5 | 0.072 |
| Length of ICU stay; h | 51.8±24.8 | 61.7±36.8 | 0.095 |
| Length of hospital stay; d | 10.8±2.9 | 11.2±3.0 | 0.431 |
Values are presented as the number (percentage), mean (SD) or median (IQR). Levels of education according to the Ministry of Science, Education and Sports of the Republic of Croatia range from elementary to secondary and higher education. General anesthesia refers to its administration within the past five years. POCD – postoperative cognitive decline; LVEF – left ventricular ejection fraction (value is the result of echocardiography, Simpson’s method); EuroSCORE – European system for cardiac operative risk evaluation; CABG – coronary artery bypass graft surgery; CPB – cardiopulmonary bypass; BIS – bispectral index; MAP – mean arterial pressure; SVRi – systemic vascular resistance index (value is the index value at the end of the surgical procedure); m.v. – mechanical ventilation; ICU – intensive care unit.
Primary and secondary outcomes
The repeated-measures GLM ANOVA with 2 factors, POCD and CPB, showed that there was no significant impact of the cortisol level. There were no significant differences between non-POCD and POCD patients in the cortisol levels at the 6 perioperative time points tested (F=0.52, P=0.690, Figure 2A). The results in Figure 2A may indicate a minor difference in cortisol levels on the 3rd postoperative morning between non-POCD and POCD patients. Although this difference is statistically significant when analyzed with a bivariate t-test (P=0.013), this result is not significant in the GLM and logistic regression analysis (odds ratio, 1.0; 95% confidence interval, 1.0–1.002; P=0.143); thus, the result of the t-test is considered a false positive. Figure 2B shows that there were no significant differences between patients with and without CPB in the cortisol levels at the 6 perioperative time points (F=2.02, P=0.103).
Figure 2.
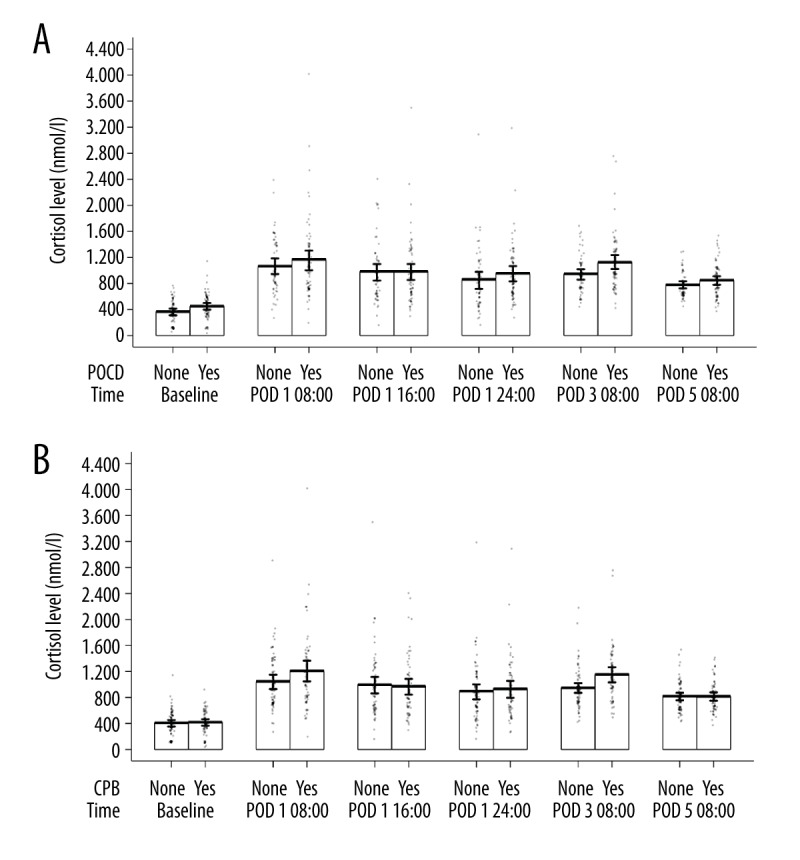
(A) Repeated measures of the cortisol levels according to the cognitive outcome. GLM ANOVA showed no statistically significant difference in the cortisol levels between non-POCD and POCD patients at 6 perioperative time points (F=0.52, P=0.690). POCD – postoperative cognitive decline; POD – postoperative day. (B) Repeated measures of the cortisol levels according to the surgical technique. GLM ANOVA showed no statistically significant difference in the cortisol levels between patients with and without CPB at 6 perioperative time points (F=2.02, P=0.103). CPB – cardiopulmonary bypass; POD – postoperative day.
Sensitivity analysis
After the outliers in the cortisol levels were removed from the analyzed data, there were still no significant differences in the cortisol levels at the 6 perioperative time points between the non-POCD and POCD patients (F=0.58, P=0.667) or between the patients with and without CPB (F=1.65, P=0.164).
Cognitive outcome
On the 6th postoperative day, 66 of the 120 (55.0%) patients fulfilled the diagnostic criteria for POCD. The patient scores on the different neuropsychological tests are presented in Table 2. GLM ANOVA showed a statistically significant interaction effect between the repeated measures and POCD factors in the MMSE, RAVLT delayed recall, Digit Span Backward task, SDMT, TMT A and simple reaction time. The post hoc analysis of the neuropsychological test battery results did not show significant differences in any baseline test between the non-POCD and POCD patients. However, analysis on the 6th postoperative day showed significant differences in the MMSE score, Visual Memory Span score, Digit Span Backward score, SDMT result and simple reaction time between the non-POCD and POCD patients. Finally, after a strict POCD definition was applied, cognitive impairment was present in 23 of the 120 patients (19.2%) and in 9 of the 120 patients (7.5%).
Table 2.
Neuropsychological test results.
| Test | Baseline | p Value* | Postoperative day 6 | p Value* | p Value** | ||
|---|---|---|---|---|---|---|---|
| Non-POCD patients (n=54) | POCD patients (n=66) | Non-POCD patients (n=54) | POCD patients (n=66) | ||||
| MMSE | 28.3±1.2 | 28.2±1.2 | 0.999 | 28.2±1.1 | 27.0±1.6 | <0.001 | <0.001 |
| RAVLT immediate recall score | 41.6±8.4 | 40.8±9.4 | 0.999 | 38.0±9.1 | 34.2±10.2 | 0.160 | 0.071 |
| RAVLT delayed recall score | 7.4±3.2 | 7.6±2.9 | 0.999 | 5.8±2.9 | 4.8±3.1 | 0.455 | 0.026 |
| WB sp-Visual Memory Span | 10.1±3.1 | 8.8±3.6 | 0.246 | 10.1±3.4 | 7.7±4.0 | 0.002 | 0.056 |
| WB sp-Digit Span Forward | 6.9±1.2 | 7.0±1.2 | 0.999 | 6.9±1.2 | 6.7±1.0 | 0.999 | 0.218 |
| WB sp-Digit Span Backward | 5.0±1.2 | 5.0±1.4 | 0.999 | 5.2±1.3 | 4.3±1.6 | 0.004 | <0.001 |
| SDMT | 33.1±12.3 | 31.7±10.5 | 0.999 | 32.8±12.7 | 25.7±10.2 | 0.005 | <0.001 |
| TMT A | 40.5±17.7 | 44.6±25.9 | 0.999 | 40.3±19.5 | 50.6±26.3 | 0.093 | 0.002 |
| Simple reaction time | 1252.8±378.5 | 1321.8±454.2 | 0.999 | 1276.5±367.8 | 1594.4±584.9 | 0.002 | 0.001 |
Values are presented as the mean (SD). Higher scores indicate better test performance, except for TMT A and simple reaction time, for which a shorter time indicates a better performance.
P value from the GLM ANOVA with Bonferroni adjustment refers to comparison of the neuropsychological test results between non-POCD and POCD patients at baseline and the 6th postoperative day.
P value from the GLM ANOVA refers to the interaction effect between the repeated measures and POCD factors.
POCD – postoperative cognitive decline; MMSE – Mini Mental State Examination; RAVLT – Rey Auditory Verbal Learning Test; WB sp – Wechsler Memory Scale; SDMT – Symbol Digit Modalities Test; TMT A – Trail Making Test A.
Predictors of POCD
Variables that were significantly associated with an occurrence of POCD after univariate analysis included a high cortisol level on the 3rd postoperative morning, old age, CABG as the surgery type, low hematocrit, vasopressor or inotropic agent administration, and blood transfusion during the surgical procedure. However, after a multivariate logistic regression analysis was conducted, no variables were significantly associated with the occurrence of POCD.
Discussion
The current prospective cohort study including 125 cardiac surgery patients showed that there is no association between perioperative cortisol levels and POCD occurrence on the 6th postoperative day. In addition, this study demonstrated that cardiac surgery stimulates a prolonged and extremely pronounced cortisol response disrupting the circadian rhythm regardless of the applied surgical technique.
Previous studies have noted that aging is associated with the loss of neurons and glucocorticoid receptors in the hippocampal region and with impaired hypothalamic-pituitary-adrenal axis feedback inhibition [20,21]. Hence, the primary obstacle in achieving endocrine homeostasis in the elderly population might be a prolonged response to stress. This stress response could be important in the development of POCD following cardiac surgery [14], given that prolonged exposure to high concentrations of glucocorticoids can be toxic to neural structures, particularly the glucocorticoid receptor-rich hippocampus [13,22], which is essential for certain types of memory [23].
To our knowledge, this is the 2nd study to investigate the association between cortisol levels and cognitive outcome in cardiac surgical patients. Mu et al. reported that patients with higher cortisol levels on the 1st postoperative morning exhibited an increased risk of early POCD [14]. Furthermore, the same investigators also found a correlation between higher postoperative cortisol levels and the development of delirium [24], which is closely related to the occurrence of early POCD [4,25]. However, previous studies did not monitor preoperative cortisol levels, and the postoperative cortisol levels were only examined at 1 time point after cardiac surgery [14,24]. Contrary to Mu et al., analysis of the repeated cortisol measurements in the current study did not show a correlation among the cortisol levels and POCD. Significantly, our results showed that, regardless of the cognitive outcome, the cortisol levels of the patients were heterogeneous, as exhibited by the large SDs and confidence intervals at each postoperative time point. Using a bivariate t-test, we observed a statistically significant difference in cortisol levels between non-POCD and POCD patients at only 1 time point, the 3rd postoperative morning, but that difference lacked significance when other factors, such as genuine outliers, confidence intervals, post hoc correction within the GLM model, sensitivity analysis without outliers, and logistic regression were considered. Therefore, in our opinion, it is unlikely that the stress response to cardiac surgery represents an exclusive trigger for POCD occurrence; however, recent findings indicate that interdependent mechanisms, such as inflammatory and stress responses, through interaction effects, may be involved in the POCD pathogenesis [1,6,18,26].
In unstressed healthy individuals, cortisol secretion follows a typical circadian rhythm, with a sharp rise after awakening and a slow decline thereafter, with the lowest levels around midnight [19]. Here, we noted a narrow cortisol oscillation during the 1st postoperative day with a level thirteenfold higher than the normal range at midnight. Cortisol levels on the 5th postoperative morning remained 2-fold higher than the preoperative levels; thus, our results support and extend previous knowledge that cardiac surgery stimulates a prolonged and severe cortisol response with an altered circadian pattern [12].
We did not identify significant differences in the postoperative cortisol levels between patients with and without CPB. Our results, in contrast to those of Hoda et al. [27], support the findings of Song et al. [28], who found that cardiac manipulation during beating-heart surgery may lead to significant hemodynamic impairment reflected by a pronounced cortisol response, which therefore negates the benefits of avoiding CPB in terms of the stress hormone response.
In the current study, the incidence of POCD was well within the range recently reported [1–3]. At baseline, the non-POCD and POCD patients had identical neuropsychological test results, supporting the hypothesis that the surgical procedure has a significant impact on the occurrence of POCD [1,6]. The follow-up analysis showed significant differences between the non-POCD and POCD patients in global cognitive status and on the domains of psychomotor speed, attention, visual short-term and working memory. Interestingly, some tests showed significant differences in certain domains between the non-POCD and POCD patients, whereas similar tests were unable to do so, probably because each test measures a series of cognitive functions with different sensitivity [4,16]. We also showed that applying a strict definition of cognitive deterioration significantly reduced the incidence of POCD, suggesting that skewed results may be produced by excluding truly declined patients; therefore, this approach is too conservative for studies with this or a similar design pattern.
A strength of the current study is that no patients refused to participate on the 6th postoperative day, and only five (4%) patients were lost to follow-up for reasons such as death or stroke, which is comparable to recently reported results [2,14,18].
The current study has several possible limitations. We examined the association of the cortisol response to cardiac surgery coincident with the occurrence of only early POCD, because the cause of later cognitive impairment might include not only the effects of the surgery but also the effects of natural aging, the progression or new silent incidence of cardiovascular disease or cerebrovascular disease, or the development of dementia [1,4,29,30]. Additionally, some might argue that the different surgical techniques have varying risks for POCD, but the current study, as well as several recent studies, did not identify a difference in the rates of POCD after cardiac surgery with or without CPB [5–9]. Finally, this study did not include a non-surgical control group, given that the inclusion of a non-surgical control group may not be appropriate in light of the complicated physiological processes attributed to the surgical procedure [31].
Conclusions
Although this prospective cohort study with a longitudinal assessment of the perioperative cortisol level in cardiac surgical patients did not reveal a significant correlation between prolonged and pronounced cortisol responses to surgical procedure and occurrence of early POCD, we cannot exclude the possibility that a synergistic effect of overlapping processes, such as inflammatory and stress responses, may underlie POCD. Therefore, we believe that our findings should encourage future research to further investigate the role of complicated and interdependent mechanisms in the development of POCD following cardiac surgery.
Supplementary Table
Supplementary Table 1.
Neuropsychological test battery.
| Task | Domain | Explanation |
|---|---|---|
| Mini Mental State Examination (MMSE) [16] | Global measure of cognition | This test includes 30 simple questions and tasks related to several topics (orientation in time and place, repetition and recall of a list of words, arithmetic, language use and comprehension, and non-verbal memory). The summary score was analyzed |
| Rey Auditory Verbal Learning Test (RAVLT) [16] | Verbal memory, learning, recall efficiency | A list of 15 meaningful monosyllabic words is presented in 5 trials. Each trial ends with a free recall of the words (immediate recall). After a period of 20 min following the 5th trial, the participant is requested to recall as many words as possible (delayed recall). The main variables used are the total number of correct words in the 5 immediate trials and the total number of correct words in the delayed recall trial |
| Wechsler Memory Scale (WB sp) – Visual Memory Span [16] WB sp-Digit Span Forward [16] WB sp-Digit Span Backward [16] |
Visual short-term memory Attention, short-term memory Attention, working memory |
This test requires subjects to reproduce a series of three geometric shapes from memory after 10 sec of exposure to the shapes. Increasingly long sequences of random numbers are read to the participant, who is then asked to recall the sequence. This test terminates when a participant fails to recall two sequences of the same length. In the backward subtest, participants must repeat the number sequence in reverse order |
| Symbol Digit Modalities Test (SDMT) [16] | Psychomotor speed, attention | The participant must fill in blanks according to a key in which a symbol corresponds to a digit. The number of filled blanks within 90 sec is recorded |
| Trail Making Test A (TMT A) [16] | Psychomotor speed, attention, concentration | The participant must draw a trail with a pencil, connecting the numbered circles in numerical order. The time to complete the task was analyzed |
| PsychE-simple reaction time [17] | Psychomotor speed, reaction time | The subject holds down the spacebar on the keyboard. After a random interval of 1–10 sec, a small ‘sun’ icon appears at a random position on the screen. The subject is required to lift his or her finger as quickly as possible from the spacebar and use it to press numeric key 4. For each response, the total reaction time is recorded with an accuracy of 1 ms |
Acknowledgements
The investigators gratefully acknowledge Lidija Sodic (Department of Neurology, University Hospital of Split, Croatia) for her help with the neuropsychological assessments and Daniela Supe-Domic (Department of Medical Laboratory Diagnostics, University Hospital of Split, Croatia) for her help with the laboratory analyses
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Patel N, Minhas JS, Chung EM. Risk factors associated with cognitive decline after cardiac surgery: A systematic review. Cardiovasc Psychiatry Neurol. 2015;2015:370612. doi: 10.1155/2015/370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 3.Knipp SC, Matatko N, Wilhelm H, et al. Cognitive outcomes three years after coronary artery bypass surgery: Relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85:872–79. doi: 10.1016/j.athoracsur.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 4.Polunina AG, Golukhova EZ, Guekht AB, et al. Cognitive dysfunction after on-pump operations: Neuropsychological characteristics and optimal core battery of tests. Stroke Res Treat. 2014;2014:302824. doi: 10.1155/2014/302824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinmetz J, Rasmussen LS. Peri-operative cognitive dysfunction and protection. Anaesthesia. 2016;71(Suppl 1):58–63. doi: 10.1111/anae.13308. [DOI] [PubMed] [Google Scholar]
- 6.van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67:280–93. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel N, Minhas JS, Chung EM. Intraoperative embolization and cognitive decline after cardiac surgery: A systematic review. Semin Cardiothorac Vasc Anesth. 2016;20:225–31. doi: 10.1177/1089253215626728. [DOI] [PubMed] [Google Scholar]
- 8.Lamy A, Devereaux PJ, Prabhakaran D, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013;368:1179–88. doi: 10.1056/NEJMoa1301228. [DOI] [PubMed] [Google Scholar]
- 9.Kozora E, Kongs S, Collins JF, et al. Cognitive outcomes after on- versus off-pump coronary artery bypass surgery. Ann Thorac Surg. 2010;90:1134–41. doi: 10.1016/j.athoracsur.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 10.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 11.Chernow B, Alexander HR, Smallridge RC, et al. Hormonal responses to graded surgical stress. Arch Intern Med. 1987;147:1273–78. [PubMed] [Google Scholar]
- 12.Gibbison B, Spiga F, Walker JJ, et al. Dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit Care Med. 2015;43:791–800. doi: 10.1097/CCM.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupien SJ, Maheu F, Tu M, et al. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Mu DL, Li LH, Wang DX, et al. High postoperative serum cortisol level is associated with increased risk of cognitive dysfunction early after coronary artery bypass graft surgery: Aa prospective cohort study. PLoS One. 2013;8:e77637. doi: 10.1371/journal.pone.0077637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–95. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 16.Lezak MD. Neuropsychological Assessment. 3rd ed. New York (NY): Oxford University Press; 1995. [Google Scholar]
- 17.Hope AT, Woolman PS, Gray WM, et al. A system for psychomotor evaluation; Design, implementation and practice effects in volunteers. Anaesthesia. 1998;53:545–50. doi: 10.1046/j.1365-2044.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 18.Glumac S, Kardum G, Sodic L, et al. Effects of dexamethasone on early cognitive decline after cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2017;34:776–84. doi: 10.1097/EJA.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 19.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–54. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hokkanen SRK, Hunter S, Polvikoski TM, et al. Hippocampal sclerosis, hippocampal neuron loss patterns and Tdp-43 in the aged population. Brain Pathol. 2017 doi: 10.1111/bpa.12556. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson CW, Peskind ER, Raskind MA. Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology. 1997;65:79–90. doi: 10.1159/000127167. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky RM. Stress and the brain: Individual variability and the inverted-U. Nat Neurosci. 2015;18:1344–46. doi: 10.1038/nn.4109. [DOI] [PubMed] [Google Scholar]
- 23.Wolf OT, Atsak P, de Quervain DJ, et al. Stress and memory: A selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. J Neuroendocrinol. 2016;28(8) doi: 10.1111/jne.12353. [DOI] [PubMed] [Google Scholar]
- 24.Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: A prospective cohort study. Crit Care. 2010;14:R238. doi: 10.1186/cc9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauër AC, Veldhuijzen DS, Ottens TH, et al. Association between delirium and cognitive change after cardiac surgery. Br J Anaesth. 2017;119:308–5. doi: 10.1093/bja/aex053. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E, Vig K, Racz K, et al. Influence of the postoperative inflammatory response on cognitive decline in elderly patients undergoing on-pump cardiac surgery: A controlled, prospective observational study. BMC Anesthesiol. 2017;17:113. doi: 10.1186/s12871-017-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoda MR, El-Achkar H, Schmitz E, et al. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2179–86. doi: 10.1016/j.athoracsur.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 28.Song SW, Yi G, Lee S, et al. Perioperative indicators of stress response and postoperative inflammatory complications in patients undergoing off-pump coronary artery bypass surgery: A prospective observational study. Circ J. 2008;72:1966–74. doi: 10.1253/circj.cj-08-0291. [DOI] [PubMed] [Google Scholar]
- 29.van Dijk D, Moons KG, Nathoe HM, et al. Cognitive outcomes five years after not undergoing coronary artery bypass graft surgery. Ann Thorac Surg. 2008;85:60–64. doi: 10.1016/j.athoracsur.2007.08.068. [DOI] [PubMed] [Google Scholar]
- 30.Kok WF, Koerts J, Tucha O, et al. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia. 2017;72:359–69. doi: 10.1111/anae.13712. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal JA, Madden DJ, Burker EJ, et al. A preliminary study of the effects of cardiac procedures on cognitive performance. Int J Psychosom. 1991;38:13–16. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Neuropsychological test battery.
| Task | Domain | Explanation |
|---|---|---|
| Mini Mental State Examination (MMSE) [16] | Global measure of cognition | This test includes 30 simple questions and tasks related to several topics (orientation in time and place, repetition and recall of a list of words, arithmetic, language use and comprehension, and non-verbal memory). The summary score was analyzed |
| Rey Auditory Verbal Learning Test (RAVLT) [16] | Verbal memory, learning, recall efficiency | A list of 15 meaningful monosyllabic words is presented in 5 trials. Each trial ends with a free recall of the words (immediate recall). After a period of 20 min following the 5th trial, the participant is requested to recall as many words as possible (delayed recall). The main variables used are the total number of correct words in the 5 immediate trials and the total number of correct words in the delayed recall trial |
| Wechsler Memory Scale (WB sp) – Visual Memory Span [16] WB sp-Digit Span Forward [16] WB sp-Digit Span Backward [16] |
Visual short-term memory Attention, short-term memory Attention, working memory |
This test requires subjects to reproduce a series of three geometric shapes from memory after 10 sec of exposure to the shapes. Increasingly long sequences of random numbers are read to the participant, who is then asked to recall the sequence. This test terminates when a participant fails to recall two sequences of the same length. In the backward subtest, participants must repeat the number sequence in reverse order |
| Symbol Digit Modalities Test (SDMT) [16] | Psychomotor speed, attention | The participant must fill in blanks according to a key in which a symbol corresponds to a digit. The number of filled blanks within 90 sec is recorded |
| Trail Making Test A (TMT A) [16] | Psychomotor speed, attention, concentration | The participant must draw a trail with a pencil, connecting the numbered circles in numerical order. The time to complete the task was analyzed |
| PsychE-simple reaction time [17] | Psychomotor speed, reaction time | The subject holds down the spacebar on the keyboard. After a random interval of 1–10 sec, a small ‘sun’ icon appears at a random position on the screen. The subject is required to lift his or her finger as quickly as possible from the spacebar and use it to press numeric key 4. For each response, the total reaction time is recorded with an accuracy of 1 ms |


