Abstract
Background
Facet joint degeneration (FJD) is one of the common causes of low back pain (LBP), and estrogen deficiency is one of the triggers for FJD. Calcitonin may possess the potential for treating osteoarthritis, but to date the hormone has not been studied in the treatment of FJD. Therefore, the aim of this study was to investigate the effects of salmon calcitonin (sCT) on FJD induced by estrogen deficiency after ovariectomy (OVX).
Material/Methods
Thirty female Sprague-Dawley rats were randomly assigned to 3 groups: the OVX group received bilateral OVX, the OVX + sCT group received subcutaneous administration of sCT (16 IU/kg/2 days) following bilateral OVX, and the Sham group received sham surgery. All rats were euthanized at 12 weeks post-OVX. Serum COMP level, cartilage degradation, and subchondral bone micro-architecture were evaluated.
Results
sCT relieved cartilage surface lesions, reduced histological score, and significantly increased cartilage thickness. The OVX + sCT group exhibited significantly increased expression of aggrecan, as well as significantly decreased levels of ADAMTS-4, MMP-13, and caspase-3. The results of micro-computed tomography analysis revealed that the OVX + sCT group exhibited higher BMD, BV/TV, and Tb.Th values but a lower Tb.Sp value than that of the OVX group. Serum COMP concentrations were significantly correlated with histological score and cartilage thickness.
Conclusions
sCT can inhibit the progression of FJD in OVX rats, which is attributed to its inhibitory effects on cartilage metabolism imbalance, chondrocyte apoptosis, and subchondral bone remodeling. Serum COMP has diagnostic potential for FJD.
MeSH Keywords: Calcitonin; Cartilage; Models, Animal; Ovariectomy
Background
Facet joints (FJs) are synovial joints, similar to knee joints, consisting of hyaline articular cartilage, a synovial membrane, and a joint capsule. FJs and intervertebral discs constitute a ‘three-joint complex’, a critical component of spinal motion segments, and plays an important role when the spine completes multiple motions, such as flexion, intension, and rotation [1]. Low back pain (LBP) is a major cause of activity limitation and work absence, and more than 70% of adults suffer from LBP at some point during their lifetime [2,3]. FJ degeneration (FJD), also referred to as ‘facet joint syndrome’, is regarded as one of the common causes of LBP [4].
Multiple risk factors have been proposed for FJD, such as age, overweight, and smoking [1,5]. In recent studies, researchers found FJD in ovariectomy (OVX) mice [6,7], which indicated that estrogen deficiency may also be a risk factor for FJD. Cartilage is sensitive to sex hormones [8]. Estrogen receptors have been found in lumbar FJ cartilage, and increased expression of estrogen receptors has been associated with the severity of FJD [9]. Therefore, estrogen replacement therapy appears to be a superb choice for FJD induced by estrogen deficiency. However, since the risks of hormone use remain uncertain [10], it is necessary to explore a new drug that targets FJD induced by estrogen deficiency.
Calcitonin (CT) is currently clinically used to treat osteoporosis due to its antiresorptive effects by inhibiting osteoclast activity [11]. Interestingly, CT may also have therapeutic potential in the treatment of joint degeneration. Previous experimental studies of knee osteoarthritis found that CT significantly retarded cartilage degeneration by preventing the loss of collagen, hyaluronan, and proteoglycan and suppressing the expression of matrix metalloprotease 13 (MMP-13), a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) [12–14]. Clinical studies have also indicated that CT can reduce cartilage lesions and the concentrations of biomarkers of joint degeneration in patients with knee osteoarthritis [15–17]. In view of the protective effects of CT on knee osteoarthritis, FJD may also be alleviated by CT treatment. However, to date, no in vivo study has explored the therapeutic effects of CT on FJD induced by estrogen deficiency.
The purpose of this research was to further explore the pathogenesis of FJD induced by estrogen deficiency in OVX rats and to investigate the therapeutic effects of sCT on FJD and the diagnostic value of cartilage oligomeric matrix protein (COMP) for FJD.
Material and Methods
Experimental design
All experimental procedures in this research were approved by the Institutional Animal Care and Use Committee. Thirty female Sprague-Dawley rats (age: 3 months old; Vital River Laboratory Animal Technology Co., Ltd., China) were used in this research. Each rat received either sham surgery (Sham group, n=10) or bilateral ovariectomy (OVX; n=20). The OVX animals were randomly assigned to the OVX group (n=10) or the OVX + sCT group (n=10) immediately after the OVX operation. Animals in the OVX + sCT group received subcutaneous administration of sCT (Novartis AG, Switzerland) (dosage: 16 IU/kg, every 2 days) [18]. All rats were euthanized at 12 weeks post-OVX to collect lumbar spine and blood.
Throughout the course of the experiment, all animals were housed at 21±1°C with a 12-h light/dark cycle. Intake of water and a sterilized diet (HFK Bioscience Co., Ltd., Beijing, China) was allowed ad libitum.
Biomarker assays
Blood samples were obtained when animals were euthanized, and serum was separated by centrifugation and stored at −80°C. Serum level of COMP was analyzed using enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech Co., Ltd., China) in accordance with the manufacturer’s instructions. The data were measured using an iMARK Reader (BioRad Laboratories Inc., USA).
Micro-CT analysis
To investigate the changes in subchondral bone architecture, L4–L5 FJs were scanned by a high-resolution micro-CT (SkyScan1176, Bruker, Belgium) after all rats were euthanized. In the right L4–L5 FJ, the subchondral cancellous bone located under the subchondral bone plate was regarded as the region of interest (ROI). Bone mineral density (BMD), trabecular separation (Tb.Sp), trabecular number (Tb.N), bone volume/trabecular volume (BV/TV), and trabecular thickness (Tb.Th) were calculated.
Histological assessments
The L4–L5 spine segments (including the FJs) were sequentially fixed in 10% formalin solution, decalcified with 10% EDTA-2Na for 10 weeks, embedded in paraffin, and finally cut into 6-μm sections, which were stained with toluidine blue for histological observation. Degenerative changes in the right L4–L5 FJ were evaluated according to a modified Mankin grading system [19] (Grade 0: intact surface; Grade 1: surface fissure; Grade 2: surface fissure to mid-zone; Grade 3: surface fissures to deep zone; Grade 4: complete destruction). The cartilage thickness of the right L4–L5 FJ was also calculated using these sections. Histological scoring and cartilage thickness calculation were both performed by 2 independent researchers in a blinded manner.
Immunohistochemical analysis
The expression levels of aggrecan (1: 300; Abbiotec LLC, San Diego, CA, USA), caspase-3 (1: 200; Boster Co., Ltd., Wuhan, China), MMP-13 (1: 200; Gene Tex Inc., USA), and ADAMTS-4 (1: 200; Abcam Inc., USA) in cartilage were analyzed. Specifically, sections were rehydrated with ethanol following deparaffinization with xylene. After antigen retrieval with 0.05% trypsin and inactivation of endogenous peroxidases with 0.3% H2O2, incubation with the target protein was performed overnight at 4°C. The remaining experimental procedures were conducted in accordance with the protocols provided with the PV-6000 DAB detection kit and ZLI-9018 DAB kit (all from ZSGB-BIO Corp., China). Finally, these sections were counter-stained with hematoxylin.
Immunohistochemical images were captured using a BX53 microscope (Olympus, Tokyo, Japan) at 100× magnification and then semiquantitatively analyzed with Image-Pro Plus version 6.0 software (Media Cybernetics, USA). The average optical density intensity of each protein, expressed as IOD/mm2, was defined as the value of the integrated optical density divided by the cartilage area.
Statistical analysis
All data are expressed as the means ± standard error of the mean (SEM) and were analyzed using SPSS software (SPSS 20.0, SPSS Inc.; Chicago, IL). The Kolmogorov-Smirnov test was used to assess whether the results presented a normal distribution. One-way analysis of variance (ANOVA) was performed to confirm the differences in data with a normal distribution among groups, followed by Fisher’s least significant difference (LSD) test, which was used to perform pairwise comparisons. The differences in modified Mankin scores among groups were determined with Kruskal-Wallis H tests, and the Dunn-Bonferroni post hoc test was then used for pairwise comparisons. Spearman’s rank correlation analysis and Pearson correlation analysis were used to evaluate the correlations between the serum COMP level and modified Mankin score and between the serum COMP level and cartilage thickness, respectively. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Biomarker analysis
A significantly higher serum COMP concentration was observed in the OVX group than in the Sham group (P<0.001), whereas after sCT treatment, rats in the OVX + sCT group showed a significantly lower serum COMP concentration than that observed in the OVX group (P<0.05). No significant difference in serum COMP was observed between the Sham group and the OVX + sCT group (P>0.05) (Figure 1).
Figure 1.
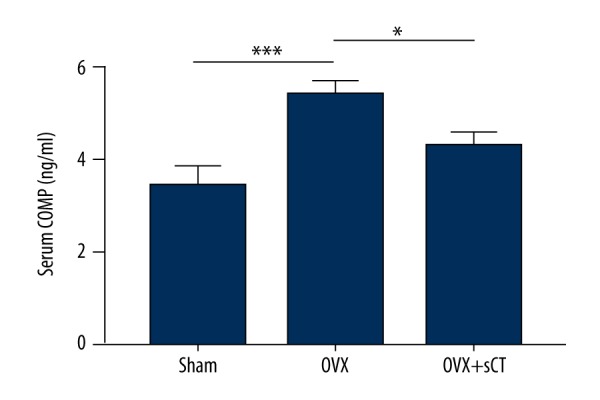
COMP levels in serum of the Sham group, the OVX group, and the OVX + sCT group. Data represented as mean ±SEM. P values were determined by Fisher’s LSD test following one-way ANOVA. * P<0.05; *** P<0.001.
Micro-CT parameters of subchondral bone
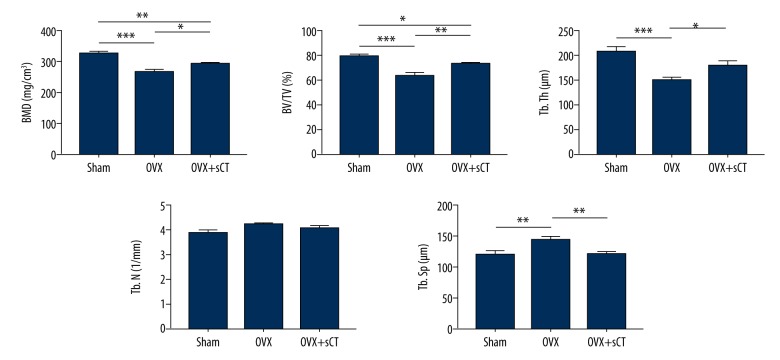
The OVX group exhibited significantly lower BMD, BV/TV, and Tb.Th values but a significantly higher Tb.Sp value than in the Sham group (P<0.001, P<0.001, P<0.001, and P<0.01, respectively). Compared with those of the OVX group, the BMD, BV/TV, and Tb.Th values of the OVX + sCT group were significantly higher, but the Tb.Sp value was significantly lower (P<0.05, P<0.01, P<0.05, and P<0.01, respectively). Compared with the Sham group, the OVX + sCT group showed significantly lower BMD and BV/TV values (P<0.01 and P<0.05, respectively), whereas there was no significant difference in Tb.Th and Tb.Sp values between the 2 groups (all P>0.05). No significant difference in the Tb.N value was observed between groups (P>0.05) (Figures 2, 3).
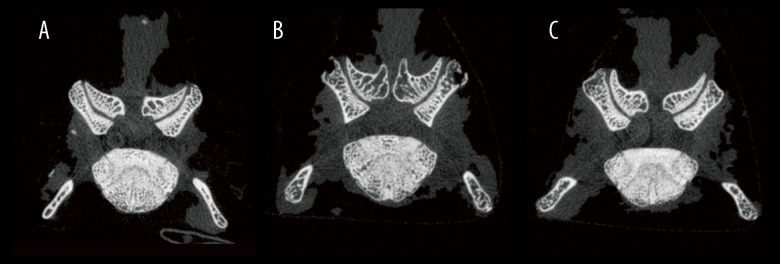
Figure 2.
Micro-CT images of L4–L5 FJ in every group: (A) the Sham group, (B) the OVX group, and (C) the OVX + sCT group.
Figure 3.
Micro-architecture parameters of FJs analyzed by micro-CT. Data are represented as mean ±SEM. P values were determined by Fisher’s LSD test following one-way ANOVA. * P<0.05; ** P<0.01; *** P<0.001.
Histological observation in articular cartilage
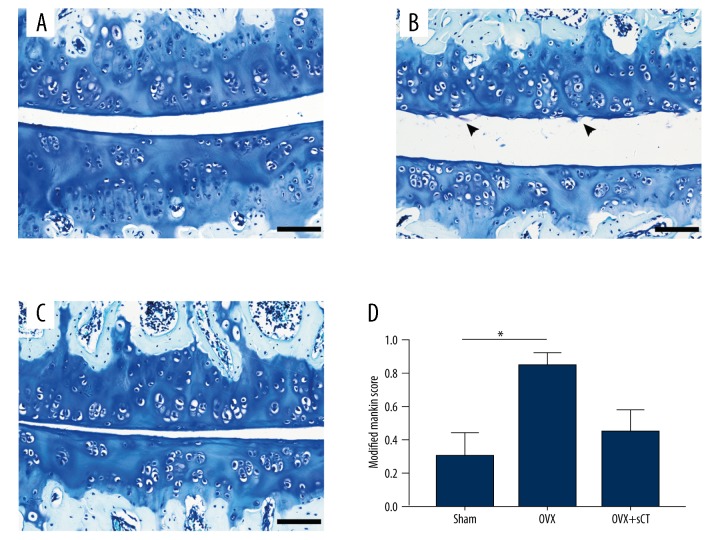
In the Sham group, the cartilage surface of FJs was intact, the structures of chondrocytes were normal, and toluidine blue staining of extracellular matrix (ECM) was evenly distributed (Figure 4A). Distinct cartilage degeneration was observed in the OVX group, characterized by surface irregularities and small fissures (Figure 4B). After treatment with sCT, cartilage lesions of OVX rats were alleviated to some extent (Figure 4C). The OVX group showed a significantly higher modified Mankin score than did the Sham group (P<0.05). No significant differences were found between the OVX group and the OVX + sCT group or between the Sham group and the OVX + sCT group (all P>0.05).
Figure 4.
Toluidine blue staining and histological score for every group: (A) the Sham group, (B) the OVX group, and (C) the OVX + sCT group. The arrowhead indicates small fissures of cartilage. Data represented as mean ±SEM. P values were determined by Dunn-Bonferroni post hoc test following Kruskal-Wallis H test. * P<0.05. Bars=100 μm.
The cartilage thickness of the OVX group was decreased significantly compared with that of the Sham group (P<0.001). The OVX + sCT group presented a significantly greater cartilage thickness than that of the OVX group (P<0.05) but displayed significantly thinner cartilage than that of the Sham group (P<0.01) (Figure 5).
Figure 5.
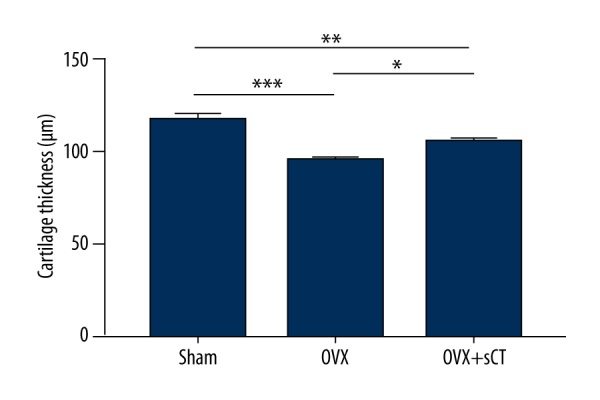
Measurements of cartilage thickness of the Sham group, the OVX group, and the OVX + sCT group. Data represented as mean ±SEM. P values were determined by Fisher’s LSD test following one-way ANOVA. * P<0.05; ** P<0.01; *** P<0.001.
Immunohistochemical assessments
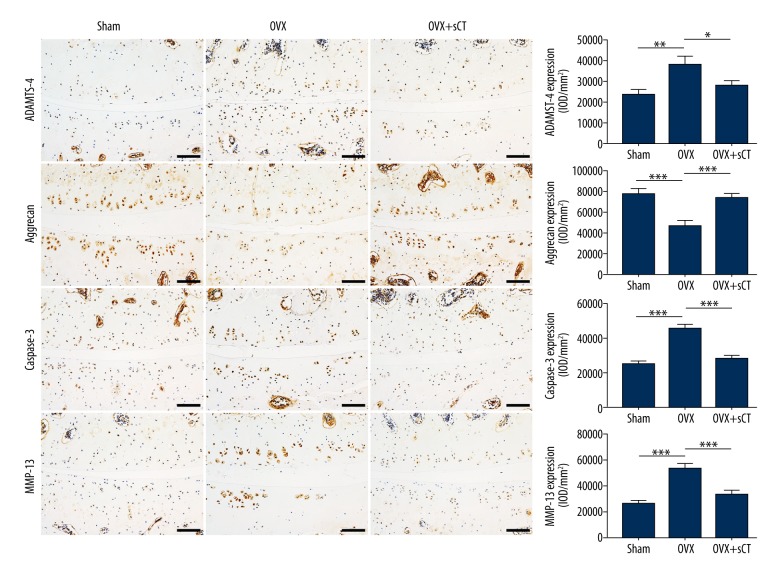
Aggrecan expression was significantly lower in the OVX group than in the Sham group (P<0.001), whereas aggrecan expression was significantly higher in the OVX + sCT group than in the OVX group (P<0.001). Significantly higher ADAMTS-4, MMP-13, and caspase-3 expression levels were observed in the OVX group than in the Sham group (P<0.01, P<0.001, and P<0.001, respectively), whereas significantly lower levels of ADAMTS-4, MMP-13, and caspase-3 expression were observed in the OVX + sCT group than in the OVX group (P<0.05, P<0.001, and P<0.001, respectively). The OVX + sCT group showed no significant differences in the expression levels of ADAMTS-4, MMP-13, caspase-3, and aggrecan compared with the Sham group (all P>0.05) (Figure 6).
Figure 6.
Immunohistochemical assay for ADAMTS-4, aggrecan, caspase-3, and MMP-13 for every group. Data are represented as mean ±SEM. P values were determined by Fisher’s LSD test following one-way ANOVA. * P<0.05; ** P<0.01; *** P<0.001. Bars=100 μm.
Correlation analysis
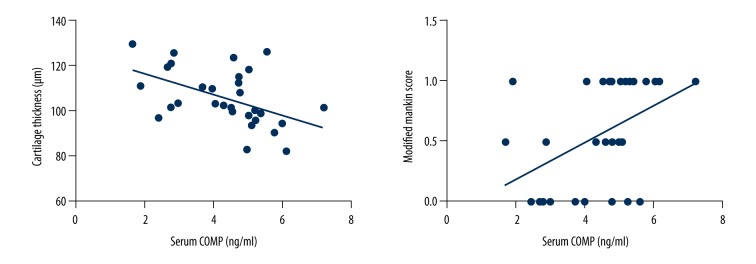
The serum COMP concentration showed a significant positive correlation with the modified Mankin score (r=0.467, P<0.05) but had a significant negative correlation with cartilage thickness (r=−0.485, P<0.05) (Figure 7)
Figure 7.
Results of Spearman’s rank correlation analysis comparing serum COMP level and modified Mankin score, and Pearson correlation analysis comparing serum COMP level and cartilage thickness. The regression line (solid) is shown.
Discussion
The results of the present study demonstrate that estrogen deficiency induced by OVX leads to subchondral bone loss and cartilage degeneration, particularly a notable decrease in cartilage thickness. All of these degenerative changes in the model used in this study were retarded by sCT inhibiting over-activated subchondral bone remodeling and cartilage catabolic metabolism.
In this study, the OVX group displayed distinct cartilage degeneration and a higher histological score than in the Sham group. This finding is similar to the results reported in previous studies of FJD in mouse models [6,7]. However, the histological score of the OVX group was only approximately equivalent to Grade 1 (surface fissure), and the degenerative cartilage changes in OVX rats were mainly characterized by small fissures limited to the cartilage surface, without fissures extending into the middle or deep zone of cartilage, which indicates a different severity of cartilage lesions from that observed in other joint degeneration models secondary to mechanical instability or chemical stimulation [20–22]. The cartilage of FJs in the OVX group was significantly thinner than that in the Sham group, which is a major feature of articular degeneration [20,21,23]. Thus, based on the results of the present study and previous studies [7,21], the most specific degenerative change in this model is the loss of cartilage thickness, not damage to superficial cartilage.
To further explore the mechanism underlying the loss of FJ cartilage thickness in OVX rats, we detected the proteins related to cartilage metabolism and chondrocyte apoptosis. Aggrecan, an integral component of chondrocyte ECM, plays an important role in maintaining the proper function of cartilage. The hydrated gel structure provided by aggrecan imparts load-bearing properties to cartilage [24]. MMP-13 and ADAMTS-4 are important matrix-degrading enzymes involved in the process of cartilage turnover and degrading Col-II and aggrecan, respectively, in cartilage [25,26]. Caspases-3 is the executive protease of apoptosis [27], and its expression is increased during chondrocyte apoptosis [28]. The immunohistochemical results obtained in this study indicate that the expression levels of MMP-13, ADAMTS-4 and caspase-3 were significantly increased, whereas the expression of aggrecan was significantly decreased in OVX rats. These findings indicate that estrogen deficiency leads to enhanced catabolism of cartilage and apoptosis of chondrocytes, which may be the primary cause of reduced cartilage thickness in the model used in the present study.
Increased subchondral bone turnover is closely related to the development of joint degeneration [29]. In our study, OVX rats displayed significantly decreased BMD, BV/TV, and Tb.Th values in subchondral bone but a significantly increased Tb.Sp value, indicating that bone remodeling occurred in the subchondral bone of FJs. Preclinical and clinical studies have found that both high and low BMD, including osteoporosis, can cause osteoarthritis [30]. It is conceivable that deleterious alterations of subchondral bone may transmit abnormal stress to articular cartilage and cause secondary lesions, thereby causing the deterioration of cartilage to transmit increased loads to subchondral bone, which forms a vicious cycle sustaining joint degeneration [31].
Based on the results in this study, the mechanism whereby FJD occurred in OVX rats appears to be mainly due to the dual role of estrogen deficiency in cartilage and subchondral bone metabolism. Firstly, estrogen deficiency caused increased chondrocyte apoptosis and imbalanced metabolism of cartilage matrix, and further led to cartilage loss and degradation. Secondly, over-activated subchondral bone remodeling caused changes of biomechanical environments, which transmitted abnormal stress to articular cartilage and resulted in its secondary lesions [31]. Therefore, we speculate that FJD caused by estrogen deficiency may be a type of ‘metabolic disorder’. A new focus of studies on joint degeneration is the classification of the disease into distinguishable phenotypes [32]. Defining the different phenotypes of articular degeneration will deepen the recognition of the pathophysiology of this disease and develop targeted treatments for different patients [32,33]. A previous study has indicated that bone-acting agents (including CT) may be beneficial for postmenopausal patients with articular degeneration [33].
CT is an antiresorptive drug and is currently used for treating osteoporosis in clinical practice. Due to its relatively good safety profile, CT may be more suitable for elderly female patients with osteoporosis [34]. Our study and other previous studies indicate that CT exerts protective effects on knee or intervertebral disc degeneration [12–14,18]. In the present study, histological observation revealed that the cartilage lesions of OVX rats were alleviated by treatment with sCT, and the histological score was lower in the OVX + sCT group than in the OVX group, but the differences were not statistically significant. Moreover, sCT treatment significantly inhibited the reduction of cartilage thickness. These results indicate that sCT helps protect the morphological structure of cartilage, similar to the findings of experimental studies of knee osteoarthritis [12,13].
The protective effects of CT on cartilage metabolism have been reported in previous in vitro studies [35,36] and in vivo studies [12,13] of knee osteoarthritis. Our results demonstrate that intervention with sCT significantly decreased the expression levels of MMP-13, ADAMTS-4, and caspase-3 in the FJ cartilage of OVX rats and significantly increased the expression of aggrecan. These findings indicate that sCT has positive effects on maintaining the balance of cartilage ECM metabolism and inhibiting the apoptosis of chondrocytes in FJ OA. Inhibiting chondrocyte apoptosis is another positive function of CT [36,37]. Although the exact details of the mechanism are not clear, this function may be associated with the effects of CT on deactivation of the MAPK/Wnt/NF-κB pathways in chondrocytes [37]. Therefore, the direct effects of sCT in maintaining structural integrity of cartilage and inhibiting loss of cartilage thickness may be attributed to its protection of cartilage chondrocytes and ECM metabolism.
COMP is an important component of the cartilage ECM and is indispensable in preserving the structural integrity of cartilage and in regulating cellular functions [38]. Many studies have shown that serum COMP is a biomarker that is useful in assessing the incidence and progression of knee and hip degeneration [39–44]. However, it remains unclear whether serum COMP is influenced by estrogen deficiency and whether serum COMP is a diagnostic biomarker for FJ degeneration. In our study, we found that the serum COMP concentration in the OVX group was significantly higher than that in the Sham group. After administration of sCT, the serum COMP concentration of OVX rats was significantly reduced, which provides indirect evidence that sCT has protective effects on cartilage. Moreover, serum COMP concentration was significantly correlated with the cartilage thickness and histological score of FJs. Therefore, we believe that estrogen deficiency led to the increase in serum COMP concentration and that serum COMP has diagnostic potential for FJ degeneration.
In the present study, the deterioration of subchondral bone micro-architecture was remarkably ameliorated by treatment with sCT: BMD, BV/TV, and Tb.Th values significantly increased, and Tb.Sp values significantly decreased. These results demonstrate the ability of sCT to prevent subchondral bone remodeling, which is attributed to the effect of sCT in treating osteoporosis. Inhibition of subchondral bone degeneration also exerts indirect protective effects on cartilage by avoiding secondary damage caused by abnormal stress. Furthermore, because cartilage may be damaged by prostaglandins, leukotrienes, and certain growth factors produced by osteoblasts during subchondral bone remodeling [45], suppression of osteoclast activity with sCT may alleviate cartilage degeneration caused by these factors. Therefore, the normal biomechanical and biochemical environments of FJ in OVX rats were preserved by treatment with sCT.
Conclusions
In summary, estrogen deficiency can lead to FJD, characterized by enhanced cartilage catabolism and subchondral bone remodeling. Treatment with sCT can inhibit FJ degeneration by maintaining metabolic balances of cartilage, suppressing chondrocyte apoptosis, and alleviating subchondral bone remodeling. In addition, sCT can reduce high serum COMP levels caused by estrogen deficiency, and serum COMP possesses diagnostic potential for FJD.
Footnotes
Conflict of interest
None.
Source of support: The study was supported by grants from the National Natural Science Foundation of China (NSFC 31671235), the Major Program of Natural Science Foundation of Hebei Province (H2016209176), the Young Talent Support Foundation of Hebei Province, and the Scientific Innovation Team Foundation of Tangshan City (15130208C)
References
- 1.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: The facet joints. Nat Rev Rheumatol. 2013;9:216–24. doi: 10.1038/nrrheum.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrow LJ, Smith S. The management of lower back pain. J R Nav Med Serv. 2014;100:282–87. [PubMed] [Google Scholar]
- 3.Becker A, Held H, Redaelli M, et al. Low back pain in primary care: Costs of care and prediction of future health care utilization. Spine (Phila Pa 1976) 2010;35:1714–20. doi: 10.1097/brs.0b013e3181cd656f. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Ali MH, Wydra F, et al. Characterization of degenerative human facet joints and facet joint capsular tissues. Osteoarthritis Cartilage. 2015;23:2242–51. doi: 10.1016/j.joca.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: A review. Semin Arthritis Rheum. 2007;37:69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Zhu H, Zhang K, et al. Estrogen deficiency accelerates lumbar facet joints arthritis. Sci Rep. 2017;7:1379. doi: 10.1038/s41598-017-01427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Ni S, Cao Y, et al. Three-dimensional visualization and pathologic characteristics of cartilage and subchondral bone changes in the lumbar facet joint of an ovariectomized mouse model. Spine J. 2017 doi: 10.1016/j.spinee.2017.11.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Rosner IA, Goldberg VM, Moskowitz RW. Estrogens and osteoarthritis. Clin Orthop Relat Res. 1986;(213):77–83. [PubMed] [Google Scholar]
- 9.Ha KY, Chang CH, Kim KW, et al. Expression of estrogen receptor of the facet joints in degenerative spondylolisthesis. Spine (Phila Pa 1976) 2005;30:562–66. doi: 10.1097/01.brs.0000154674.16708.af. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Naot D, Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43:813–18. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen RH, Bay-Jensen AC, Byrjalsen I, Karsdal MA. Oral salmon calcitonin reduces cartilage and bone pathology in an osteoarthritis rat model with increased subchondral bone turnover. Osteoarthritis Cartilage. 2011;19:466–73. doi: 10.1016/j.joca.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T, Zhang L, Fu X, et al. The potential protective effects of calcitonin involved in coordinating chondrocyte response, extracellular matrix, and subchondral trabecular bone in experimental osteoarthritis. Connect Tissue Res. 2013;54:139–46. doi: 10.3109/03008207.2012.760549. [DOI] [PubMed] [Google Scholar]
- 14.El Hajjaji H, Williams JM, Devogelaer JP, et al. Treatment with calcitonin prevents the net loss of collagen, hyaluronan and proteoglycan aggregates from cartilage in the early stages of canine experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:904–11. doi: 10.1016/j.joca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Bagger YZ, Tanko LB, Alexandersen P, et al. Oral salmon calcitonin induced suppression of urinary collagen type II degradation in postmenopausal women: A new potential treatment of osteoarthritis. Bone. 2005;37:425–30. doi: 10.1016/j.bone.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Manicourt DH, Azria M, Mindeholm L, et al. Oral salmon calcitonin reduces Lequesne’s algofunctional index scores and decreases urinary and serum levels of biomarkers of joint metabolism in knee osteoarthritis. Arthritis Rheum. 2006;54:3205–11. doi: 10.1002/art.22075. [DOI] [PubMed] [Google Scholar]
- 17.Karsdal MA, Byrjalsen I, Henriksen K, et al. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: A 14-day randomized study. Osteoarthritis Cartilage. 2010;18:150–59. doi: 10.1016/j.joca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Tian FM, Yang K, Wang WY, et al. Calcitonin suppresses intervertebral disk degeneration and preserves lumbar vertebral bone mineral density and bone strength in ovariectomized rats. Osteoporos Int. 2015;26:2853–61. doi: 10.1007/s00198-015-3202-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Ahmadinia K, Li X, et al. Development of an experimental animal model for lower back pain by percutaneous injury-induced lumbar facet joint osteoarthritis. J Cell Physiol. 2015;230:2837–47. doi: 10.1002/jcp.25015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Dai MW, Chu JG, Tian FM, et al. Parathyroid hormone(1–34) exhibits more comprehensive effects than celecoxib in cartilage metabolism and maintaining subchondral bone micro-architecture in meniscectomized guinea pigs. Osteoarthritis Cartilage. 2016;24:1103–12. doi: 10.1016/j.joca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Iijima H, Aoyama T, Ito A, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22:1036–43. doi: 10.1016/j.joca.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Yeh TT, Wen ZH, Lee HS, et al. Intra-articular injection of collagenase induced experimental osteoarthritis of the lumbar facet joint in rats. Eur Spine J. 2008;17:734–42. doi: 10.1007/s00586-008-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karvonen RL, Negendank WG, Teitge RA, et al. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21:1310–18. [PubMed] [Google Scholar]
- 24.Kiani C, Chen L, Wu YJ, et al. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg WB. Osteoarthritis year 2010 in review: Pathomechanisms. Osteoarthritis Cartilage. 2011;19:338–41. doi: 10.1016/j.joca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1:33–47. doi: 10.1007/s13238-010-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvesen GS. Caspases: Opening the boxes and interpreting the arrows. Cell Death Differ. 2002;9:3–5. doi: 10.1038/sj.cdd.4400963. [DOI] [PubMed] [Google Scholar]
- 28.Xue H, Tu Y, Ma T, et al. Lactoferrin inhibits IL-1beta-induced chondrocyte apoptosis through AKT1-induced CREB1 activation. Cell Physiol Biochem. 2015;36:2456–65. doi: 10.1159/000430206. [DOI] [PubMed] [Google Scholar]
- 29.Bailey AJ, Buckland-Wright C, Metz D. The role of bone in osteoarthritis. Age Ageing. 2001;30:374–78. doi: 10.1093/ageing/30.5.374. [DOI] [PubMed] [Google Scholar]
- 30.Herrero-Beaumont G, Roman-Blas JA, Largo R, et al. Bone mineral density and joint cartilage: Four clinical settings of a complex relationship in osteoarthritis. Ann Rheum Dis. 2011;70:1523–25. doi: 10.1136/ard.2011.151233. [DOI] [PubMed] [Google Scholar]
- 31.Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: An update with relevance for clinical practice. Lancet. 2011;377:2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 33.Roman-Blas JA, Castaneda S, Largo R, et al. An OA phenotype may obtain major benefit from bone-acting agents. Semin Arthritis Rheum. 2014;43:421–28. doi: 10.1016/j.semarthrit.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Silverman S, Christiansen C. Individualizing osteoporosis therapy. Osteoporos Int. 2012;23:797–809. doi: 10.1007/s00198-011-1775-y. [DOI] [PubMed] [Google Scholar]
- 35.Sondergaard BC, Wulf H, Henriksen K, et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage. 2006;14:759–68. doi: 10.1016/j.joca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Greco KV, Nalesso G, Kaneva MK, et al. Analyses on the mechanisms that underlie the chondroprotective properties of calcitonin. Biochem Pharmacol. 2014;91:348–58. doi: 10.1016/j.bcp.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LB, Man ZT, Li W, et al. Calcitonin protects chondrocytes from lipopolysaccharide-induced apoptosis and inflammatory response through MAPK/Wnt/NF-kappaB pathways. Mol Immunol. 2017;87:249–57. doi: 10.1016/j.molimm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Acharya C, Yik JH, Kishore A, et al. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–11. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Hosnijeh FS, Runhaar J, van Meurs JB, Bierma-Zeinstra SM. Biomarkers for osteoarthritis: Can they be used for risk assessment? A systematic review. Maturitas. 2015;82:36–49. doi: 10.1016/j.maturitas.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Golightly YM, Marshall SW, Kraus VB, et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63:2276–83. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobasheri A, Bay-Jensen AC, van Spil WE, et al. Osteoarthritis year in review 2016: Biomarkers (biochemical markers) Osteoarthritis Cartilage. 2017;25:199–208. doi: 10.1016/j.joca.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Sharif M, Kirwan JR, Elson CJ, et al. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–88. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 43.Van Spil WE, Welsing PM, Bierma-Zeinstra SM, et al. The ability of systemic biochemical markers to reflect presence, incidence, and progression of early-stage radiographic knee and hip osteoarthritis: Data from CHECK. Osteoarthritis Cartilage. 2015;23:1388–97. doi: 10.1016/j.joca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: Evidence from the Rotterdam study cohort. Arthritis Res Ther. 2016;18:81. doi: 10.1186/s13075-016-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: A biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol. 2003;15:628–33. doi: 10.1097/00002281-200309000-00018. [DOI] [PubMed] [Google Scholar]