Abstract
Background
The timely evaluation and initiation of treatment for acute ischemic stroke (AIS) is critical to optimal patient outcomes. However, clinical practice often falls short of guideline-established goals. Hospitals in rural regions of the USA, and notably those in the Stroke Belt, are particularly challenged to meet timing goals since the vast majority of primary stroke centers (PSCs) are concentrated in urban academic institutions.
Methods
Between May 2015 and May 2017, emergency department (ED) teams from 5 non-PSC hospitals in the Stroke Belt participated in a quality improvement (QI) initiative. The intervention included a baseline practice assessment survey, repeat audit-and-feedback cycles with patient data on AIS treatment timing, personalized Continuing Medical Education/Continuing Education-certified grand rounds sessions at each participating site with expert study faculty, targeted reinforcement of best practices, and follow-up to evaluate the benefits and limitations of the intervention.
Results
At the start of the initiative, clinical staff from participating EDs overestimated the proportion of patients with AIS who received alteplase within the guideline-recommended 60-minute door-to-needle window at their facility. At the end of the 6-month intervention period, significantly more patients were treated with alteplase within 60 minutes of ED arrival compared to baseline across the entire sample (1.9% of patients at baseline vs. 5.2% at 6 months; P < 0.01). Similarly, there was a trend toward a decrease in the percentage of patients whose alteplase treatment was initiated more than 60 minutes after their arrival at the ED (67.3% at baseline vs. 22.2% at 6 months).
Conclusion
Structured QI interventions that engage ED care teams to reflect on processes related to AIS diagnosis and treatment and deploy repeat audit-and-feedback cycles with real-time patient data have the potential to support an increase in the number of patients who receive alteplase within the guideline-recommended timeframe of 60 minutes from hospital arrival.
Keywords: AIS, neurology, CME, quality improvement, alteplase
Introduction
Approximately 795,000 Americans experience a new or recurrent stroke each year, contributing to a substantial share of morbidity, mortality, and serious long-term disability in the USA.1 Although the number of stroke deaths nationwide has been steadily declining for decades, stroke mortality rates vary substantially by geographic region.1–3 Counties with particularly elevated stroke mortality rates are concentrated in the so-called Stroke Belt, an 8-state region that includes Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee.1,3
Extensive clinical evidence demonstrates the importance of timely evaluation and initiation of treatment to optimal short- and long-term outcomes for patients with acute ischemic stroke (AIS).4 However, clinical practice often falls short of guideline-established goals. For example, Adeoye et al found that while alteplase use roughly doubled from 2005 to 2009, the rate of patients receiving alteplase in 2009 remained low at 3.4%–5.2%, correlating to ~23,800–36,000 of the over 700,000 patients with AIS.5 Furthermore, data from Get with the Guidelines (GWTG)-Stroke, a quality improvement (QI) project within the American Heart Association (AHA)/American Stroke Association’s (ASA) Target: Stroke initiative, demonstrated a significant increase in the number of patients with a door-to-needle (DTN) time of ≤60 minutes between the 4th quarter of 2009 and the 3rd quarter of 2013; however, only 53% of patients received alteplase treatment within the guideline-recommended 60-minute DTN window.6
Hospitals in rural areas of the country are particularly challenged to meet these critical goals related to treatment timing. Although more than 1,000 hospitals in all 50 states have been recognized by The Joint Commission as a primary stroke center (PSC), the percentage of individuals who live within 60 minutes of a PSC is markedly lower in the Stroke Belt than it is nationally (44% vs. 69%).7 Correspondingly, significantly fewer individuals with AIS in this region are evaluated at a PSC compared to the rest of the country (14.7% vs. 27.3%; P < 0.001).8 Furthermore, data from the GWTG-Stroke dataset suggest that hospitals located in the South or in rural regions, as well as those that are not a designated PSC or teaching hospital, are less likely to administer alteplase to eligible patients.9
The implementation of recommended time-to-treatment parameters has been the focus of several QI initiatives over the past decade. Hospitals participating in the Paul Coverdell National Acute Stroke Registry demonstrated significant improvements in 9 of 10 AHA/ASA-endorsed stroke performance measures from 2005 to 2009, including an 11% increase in the average annual use of alteplase.10 Additionally, hospitals participating in the AHA/ASA GWTG-Stroke QI program have demonstrated significant improvement in adherence to stroke performance measures over a 5-year period, including the use of alteplase within 2 hours (42.1%–72.8%; P < 0.0001). Furthermore, centers that follow GWTG have shown good outcomes compared to those that do not follow GWTG, and those with better adherence to GWTG measures have shown better outcomes.6
Therefore, to bring the demonstrated benefits of structured, data-driven QI interventions to non-PSC-designated community hospitals in the South, this initiative was designed to engage 5 emergency department (ED) teams to reflect on current processes and performance related to the administration of alteplase to patients presenting to their facility with AIS and improve the percentage of individuals receiving treatment within 60 minutes of ED arrival.
Methods
Hospital recruitment
Five hospitals located in Stroke Belt states across the southeastern USA were recruited based on expressed interest in conducting a QI initiative in their ED. None of the hospitals were designated as a PSC at the time of study initiation. Participation required the involvement of a designated site champion and clinical team members who cared for patients presenting with symptoms of AIS from each site, including at least 1 physician or advance practice provider involved in making treatment decisions.
Initiative components
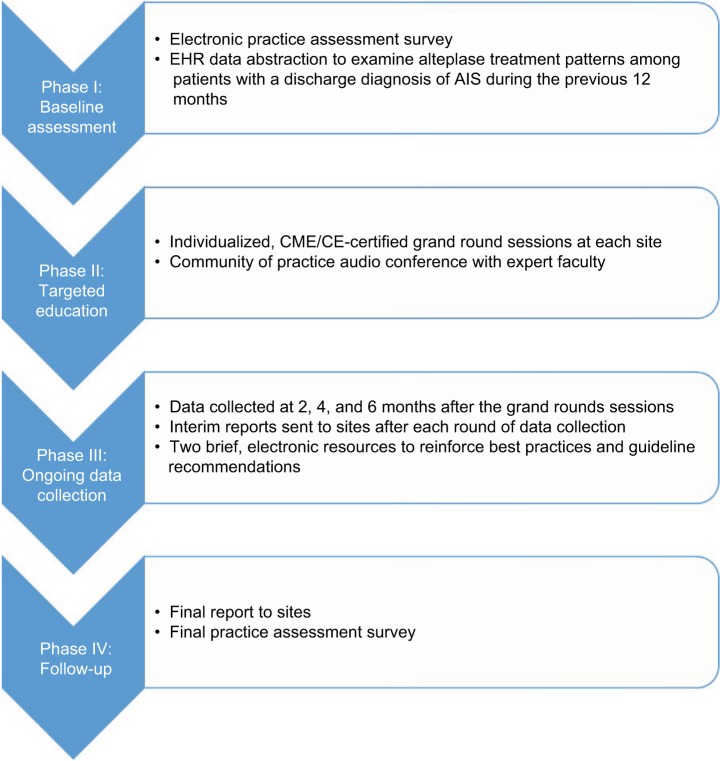
The QI initiative was implemented in 4 phases between May 2015 and May 2017 (Figure 1). The protocol for this project was approved by the Chesapeake Institutional Review Board. All participants in this study provided written informed consent.
Figure 1.
Initiative components.
Abbreviations: EHR, electronic health record; AIS, acute ischemic stroke; CME/CE, Continuing Medical Education/Continuing Education.
Phase I: Baseline assessment
A practice assessment survey, developed in conjunction with multidisciplinary study faculty, was distributed via email to ED teams from each participating site. Participants received a nominal stipend in return for their time. The practice assessment survey used multiple-choice questions, self-efficacy ratings, and open-ended barrier questions to facilitate reflection on current processes and frontline challenges, and served to identify potential areas for improvement related to the evaluation of patients arriving at the ED with symptoms of AIS and the timely initiation of treatment.
In addition to the electronic survey, the baseline assessment also included a retrospective abstraction of data from the electronic health record (EHR) at each participating site conducted by appropriate local designees. This sample identified continuous ED admissions over the course of the previous 12 months with a discharge diagnosis of AIS. From this dataset, local designees calculated the percentage of patients who: 1) had documentation of treatment with alteplase, 2) were treated with alteplase within 3 hours of last known well time, and 3) received alteplase within 60 minutes of hospital arrival.
Results of the electronic practice assessment survey and retrospective EHR data abstraction were compiled into a baseline summary report that was distributed to ED clinicians at each hospital site-by-site champions. EHR data of individual sites were presented in comparison with de-identified results from other participating sites and put into context of the practice assessment survey findings.
Phase II: Targeted education
Following the distribution of the baseline assessment report, five, 60-minute, Continuing Medical Education (CME)/Continuing Education (CE)-certified grand rounds presentations were conducted by study faculty, one at each hospital site. In addition to an overview of available clinical evidence and guideline recommendations for the identification and timely treatment of AIS in the ED, the sessions highlighted insights from the electronic practice assessment survey and site-specific performance metrics from the baseline EHR data abstraction. Study faculty facilitated a discussion with each ED team regarding site-specific challenges hindering timely alteplase administration and recommendations for process improvements.
Approximately 1 month after the grand rounds sessions, study faculty facilitated a Community of Practice audioconference with participating ED teams. This audioconference provided a forum for participants to further engage with faculty on their site’s baseline data and troubleshoot specific barriers to the timely initiation of alteplase for patients with AIS.
Phase III: Ongoing data collection
Ongoing audit-and-feedback related to each participating ED’s patterns of alteplase use for the treatment of patients with AIS was provided on an every-other-month basis (eg, 2, 4, and 6 months) following the distribution of the baseline summary report. These interim data reports were shared with participating sites via site champions as a reinforcement and engagement tactic for clinicians practicing at these locations.
In addition, over the course of the 6-month data collection period, each participating site received a series of brief electronic resources (2 in total) via email that were designed to reinforce earlier training conducted in Phase II of the initiative. Topics discussed included strategies to support the rapid diagnosis of AIS, the benefits of timely treatment with tissue plasminogen activator (alteplase), the appropriate use of endovascular therapy, and ways to overcome barriers to the timely treatment of AIS.
Phase IV: Follow-up
At the conclusion of the initiative, the participating sites received a final summary data report detailing performance changes from baseline to initiative completion in patterns of alteplase use across the entire cohort of participating hospitals and within individual EDs. Furthermore, clinicians from participating ED teams were given an opportunity to complete a final practice assessment survey to assess changes in knowledge, competence, and practice patterns related to the timely treatment of patients with AIS in their setting. Respondents received a nominal stipend in return for their time.
Data analysis
Electronic baseline and follow-up practice survey data were tabulated to visually assess trends in attitudes and practice behaviors, and the results described herein are descriptive. Administration of alteplase after ED presentation with symptoms of AIS at baseline vs. 6 months was subject to statistical analysis (chi-square, 2-tailed) according to following cut-points: ever, within 3 hours, and within 60 minutes. P values less than 0.05 were considered statistically significant.
Results
Five community hospital EDs located in Arkansas and North Carolina participated in the QI initiative. The baseline practice assessment survey had 45 respondents, including physicians, nurses, nurse practitioners, physician assistants, a certified nurse assistant, and an ED greeter (Table 1). The average number of respondents per facility was 9 (range: 6–13).
Table 1.
Emergency department team members responding to baseline electronic survey by degree type (N = 45)
| Respondent | n (%) |
|---|---|
| MD/DO | 14 (31.1) |
| NP/PA | 6 (13.3) |
| Nurse | 23 (51.1) |
| Other | 2 (4.4) |
Abbreviations: MD, medical doctor; DO, doctor of osteopathy; NP, nurse practitioner; PA, physician assistant.
The baseline practice assessment survey assessed ED teams’ perceptions of the quality of stroke care provided in their facility. Across all 5 sites, respondents estimated that an average 71% of patients with AIS who are treated with alteplase receive it within the guideline-recommended 60-minute window (Table 2). When asked about what barriers they face in improving DTN times for patients with AIS, the most frequently cited challenges among the 37 open-ended responses provided related to coordinating across the stroke team, recognizing symptoms of AIS by triage staff, and obtaining a neurology consult. Three of the sites had access to neurologists at larger stroke centers via telestroke. Clinician education and increased staffing were most frequently cited as strategies to improve DTN times in their facility (accounting for 32% and 24% of responses, respectively).
Table 2.
Clinicians’ baseline estimation of the percentage of patients with acute ischemic stroke treated with alteplase within 60 minutes of emergency department arrival
| Number of respondents | Average percentage of patients receiving alteplase ≤60 minutes | |
|---|---|---|
| Overall* | 43 | 69.2% |
| By clinician type | ||
| MD/DO | 14 | 80.6% |
| Nurse | 23 | 60.9% |
| NP/PA | 6 | 74.2% |
Note:
Does not include 2 respondents who were nonclinician emergency department staff.
Abbreviations: MD, medical doctor; DO, doctor of osteopathy; NP, nurse practitioner; PA, physician assistant.
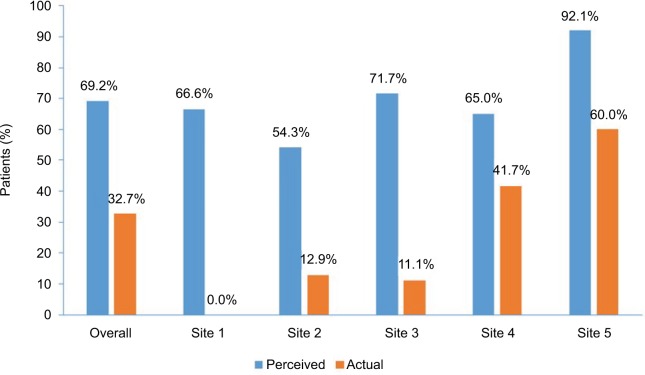
The CME/CE-certified grand rounds sessions held at each of the 5 participating hospitals in Phase II of the intervention were attended by 88 ED team members. Sessions included an overview of available guideline recommendations and current evidence from recent clinical trials that outlined the importance of identification and timely treatment of patients with AIS, as well as strategies that the participating hospitals could adopt in an effort to decrease DTN times. Each session then concluded with the study faculty presenting 2 data points related to the frequency at which patients receive alteplase in 60 minutes or less: the perceived percentage of patients with AIS who are treated within this timeframe based on self-reports from their colleagues who responded to the baseline electronic survey, and the actual percentage of patients achieving this goal based on data abstracted from the EHR. Across all groups, the differential between these 2 data points was substantial; the actual percentage of patients who received alteplase within 60 minutes based on patient chart data abstracted from all 5 hospitals was approximately half of survey respondents’ estimates (Figure 2).
Figure 2.
Perceived vs. actual percentage of patients with acute ischemic stroke who receive alteplase within 60-minute door-to-needle window at the start of the initiative.
The grand rounds sessions evaluation form posed an optional open-ended question about what participants planned to do differently in practice as a result of the education; among the 23 responses submitted, the 2 most common themes were more quickly evaluating patients with suspected AIS (30%) and taking steps to treat more patients with alteplase when appropriate (22%). In addition, when asked how they planned to implement these changes in practice, 40% of responses focused on better communication with colleagues and 30% of responses indicated ongoing education for themselves and their team (N = 20 responses).
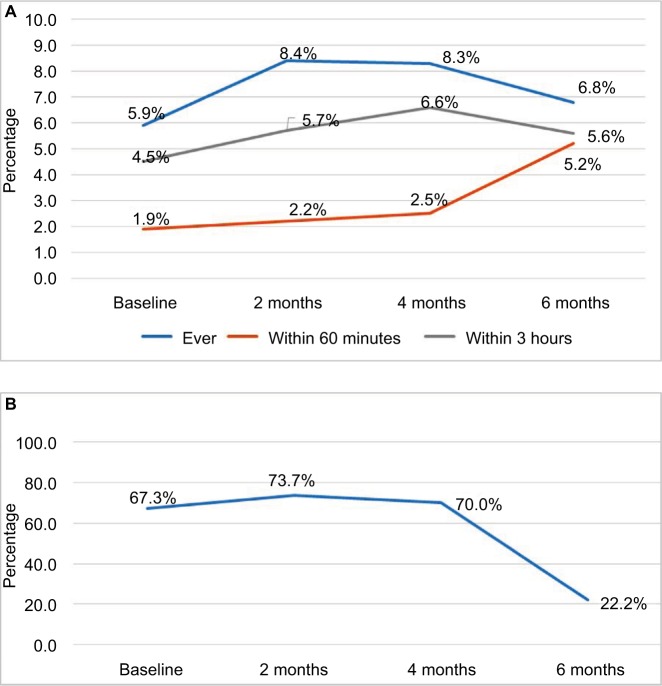
Data abstracted from the EHR of patients discharged with a diagnosis of AIS from the 5 participating EDs in Phase III of the initiative are depicted in Figure 3. In the 12 months prior to the start of the intervention (baseline), there were 1,651 patients with AIS from all 5 sites who came to the ED with symptoms of AIS; 1.9% of these patients received alteplase within 60 minutes of ED arrival. After the 6-month intervention period, 5.2% of the 266 eligible patients received alteplase within 60 minutes (P < 0.01; Figure 3A). Similarly, there was a nonsignificant trend toward a reduction in the percentage of patients with AIS who were treated with alteplase outside of the target DTN window of less than 60 minutes from ER arrival (67.3% of patients receiving alteplase greater than 60 minutes after ED presentation with symptoms of AIS at baseline vs. 22.2% of patients at 6 months; Figure 3B). Interestingly, DTN outcomes were no different at sites with telestroke capabilities compared with those without telestroke capabilities.
Figure 3.
Patients with acute ischemic stroke receiving alteplase over the 6-month intervention period (all participating emergency departments combined).
Notes: (A) Percentage of eligible patients receiving alteplase ever, within 3 hours, and within 60 minutes. (B) Percentage of eligible patients receiving alteplase more than 60 minutes after arrival in the emergency department.
Discussion
The results of this initiative reaffirm the value of data-driven, structured QI interventions to improve the administration of alteplase within 60 minutes for patients presenting to community-based EDs with symptoms of AIS. The provision of evidence-based acute stroke care at hospitals throughout the Stroke Belt is critically important to mitigate the negative effects of attendant morbidity and mortality, as residents of the southeastern USA generally have significantly less timely access to PSCs than individuals living in other regions of the country.
One practical challenge surrounding the optimal use of alteplase in clinical practice is related to the therapeutic window of this agent. The debate continues over whether the time-frame for alteplase administration can be extended beyond 3 hours after symptom onset, with contradictory actions from regulatory authorities. While the European Medicines Agency approved the expansion of the timeframe for intravenous alteplase administration from 3 to 4.5 hours, the FDA declined to extend the timeframe beyond the currently approved 3-hour window. The 2013 AHA/ASA guidelines specify that the findings of the ECASS III trial, a randomized, placebo-controlled Phase III study prospectively designed to assess the safety and efficacy of alteplase administered to patients with AIS between 3 and 4.5 hours after symptom onset, support the recommendation that alteplase may be considered in this extended timeframe in select patients.11 However, ED physicians in the USA face the difficult task of weighing the risk/benefit profile of off-label alteplase administration for patients who fall into this category. Expanding the window for alteplase administration to 4.5 hours after symptom onset may be beneficial in rural regions of the USA where patients must travel longer distances to reach their local hospital, though additional research is needed to better understand whether the potential clinical benefits of broader alteplase use justify the associated risks of treatment-related adverse events.
Sustainability of practice behaviors that support clinical care improvements following the completion of a targeted QI intervention is of utmost importance in fulfilling the promise of high-quality care and optimal outcomes for patients interacting with the health care system. Specific to AIS identification and management, the GWTG-Stroke QI project within the AHA/ASA’s Target: Stroke initiative, which provided participating hospital teams with various practice resources and regularly shared real-time performance data and feedback, resulted in a significant improvement in the number of patients with a DTN time of ≤60 minutes over a 10-year period.6 While findings from the present study suggest an improvement in the timely administration of alteplase in participating EDs in the Stroke Belt, an evaluation of whether this positive trend will be sustained after the intervention is beyond the scope of the project. However, the best practices described in the targeted education delivered in Phase II, together with the data abstraction method used at baseline and over the course of the intervention, provide participating hospitals with enduring tools that enable future audit-and-feedback cycles and support continuous, data-driven improvement efforts. Furthermore, the recommendations and methods deployed in this project could be informally shared by the participating ED staff with the other non-PSC institutions in the region as a means to scale the initiative beyond the scope of the current study.
The findings of this initiative were limited by several factors. Only a small number of EDs in the Stroke Belt region participated in the initiative; therefore, the conclusions drawn from the data may not be broadly applicable to other rural hospitals in this part of the country. Additionally, a systematic review of CME-based interventions by Tian et al suggests that study periods of less than 12 months may not be sufficient for new knowledge or skills to be incorporated into practice.12
Conclusion
Stroke remains a common cause of morbidity, mortality, and serious long-term disability, particularly in the Stroke Belt region of the southeastern USA. Extensive clinical evidence demonstrates the importance of timely evaluation and initiation of treatment to optimal short- and long-term outcomes for patients with AIS. Structured QI interventions that engage ED care teams to reflect on processes related to AIS diagnosis and treatment, as well as deploy repeat audit-and-feedback cycles with real-time patient data, have the potential to support an increase in the number of patients who receive intravenous alteplase within the guideline-recommended timeframe of 60 minutes from hospital arrival and mitigate both the financial and human burden of this often life-altering event.
Acknowledgments
The authors thank Whitney Stevens for project management, Jason Olivieri for data analysis and interpretation, and Laura Rafferty for editorial assistance. This initiative was supported by an unrestricted educational grant from Genentech, Inc. The funding source had no role in the execution, analysis, or development of the resulting manuscript associated with this initiative.
Footnotes
Disclosure
JL Blum and AJ Gardner are employees of Med-IQ, and DY Huang and EC Jauch received honoraria for participating as study faculty. The authors report no other conflicts of interest in this work.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229–e445. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lackland DT, Roccella EJ, Deutsch AF, et al. American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Quality of Care and Outcomes Research. Council on Functional Genomics and Translational Biology Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karp DN, Wolff CS, Wiebe DJ, Branas CC, Carr BG, Mullen MT. Reassessing the Stroke Belt: using small area spatial statistics to identify clusters of high stroke mortality in the United States. Stroke. 2016;47(7):1939–1942. doi: 10.1161/STROKEAHA.116.012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 5.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42(7):1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 7.Mullen MT, Wiebe DJ, Bowman A, et al. Disparities in accessibility of certified primary stroke centers. Stroke. 2014;45(11):3381–3388. doi: 10.1161/STROKEAHA.114.006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullen MT, Judd S, Howard VJ, et al. Disparities in evaluation at certified primary stroke centers: reasons for geographic and racial differences in stroke. Stroke. 2013;44(7):1930–1935. doi: 10.1161/STROKEAHA.111.000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87(15):1565–1574. doi: 10.1212/WNL.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Use of a registry to improve acute stroke care: seven states, 2005–2009. MMWR Morb Mortal Wkly Rep. 2011;60(7):206–210. [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 12.Tian J, Atkinson NL, Portnoy B, Gold RS. A systematic review of evaluation in formal continuing medical education. J Contin Educ Health Prof. 2007;27(1):16–27. doi: 10.1002/chp.89. [DOI] [PubMed] [Google Scholar]