Abstract
Purpose of Review
Bone is a structurally unique microenvironment that presents many challenges for the development of 3D models for studying bone physiology and diseases, including cancer. As researchers continue to investigate the interactions within the bone microenvironment, the development of 3D models of bone has become critical.
Recent Findings
3D models have been developed that replicate some properties of bone, but have not fully reproduced the complex structural and cellular composition of the bone microenvironment. This review will discuss 3D models including polyurethane, silk, and collagen scaffolds that have been developed to study tumor-induced bone disease. In addition, we discuss 3D printing techniques used to better replicate the structure of bone.
Summary
3D models that better replicate the bone microenvironment will help researchers better understand the dynamic interactions between tumors and the bone microenvironment, ultimately leading to better models for testing therapeutics and predicting patient outcomes.
Keywords: 3D models, Bone tumors, Bone, 3D printing, Tumor microenvironment
Introduction
Tumor cells frequently reside in the bone microenvironment due to primary (osteosarcomas), invasive (melanoma, myeloma), or metastatic disease (breast, prostate, lung, and renal cancers). Once tumors establish in bone, they interact with the physical microenvironment as well as the resident bone cells to cause bone destruction known as tumor-induced bone disease. While these interactions have been well-established by in vitro and in vivo studies, it has been challenging to investigate dynamic tumor-bone interactions due to a lack of appropriate 3D models. Thus, many groups have developed new models for studying tumor-induced bone disease that use both tumor cells and bone-resident cells (osteoblasts, osteoclasts). The development of these models has relied heavily on collaborations between biologists, clinicians, and engineers. These 3D models have allowed scientists to better understand the signaling pathways that drive tumor-induced bone disease, the interactions between different cell types, and the influence of the physical bone microenvironment. Furthermore, these models can serve as valuable platforms for the discovery and development of novel therapeutics to target tumors in bone. In this review, we will briefly discuss common 3D culture methods used in cancer research (hydrogels, spheroids) while focusing on current 3D models for studying bone and tumor metastasis to bone, specifically tissue-engineered constructs (TECs).
3D Cancer Models
Since its development in the late nineteenth century, cell culture has remained an important tool for both basic biology and medical research, including cancer research. Most adherent tumor cells are cultured as a monolayer on two-dimensional (2D) substrates made of polystyrene plastic or glass. Although 2D culture systems are widely used in cancer research, an increasing body of evidence has shown that 2D cell culture does not adequately replicate the complex interactions and spatial organization of cells in the three-dimensional (3D) tumor microenvironment. Moreover, tumor cell behavior (proliferation, migration, gene expression) and response to drug treatment can differ dramatically in conventional 2D culture compared to in vivo cellular responses [1–3]. To address some of these limitations, several 3D cell culture systems have been developed in the last few decades, and the adoption of these methods in cancer cell biology is rapidly increasing. Cancer cells grown in 3D more closely resemble those in the tumor microenvironment and thus have more physiologically relevant responses. To date, the most common 3D cell culture methods in cancer research include extracellular matrix (ECM)-based hydrogels and tumor spheroids.
Hydrogels
Due to their soft tissue-like properties, hydrogels have been increasingly used to mimic the 3D extracellular matrix (ECM) of solid tumors including breast, prostate, lung, and colorectal cancers [4–7]. Hydrogels are comprised of crosslinked polymer networks derived from natural or synthetic materials. Natural hydrogels are typically formed from ECM proteins like collagen, laminin, and fibrin as well as other matrix components like hyaluronic acid [8, 9]. Collagen type I is a commonly used natural hydrogel since it is the most abundant ECM protein in tumor stroma and has been shown to support tumor growth and increase the expression of genes promoting malignant phenotypes [10, 11]. The commercially available Corning® Matrigel® matrix is perhaps the most widely used natural ECM-based hydrogel for 3D culture of tumor cells in vitro. Matrigel® is a reconstituted basement membrane isolated from murine Engelbreth-Holm-Swarm (EHS) sarcoma which contains various ECM proteins (e.g., laminin, collagen IV, heparin sulfate proteoglycans) and endogenous growth factors (e.g., TGF-β, EGF, IGF-1, PDGF) [12]. These gels are highly biocompatible and not only modulate tumor cell viability, proliferation, adhesion, and motility but also sensitivity to therapeutic agents [13]. However, the concentration of proteins and growth factors in natural hydrogels can vary between batches, and confounding factors such as undefined matrix components can influence tumor cell behavior [14].
Alternatively, hydrogels made from synthetic materials such as poly(ethylene glycol) (PEG), poly(lactic acid) (PLA), and poly(vinyl alcohol) (PVA) have relatively well-defined structures with tunable chemical compositions and mechanical properties (e.g., stiffness) [15, 16]. Synthetic hydrogels also provide 3D architectural support for tumor cells and have been shown to maintain cell viability even in the absence of endogenous matrix components; however, these gels are usually supplemented with ECM proteins, growth factors, and other bioactive molecules in order to optimize tumor cell growth and survival [17–19]. Both natural and synthetic hydrogels can be used alone or in combination with other 3D culture methods including tumor spheroids.
Tumor Spheroids
In contrast to 2D monolayers, adherent tumor cells cultured in 3D tend to self-assemble into multicellular aggregates known as spheroids. Tumor spheroids are more mimetic of solid tumors in vivo with respect to cellular heterogeneity, metabolic and proliferative gradients, and gene expression. Specifically, spheroids typically contain a well-oxygenated outer layer of proliferating cells, a hypoxic inner layer of quiescent cells, and a necrotic core [20, 21]. Multicellular spheroids may consist of tumor cells alone or as co-cultures with stromal, endothelial, and immune cells. The 3D cell culture methods used to generate tumor spheroids include both scaffold-based (e.g., hydrogels) and scaffold-free (e.g., forced floating, hanging drop) platforms.
Scaffold-based methods for spheroid growth involve embedding or encapsulating tumor cells within natural or synthetic hydrogels that mimic the ECM. As previously discussed, the presence of endogenous matrix proteins and growth factors in these gel matrices not only support the organization of tumor cells into 3D spheroids but also promote the formation of migratory and invasive structures, significantly alter gene expression patterns, and affect cellular response to anti-tumor drugs [4–7].
On the other hand, scaffold-free platforms do not use a gel matrix support. Tumor spheroids produced by these methods are generated in suspension culture. One relatively simple approach is the force floating method which utilizes an ultra-low attachment plate to prevent tumor cell adhesion to the surface. Instead, cells aggregate to form multicellular spheroids [22, 23]. Another scaffold-free approach is the hanging drop method during which tumor cells in suspension aggregate into spheroids under gravity. Specifically, small aliquots of cell suspension are dispensed onto a Petri dish lid that is subsequently inverted to allow droplets to hang [24]. Various spheroid culture array plates have also been developed to better stabilize hanging drops [25, 26]. This 3D tumor culture model generates a large number of spheroids with uniform size and morphology which is suitable for biochemical assays and high-throughput screening of therapeutics [25, 27, 28].
Limitations of 3D Cancer Models for Bone
Tumors originating in the breast, prostate, and lung frequently metastasize to other organs, including bone [29, 30]. In addition to interacting with bone-resident cells, tumor cells also come into contact with the mineralized bone matrix, which is orders of magnitude more rigid than soft tissues [31, 32]. Simple 3D models like hydrogels and tumor spheroids improve upon 2D culture methods used to investigate soft tissue tumors and provide structural support for 3D cell growth and adhesion that resembles that of cells in their native environment. However, these approaches fail to recapitulate the mechanical and physical properties of bone which should be considered when investigating tumor interactions with bone, especially mechanically responsive genes. Tissue engineering and scaffolding approaches have been employed to develop biomimetic 3D constructs in order to study metastatic tumors in bone. In fact, hydrogels and other organic ECM components are often combined with more rigid scaffolding materials like ceramics, polymers, and composites to better mimic the bone microenvironment (discussed later in this review).
3D Bone Models
The field of bone tissue engineering has traditionally been focused on regenerating or repairing bone through the development of bone tissue-engineered constructs (TECs). However, advances toward creating TECs for in vivo applications have led to progress in designing biomimetic in vitro models for studying bone biology, disease progression, and drug screening in the bone microenvironment. 3D in vitro bone models have been proposed to aid in bridging the gap between 2D culture and animal models for diseases and medical conditions such as osteomyelitis [33], bone fracture healing [34, 35], and, as will be discussed in this review, tumor metastases. In designing such TECs, studies investigating properties of the constructs indicated that characteristics including rigidity [32, 36], pore size [37, 38], pore shape [39], and curvature [40] all affect cell behavior. Tissue engineers have been working toward creating TECs with precisely controlled physicochemical, mechanical, and structural properties that not only replicate human bone but also allow for the systematic and parametric study of how these factors influence disease progression and drug response.
Materials and Fabrication Methods
The first step in engineering in vitro models is designing the appropriate construct considering the in vivo microenvironment of interest. Bone stands apart from other non-mineralized tissues in that the rigidity of bone (1.7–2.9 × 1010 Pa) is orders of magnitude higher than soft tissues (102–106 Pa) [31, 41]. This unique rigidity necessitates TECs with high mechanical properties not attainable by hydrogels and other ECM-mimicking materials. On the other hand, materials used for cancer models vary widely depending on the origin of the tumor and would ideally exhibit an angiogenic capacity [42, 43]. Thus, in designing TECs for bone applications and 3D cancer models, engineers employ various materials and fabrication methods to create biomimetic matrices on which appropriate cell populations may be cultured.
First and foremost, materials must be biocompatible to avoid eliciting adverse cell responses in vitro. Since cells can sense and respond to the matrix, these biocompatible materials are carefully chosen for characteristics that will have the desired effect on the cell populations to be introduced. Mechanical properties, bioactivity, biodegradability, and chemical composition are characteristics that must be considered when choosing TEC materials for cancer and bone modeling alike. The robust mechanical properties of materials like ceramics, metals, polymers, and composites have rendered them the predominant materials used in fabricating bone TECs. While 3D models for soft tissue tumors are not necessarily subjected to the stringent rigidity restrictions, conferring ECM-like properties and surface modifications with specific proteins and growth factors are desirable to mimic the cancer microenvironment [44].
Synthetic Materials
Multiple poly(α-esters) have been used extensively in bone tissue engineering and for cancer models including poly(caprolactone) (PCL) [45–47], PLA [48, 49], and poly(lactic-co-glycolic acid) (PLGA) [50, 51]. These are bio-compatible and biodegradable polymers that have other biomaterial applications such as drug delivery. They have also been combined with hydroxyapatite and other ceramics to create composite materials that exhibit more bone-like qualities [52]. However, these materials have drawbacks that can limit their effectiveness in bone applications including slow degradation time (PCL), low mechanical properties (PLGA), and low cell adhesion. Polyurethanes (PUR) are a good alternative due to their tunable rigidity, biodegradability, and physicochemical properties [53]. Furthermore, the ease of PUR processing, high mechanical properties, and biostability makes them attractive materials for biomedical bone implants and other bone-mimicking materials. Poly(propylene fumarate) (PPF) has also been incorporated into TECs due to their biocompatibility, biodegradability, and high mechanical properties [54, 55].
Natural Materials
Natural materials have been employed for bone TEC applications as well. Collagen is a versatile material that has obvious appeal for bone applications as it is the main protein constituent of bone and comprises ~10% of bone matrix [56]. Collagen can be prepared into cross-linked solids or gels with varying mechanical properties and is intrinsically resorbable and bioactive. This makes collagen useful for a variety of applications including bone TECs, skin grafts, hydrogels, and sponges for wound healing [57]. Hydroxyapatite (HA) constitutes 50–70% of bone; therefore, HA and other calcium phosphate materials are also appealing for bone applications. Since HA is a ceramic material and not easily formed into 3D structures by conventional means, it is often combined with polymeric materials to create composites that impart both the osteoinductive benefits of HA along with the malleability of polymers. These main components of bone are enticing materials to use for bone TECs due to their physiologic relevance; however, other natural materials have been pursued as bone-like substrates. Silk is another biomaterial used in several biomedical applications because of its mechanical properties and versatility through its receptivity to chemical modifications. Silk can be molecularly engineered to confer specific properties onto the material such as cellular recognition and mineralization, and multiple studies have utilized silk as a biomaterial for studying bone metastases [58–60]. Researchers have even used bone cells alone to create TECs for studying cancer progression. In one such study, an osteoid matrix was constructed by long-term culture of osteoblasts in a bioreactor system prior to co-culture with tumor cells [61].
Creating 3D Bone Morphology
In addition to substrate properties, it is important that biomaterials are able to be manipulated into relevant structures and 3D morphologies. Traditional methods to fabricate porous TECs from natural or synthetic polymers include gas foaming [62, 63], particulate leaching [52, 64, 65], or freeze-drying [50, 66]. While these methods are effective in creating porous scaffolds, they lack the control necessary to create specific architectures.
As the importance of structural properties on which cells are grown becomes more evident, biologists and engineers have looked to new avenues for creating TECs with well-defined architectures. Additive manufacturing (AM), also known as 3D printing, is perhaps the most widely used new technology for creating TECs due to its unparalleled ability to create precisely controlled geometries at increasingly fast speeds [67]. AM, defined as the layer-by-layer fabrication of parts directed by digital information from a 3D computer-aided design file, is an umbrella term that describes multiple methods for creating 3D constructs. These methods include fused deposition modeling [68, 69], stereolithography [48, 70], material jetting (inkjetting) [71], and bioprinting [72, 73]. Other AM methods such as selective laser sintering (SLS) have also been implicated for use in biomaterials applications.
In addition to creating TECs with the appropriate physicochemical, structural, and mechanical properties, researchers are using bioreactors to more closely mimic physiologic flow conditions experience by cells in vivo. To do this, media is perfused through the TECs and circulated within the system, often using a peristaltic pump. Perfusion culture allows cells to experience physiologic shear conditions which are known to affect cell behavior [74]. Furthermore, perfusion culture allows for the flow of nutrients and other soluble factors that can keep cells viable for longer durations.
3D Bone-Tumor Models
Collaborations between biologists and engineers have fostered the combining of the discussed biomaterials and fabrication methods to create 3D bone-tumor models using TECs. These biomimetic models not only aim to confer the appropriate cell-cell interactions but also the appropriate cell-matrix interactions through the creation of TECs with precisely controlled properties that more closely replicate the in vivo bone-tumor milieu.
One such study employed a 3D bioreactor culture system to first create a mineralized, multi-layered tissue of osteoblasts and subsequently co-cultured with osteoclasts and metastatic tumor cells to investigate tumor effect on matrix degradation [75•]. To create the 3D matrix, MC3T3-E1 murine osteoblasts were cultured for up to 10 months to create a 3D osteoid matrix. Osteoclasts were then introduced to the 3D culture, and subsequent matrix degradation was observed. After the addition of fresh MC3T3-E1s, the matrix was reformed, thereby suggesting that bone remodeling was occurring. Finally, metastatic MDA-MB-231 breast cancer cells were added to the 3D co-culture system. By confocal microscopy, it was observed that the cancer cells migrated toward sites of active remodeling which led to further degradation of the matrix. This study not only demonstrates the ability to study the bone remodeling process in vitro but also how this process can be disrupted in a diseased state. This system is also amenable to clinically relevant drug screening since it has a measurable physical outcome (matrix degradation).
In another high-impact study, porous silk fibroin scaffolds prepared directly from the silk fibroin protein of silk worms [76] were used in a 3D culture system to investigate the response of metastatic tumor cells to external stimuli in the presence of osteoblasts and mesenchymal stem cells (MSC) [77]. The silk fibroin scaffolds were hypothesized to be an ideal scaffold for investigating metastatic breast cancer behavior due to its mechanical properties in the range of adipose breast cancer in breast cancer patients, its inherent possession of Asp-Gly-Asp (RGD) peptide sequences known to promote cytocompatibility and cell adhesion, and based on the results of previous studies showing that MSCs undergo osteogenic differentiation on the scaffolds. Co-culture of MG-63 human osteoblast-like cells and MDA-MB-231 human breast cancer cells on the silk scaffolds resulted in a decrease in MG-63 population compared to that of the MDA-MB-231 despite being seeded at a 1:1 ratio. This suggests that the breast cancer cells were inhibiting growth of the osteoblasts, a finding that is supported by previous studies [78]. To take these findings a step further, the effect of the breast cancer cells on matrix mineralization was investigated using the same co-culture. Alizarin red staining and alkaline phosphatase activity indicated that matrix mineralization was lower on the scaffolds containing the breast cancer cells, further confirming the effect of tumor cells on osteoblast viability and function. Further studies in this system indicated that the co-culture significantly increases drug resistance, invasiveness, and angiogenicity.
In a more recent study from the same group, a similar 3D system utilizing silk fibroin scaffolds was used to screen anti-cancer drugs to understand its effect on the cellular interactions of the co-culture of MDA-MD-231 breast cancer cells and MG-63 osteoblastic cells [79•]. A targeted nanoparticle (NP) formulation for the anticancer drug, doxorubicin, was developed using a folate-conjugated silk fibroin polymer. This formulation was then introduced into the 3D co-culture system to test the efficacy of the drug in vitro as well as its targeting ability. After 14 days of co-culture, the viability of the cancer cells decreased while osteoblast morphology and density was not much affected by the presence of doxorubicin. Also noted was the IC50 of the doxorubicin which was 10-fold higher in the 3D system than in the 2D, thus illustrating that tumor cell response to drugs can be drastically different in 3D compared to 2D. Further, they found that drug treatment reduced vascular endothelial growth factor (VEGF) expression and glucose consumption, suggesting a downregulation of angiogenic factors and slowed proliferation of cancer cells, respectively. While the effect of the targeted NP formulation did not necessarily produce notably improved results over free doxorubicin, this study shows that the 3D co-culture system is capable of screening anticancer drugs and outcomes in 3D are notably different than in 2D.
Other studies have incorporated mechanical loading into the model to test how mechanical stress affects bone and cancer cell behavior. One such study employed HA-containing PLGA scaffolds under cyclic compression to investigate the interactions between MDA-MB-231 breast cancer cells and human bone marrow-derived mesenchymal stem cells (hBM-MSCs) in the mechanically stressed environment [80•]. Before investigating mechanical loading, HA-containing scaffolds were seeded with hBM-MSCs and treated with tumor-conditioned media to observe how tumor-derived soluble factors influence osteogenic behavior. Interestingly, it was found that alkaline phosphatase (ALP) activity increased in the presence of tumor-conditioned media. This finding runs contrary to previous findings concluding that tumors tend to inhibit osteogenic differentiation. The authors suggest this could be a result of the early timing at which the hBM-MSCs were exposed to the conditioned media. Upon cyclic compression of HA-containing scaffolds seeded with hBM-MSCs and supplemented with conditioned media from mechanically loaded MDA-MD-231, there was no notable effect on ALP activity as seen in the non-loaded case. However, gene expression of the osteogenic marker osteopontin (OPN) significantly increased when the hBM-MSCs and MDA-MB-231s were mechanically loaded, and these findings were corroborated by measuring OPN protein levels. These data suggest that mechanical loading influences the interaction between hBM-MSCs and tumor cells through modulation of OPN levels. This study stresses the important role mechanical loading plays in bone-tumor interactions, which emphasizes the need to further investigate the effects of dynamic mechanical forces on tumor progression in bone.
As stated earlier, additive manufacturing has become a valuable tool for in vitro modeling due to its ability to create precise 3D geometries. Considering that bone has a complex and intricate structure, it is perhaps not surprising that 3D printing has started to influence 3D bone-tumor modeling. A recent study utilized a stereolithography 3D printing technique to create HA-composite scaffolds for modeling breast cancer bone metastases [81•]. First, a PEG-based ink was printed into several pore geometries including square and hexagonal. After choosing the optimal geometry (small square pores) based on cell proliferation of MDA-MB-231 cells, HA was incorporated into the PEG ink (10% HA) and printed. The breast cancer cells grown on the HA-containing scaffolds proliferated significantly faster than the non-HA scaffolds, suggesting the HA component of bone promotes tumor proliferation. To study tumor cell migration on the HA-containing scaffolds, a non-metastatic breast cancer cell line (MCF-7) was also introduced and compared to the MDA-MB-231 cells. MDA-MB-231 cells cultured on the matrices migrated significantly farther than the MCF-7 in both the HA and non-HA scaffolds. They further tested the efficacy of the chemotherapeutic 5-FU in both the 3D and 2D environment. While 5-FU treatment did show efficacy in both environments, the efficacy was significantly less in 3D culture than in 2D. This study highlights the potential impact that 3D printing can have in in vitro modeling. It is evident from this study that curvature affects cell proliferation; therefore, it can be conjectured that 3D architecture may play an important role in tumor progression in bone.
Future of 3D Models
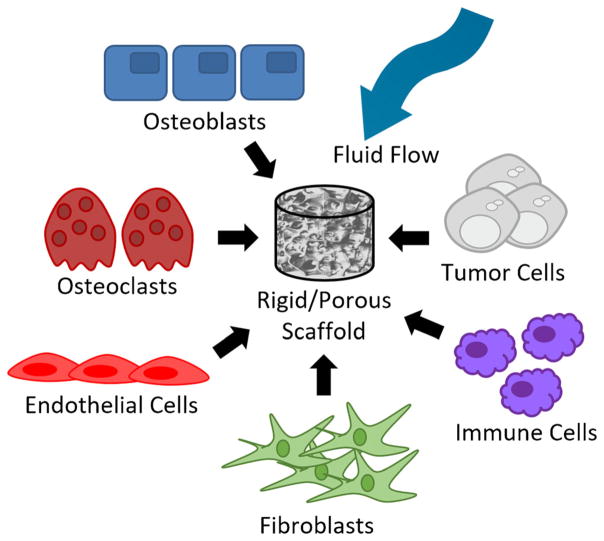
3D models have drastically improved over the past 5 years. However, existing 3D bone models only focus on a few aspects of the bone microenvironment. As researchers continue to study this niche, models will not only begin to incorporate more physical properties (rigidity, fluid flow, compression, pore size) but also different cell types (osteoblasts, osteoclasts, endothelial cells, fibroblasts, immune cells) (Fig. 1). This will be an exceedingly complex undertaking that will likely take many years to accomplish as well as a variety of expertise from different groups. Additionally, researchers have begun modeling dynamic cellular interactions and processes in bone using computational models in order to develop a more complete understanding of the bone microenvironment and to predict outcomes [82, 83]. These dynamic 3D models will significantly improve our ability to screen and develop new drugs to treat bone diseases including tumor-induced bone disease. One important objective is to increase usage of patient-derived cells in these 3D models to help predict patient outcomes to novel therapeutics.
Fig. 1.
The incorporation of additional physical and cellular components in 3D bone models will help increase our understanding of the dynamic interactions in the bone microenvironment
Acknowledgments
This work was supported by 1I01BX001957 (JAS), 1R01CA163499 (SAG/JAS), 5R01 AR064772 (SAG), and 5T32CA009592 (KAK).
Footnotes
Compliance with Ethical Standards
Conflict of Interest Kristin Kwakwa, Joseph Vanderburgh, Julie Sterling, and Scott Guelcher declare no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Ma XH, Piao S, Wang D, Mcafee QW, Nathanson KL, Lum JJ, et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17:3478–89. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JM, Mhawech-Fauceglia P, Lee N, Parsanian LC, Lin YG, Gayther SA, et al. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab Investig. 2013;93:528–42. doi: 10.1038/labinvest.2013.41. [DOI] [PubMed] [Google Scholar]
- 3.Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep. 2015;33:1837–43. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 4.Vantangoli MM, Madnick SJ, Huse SM, Weston P, Boekelheide K. MCF-7 human breast cancer cells form differentiated microtissues in scaffold-free hydrogels. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, Knuuttila M, et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One. 2010;5:e10431. doi: 10.1371/journal.pone.0010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cichon MA, Gainullin VG, Zhang Y, Radisky DC. Growth of lung cancer cells in three-dimensional microenvironments reveals key features of tumor malignancy. Integr Biol. 2012;4:440–8. doi: 10.1039/c1ib00090j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer KL, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One. 2013;8:e59689. doi: 10.1371/journal.pone.0059689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, Gupta PB. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18:1–13. doi: 10.1186/s13058-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Gurski LA, Zhang C, Harrington DA, Farach-Carson MC, Jia X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33:9049–60. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–12. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys J. 2014;107:2546–58. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Lovitt CJ, Shelper TB, Avery VM. Evaluation of chemotherapeutics in a three-dimensional breast cancer model. J Cancer Res Clin Oncol. 2015;141:951–9. doi: 10.1007/s00432-015-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 15.Chung IM, Enemchukwu NO, Khaja SD, Murthy N, Mantalaris A, García AJ. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials. 2008;29:2637–45. doi: 10.1016/j.biomaterials.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurski LA, Petrelli NJ, Jia X, Farach-Carson MC. 3D matrices for anti-cancer drug testing and development. Oncol Issues. 2010;25:20–5. [Google Scholar]
- 17.Gill BJ, Gibbons DL, Roudsari LC, Saik JE, Rizvi ZH, Roybal JD, et al. A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model. Cancer Res. 2012;72:6013–23. doi: 10.1158/0008-5472.CAN-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Bufalo F, Manzo T, Hoyos V, Yagyu S, Caruana I, Jacot J, et al. 3D modeling of human cancer: a PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus. Biomaterials. 2016;84:76–85. doi: 10.1016/j.biomaterials.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan S, Hassani I, Seeto WJ, Lipke EA. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J Biomed Mater Res Part A. 2017;105:236–52. doi: 10.1002/jbm.a.35899. [DOI] [PubMed] [Google Scholar]
- 20.Feder-Mengus C, Ghosh S, Reschner A, Martin I, Spagnoli GC. New dimensions in tumor immunology: what does 3D culture reveal? Trends Mol Med. 2008;14:333–40. doi: 10.1016/j.molmed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6:19103. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:1–20. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18:240–9. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun. 2013;433:327–32. doi: 10.1016/j.bbrc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Tung Y-C, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–8. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amann A, Zwierzina M, Gamerith G, Bitsche M, Huber JM, Vogel GF, et al. Development of an innovative 3D cell culture system to study tumour—stroma interactions in non-small cell lung cancer cells. PLoS One. 2014;9:e92511. doi: 10.1371/journal.pone.0092511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan S, Ward MR, Rowley KR, Wold RM, Takayama S, Buckanovich RJ, et al. Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol Oncol. 2015;138:181–9. doi: 10.1016/j.ygyno.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghavan S, Mehta P, Horst EN, Ward MR, Rowley KR, Mehta G. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7:16948–61. doi: 10.18632/oncotarget.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboulafia AJ, Levine AM, Schmidt D, Aboulafia D. Surgical therapy of bone metastases. Semin Oncol. 2007;34:206–14. doi: 10.1053/j.seminoncol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RW, Schipani E, Giaccia AJ. HIF targets in bone remodeling and metastatic disease. Pharmacol Ther. 2015;150:169–77. doi: 10.1016/j.pharmthera.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guelcher SA, Sterling JA. Contribution of bone tissue modulus to breast cancer metastasis to bone. Cancer Microenviron. 2011;4:247–59. doi: 10.1007/s12307-011-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page JM, Merkel AR, Ruppender NS, Guo R, Dadwal UC, Cannonier SA, et al. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials. 2015;64:33–44. doi: 10.1016/j.biomaterials.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Alvarez R, Izquierdo-Barba I, Vallet-Regí M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2016;49:113–26. doi: 10.1016/j.actbio.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Sundelacruz S, Li C, Choi YJ, Levin M, Kaplan DL. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials. 2013;34:6695–705. doi: 10.1016/j.biomaterials.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Gu Y, Wang H, Lee WY. Microfluidic 3D bone tissue model for high-throughput evaluation of wound-healing and infection-preventing biomaterials. Biomaterials. 2012;33:999–1006. doi: 10.1016/j.biomaterials.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, et al. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051–62. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo R, Lu S, Page JM, Merkel AR, Basu S, Sterling JA, et al. Fabrication of 3D scaffolds with precisely controlled substrate modulus and pore size by templated-fused deposition modeling to direct osteogenic differentiation. Adv Healthc Mater. 2015;4:1826–32. doi: 10.1002/adhm.201500099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Parker ST, Syoji D, Wang X, Lewis JA, Kaplan DL. Direct-write assembly of 3D silk/hydroxyapatite scaffolds for bone co-cultures. Adv Healthc Mater. 2012;1:729–35. doi: 10.1002/adhm.201200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Fratzl P, Dunlop JWC. Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv Healthc Mater. 2013;2:186–94. doi: 10.1002/adhm.201200159. [DOI] [PubMed] [Google Scholar]
- 40.Gamsjager E, Bidan CM, Fischer FD, Fratzl P, Dunlop JWC. Modelling the role of surface stress on the kinetics of tissue growth in confined geometries. Acta Biomater. 2013;9:5531–43. doi: 10.1016/j.actbio.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Sterling JA, Guelcher SA. Bone structural components regulating sites of tumor metastasis. Curr Osteoporos Rep. 2011;9:89–95. doi: 10.1007/s11914-011-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 44.Schuessler TK, Chan XY, Chen HJ, Ji K, Park KM, Roshan-Ghias A, et al. Biomimetic tissue-engineered systems for advancing cancer research: NCI strategic workshop report. Cancer Res. 2014;74:5359–63. doi: 10.1158/0008-5472.CAN-14-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res Part A. 2014;102:4317–25. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 46.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 47.Petrie Aronin CE, Cooper JA, Sefcik LS, Tholpady SS, Ogle RC, Botchwey EA. Osteogenic differentiation of dura mater stem cells cultured in vitro on three-dimensional porous scaffolds of poly(epsilon-caprolactone) fabricated via co-extrusion and gas foaming. Acta Biomater. 2008;4:1187–97. doi: 10.1016/j.actbio.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillaume O, Geven MA, Sprecher CM, Stadelmann VA, Grijpma DW, Tang TT, et al. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017;54:386–98. doi: 10.1016/j.actbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Gay S, Arostegui S, Lemaitre J. Preparation and characterization of dense nanohydroxyapatite/PLLA composites. Mater Sci Eng C. 2009;29:172–7. [Google Scholar]
- 50.Grinberg O, Binderman I, Bahar H, Zilberman M. Highly porous bioresorbable scaffolds with controlled release of bioactive agents for tissue-regeneration applications. Acta Biomater Acta Materialia Inc. 2010;6:1278–87. doi: 10.1016/j.actbio.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 51.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008;5:849–62. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Wu H, Wu H, Lù Z, Deng C, Hong Z, et al. RGD-conjugated copolymer incorporated into composite of poly(lactide-co-glycotide) and poly(L-lactide)-grafted nanohydroxyapatite for bone tissue engineering. Biomacromolecules. 2011;12:2667–80. doi: 10.1021/bm2004725. [DOI] [PubMed] [Google Scholar]
- 53.Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 54.Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–12. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 55.Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10:1810–7. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chattopadhyay S, Raines RT. Collagen-based biomaterials for wound healing. Biopolymers. 2015;101:821–33. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reagan MR, Mishima Y, Glavey SV, Zhang Y, Manier S, Lu ZN, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124:3250–9. doi: 10.1182/blood-2014-02-558007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon H, Kim HJ, Rice WL, Subramanian B, Park S, Georgakoudi I, et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J Tissue Eng Regen Med. 2010;4:590–9. doi: 10.1002/term.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mastro AM, Vogler EA. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer Res. 2009;69:4097–100. doi: 10.1158/0008-5472.CAN-08-4437. [DOI] [PubMed] [Google Scholar]
- 62.Thein-Han W, Xu HHK. Prevascularization of a gas-foaming macroporous calcium phosphate cement scaffold via coculture of human umbilical vein endothelial cells and osteoblasts. Tissue Eng Part A. 2013;19:1675–85. doi: 10.1089/ten.tea.2012.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Annabi N, Fathi A, Mithieux SM, Martens P, Weiss AS, Dehghani F. The effect of elastin on chondrocyte adhesion and proliferation on poly (epsilon-caprolactone)/elastin composites. Biomaterials Elsevier Ltd. 2011;32:1517–25. doi: 10.1016/j.biomaterials.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 64.Akar B, Jiang B, Somo SI, Appel AA, Larson JC, Tichauer KM, et al. Biomaterials with persistent growth factor gradients in vivo accelerate vascularized tissue formation. Biomaterials. 2015;72:61–73. doi: 10.1016/j.biomaterials.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Zhou H, Yang K, Yuan Y, Liu C. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials. 2013;34:9381–92. doi: 10.1016/j.biomaterials.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 66.Fereshteh Z, Fathi M, Bagri A, Boccaccini AR. Preparation and characterization of aligned porous PCL/zein scaffolds as drug delivery systems via improved unidirectional freeze-drying method. Mater Sci Eng C. 2016;68:613–22. doi: 10.1016/j.msec.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Vanderburgh J, Sterling JA, Guelcher SA. 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann Biomed Eng. 2016:1–16. doi: 10.1007/s10439-016-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001;55:203–16. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 69.Guo R, Merkel AR, Sterling JA, Davidson JM, Guelcher SA. Substrate modulus of 3D-printed scaffolds regulates the regenerative response in subcutaneous implants through the macrophage phenotype and Wnt signaling. Biomaterials. 2015;73:85–95. doi: 10.1016/j.biomaterials.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S-J, Nowicki M, Harris B, Zhang LG. Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng Part A. 2017 doi: 10.1089/ten.TEA.2016.0353. [DOI] [PubMed]
- 71.Li G, Cuidi L, Fangping C, Changsheng L. Fabrication and characterization of toughness-enhanced scaffolds comprising beta-TCP/ POC using the freeform fabrication system with micro-droplet jetting. Biomed Mater. 2015;10:35009. doi: 10.1088/1748-6041/10/3/035009. [DOI] [PubMed] [Google Scholar]
- 72.Kundu J, Shim JH, Jang J, Kim SW, Cho DW. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9:1286–97. doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 73.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–9. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 74.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–5. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Krishnan V, Vogler EA, Mastro AM. Three-dimensional in vitro model to study osteobiology and osteopathology. J Cell Biochem. 2015;116:2715–23. doi: 10.1002/jcb.25250. This study uses a 3D bioreactor tri-culture model to demonstrate that tumor cells migrate towards sites of active bone remodeling which results in further degradation of osteoid matrix. [DOI] [PubMed] [Google Scholar]
- 76.Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927–37. doi: 10.1016/j.biomaterials.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talukdar S, Kundu SC. Engineered 3D silk-based metastasis models: interactions between human breast adenocarcinoma, mesenchymal stem cells and osteoblast-like cells. Adv Funct Mater. 2013;23:5249–60. [Google Scholar]
- 78.Mastro AM, Gay CV, Welch DR, Donahue HJ, Jewell J, Mercer R, et al. Breast cancer cells induce osteoblast apoptosis: a possible contributor to bone degradation. J Cell Biochem. 2004;91:265–76. doi: 10.1002/jcb.10746. [DOI] [PubMed] [Google Scholar]
- 79•.Subia B, Dey T, Sharma S, Kundu SC. Target specific delivery of anticancer drug in silk fibroin based 3D distribution model of bone–breast cancer cells. ACS Appl Mater Interfaces. 2015;7:2269–79. doi: 10.1021/am506094c. This study reports that the viability, invasiveness, and angiogenic potential of cancer cells co-cultured with osteoblasts on silk scaffolds significantly decreases after nanoparticle-targeted delivery of doxorubicin; osteoblasts were mostly unaffected by treatment. [DOI] [PubMed] [Google Scholar]
- 80•.Lynch ME, Chiou AE, Lee MJ, Marcott SC, Polamraju PV, Lee Y, et al. Three-dimensional mechanical loading modulates the osteogenic response of mesenchymal stem cells to tumor-derived soluble signals. Tissue Eng Part A. 2016;22:1006–15. doi: 10.1089/ten.tea.2016.0153. This paper emphasizes the important role that mechanical stress plays on osteogenic cells cultured on HA-containing scaffolds in the presence of tumor-conditioned media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.Zhu W, Holmes B, Glazer RI, Zhang LG. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine Nanotechnol Biol Med. 2016;12:69–79. doi: 10.1016/j.nano.2015.09.010. This study utilizes a novel stereolithography-based 3D printer to fabricate nanohydroxyapatite scaffolds that promote tumor spheroid formation, proliferation, and migration; tumor cells also exhibit more chemoresistance on 3D scaffolds. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Pivonka P, Buenzli PR, Smith DW, Dunstan CR. Computational modeling of interactions between multiple myeloma and the bone microenvironment. PLoS One. 2011;6:e27494. doi: 10.1371/journal.pone.0027494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Araujo A, Cook LM, Lynch CC, Basanta D. An integrated computational model of the bone microenvironment in bone-metastatic prostate cancer. Cancer Res. 2014;14:2391–401. doi: 10.1158/0008-5472.CAN-13-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]