Significance
Shifts in the timing of species interactions are often cited as a consequence of climate change and, if present, are expected to have wide-reaching implications for ecological communities. Our knowledge about these shifts mostly comes from single systems, which have provided no clear picture, thus limiting our understanding of how species interactions may be responding overall. Using a new global database based on long-term data on the seasonal timing of biological events for pairwise species interactions, we find that the relative timing of interacting species has changed substantially in recent decades. The observed shifts are greater in magnitude than before recent climate change began, suggesting that there will be widespread warming-related shifts in the synchrony of species in the future.
Keywords: mismatch, trophic interactions, global warming, time series, baseline
Abstract
Phenological responses to climate change (e.g., earlier leaf-out or egg hatch date) are now well documented and clearly linked to rising temperatures in recent decades. Such shifts in the phenologies of interacting species may lead to shifts in their synchrony, with cascading community and ecosystem consequences. To date, single-system studies have provided no clear picture, either finding synchrony shifts may be extremely prevalent [Mayor SJ, et al. (2017) Sci Rep 7:1902] or relatively uncommon [Iler AM, et al. (2013) Glob Chang Biol 19:2348–2359], suggesting that shifts toward asynchrony may be infrequent. A meta-analytic approach would provide insights into global trends and how they are linked to climate change. We compared phenological shifts among pairwise species interactions (e.g., predator–prey) using published long-term time-series data of phenological events from aquatic and terrestrial ecosystems across four continents since 1951 to determine whether recent climate change has led to overall shifts in synchrony. We show that the relative timing of key life cycle events of interacting species has changed significantly over the past 35 years. Further, by comparing the period before major climate change (pre-1980s) and after, we show that estimated changes in phenology and synchrony are greater in recent decades. However, there has been no consistent trend in the direction of these changes. Our findings show that there have been shifts in the timing of interacting species in recent decades; the next challenges are to improve our ability to predict the direction of change and understand the full consequences for communities and ecosystems.
While the most common ecological response to climate change is an advance in seasonal timing, substantial variation has been observed within and across taxonomic groups, including between directly interacting species (1–5). One of the potential outcomes of this variation is a directional change in the relative timing of interacting species (i.e., a change in phenological synchrony). Many researchers hypothesize that climate change will lead to significant changes in synchrony, with potential negative consequences for those interacting species and their ecological communities in some (1, 2, 6, 7) but not all (8–10) contexts.
It is commonly thought that warming will lead to changes in synchrony (11–13). These changes are expected to be prevalent because (i) temperature is an important phenological cue for many taxonomic groups (14), (ii) the temperature sensitivity of the phenology of interacting species can differ (2, 15) and, (iii) global temperatures have increased, on average, by 0.85 °C since 1880 (16). Indeed, there is evidence from single systems, as well as from reviews (17, 18), that many interacting species are shifting their phenologies at different rates, leading to changes in synchrony (7, 19–21). To date, however, there have been no quantitative assessments of shifts across studies for species that directly interact, leaving open the question of how prevalent and large such shifts may be. Indeed, evidence from observations and small-scale experiments suggests that maintenance of synchrony in the context of environmental change could be common (1, 22–26). Examples from directly interacting species show that synchrony has been sustained (27, 28). Others show that the degree of changes in synchrony can vary across populations (29–31) or has been less than expected (32, 33). These examples, along with theoretical considerations (further discussion provided in SI Appendix, section 1), question whether shifts toward asynchrony should be widespread (1, 8, 10). Considered together, the evidence to date does not provide a clear picture of how prevalent and large shifts in synchrony have been in response to recent climate change. Here, we use a quantitative meta-analysis to assess shifts in the synchrony of directly interacting species to provide perspective and to estimate global trends in synchrony change.
We used published time-series data of phenological events from pairwise species interactions to test (i) whether there have been recent directional changes in synchrony and (ii) whether these shifts can be attributed to climatic warming. Establishing these links is critical for robust predictions of future shifts in synchrony due to climate change.
Our meta-analytic approach identified 27 studies with time-series phenological data for >4 years that include 970 study years and spanned the years 1951–2013 (average first year was 1984). Our dataset includes 88 species that span a wide range of taxonomic groups, from aquatic and terrestrial ecosystems across four continents, and consists of 54 pairwise species interactions (trophic and nontrophic) that vary in their interaction strengths. To estimate synchrony change, we first estimated phenological change (days/decade) for each species using a Bayesian hierarchical model. Our model estimates both species-level responses and the distribution from which they are drawn, yielding a higher-level estimate of the overall response across species. With this approach we accounted for variation in variance and sample size across species, thus accounting for methodological differences across studies. Because we are interested in phenological change in response to climate change, we used a hinge model with 1981 as the inflection point, when a major shift in the temporal trend of temperature occurred (16) (SI Appendix, Fig. S2). This allowed us to estimate change across time series more accurately with varying start dates and prevented bias toward weaker effects in longer time series (more discussion is provided in SI Appendix, section 2). We estimated the magnitude of change in synchrony by taking the absolute value of the difference between slopes (days/decade) of interacting species, with zero indicating no change in synchrony and larger differences in slope indicating a larger change in synchrony. We also evaluated the magnitude and directionality of change in synchrony (henceforth referred to as “overall shifts”) by simply taking the difference in slopes; here, negative values indicate the timings of interacting species are getting further apart and positive values signify that the two events are getting closer together.
To put the magnitude of observed changes in synchrony into context and to quantify how much variation may be due to noise (i.e., factors other than climate change), we take an important step toward testing the hypothesis that recent shifts in synchrony have diverged from some baseline level of synchrony that existed before large changes in climate occurred in the early 1980s (9). We designed two types of null models that estimate the amount of natural variation in either the magnitude or overall change in synchrony among interacting species before recent climate change began, which we then compared with changes after recent climate change started (SI Appendix, section 2).
We also assessed whether changes in synchrony were related to recent temperature change. To do so, we correlated long-term trends in temperature (°C/decade) with individual species’ phenological shifts (days/decade), as well as synchrony changes (days/decade). Temperature change was evaluated for the measure of temperature identified as the strongest phenological cue by individual studies. These analyses were done on a subset of the data, as temperature data are only available for 37 species from 22 interactions. To calculate temperature change, we used a similar hierarchical hinge model, as explained above.
Results and Discussion
Since the early 1980s, phenology advanced across species by 4.0 days/decade [95% credible interval (CI): −5.5, −2.4; n = 88], which was significantly greater than advances estimated before 1981 (2.7 days/decade; 95% CI: −6.4, 1.4; n = 31; difference = 0.94 days/decade; 95% CI: 0.86, 1.0). Our observed magnitude of phenological change is similar to other recent syntheses (34–36).
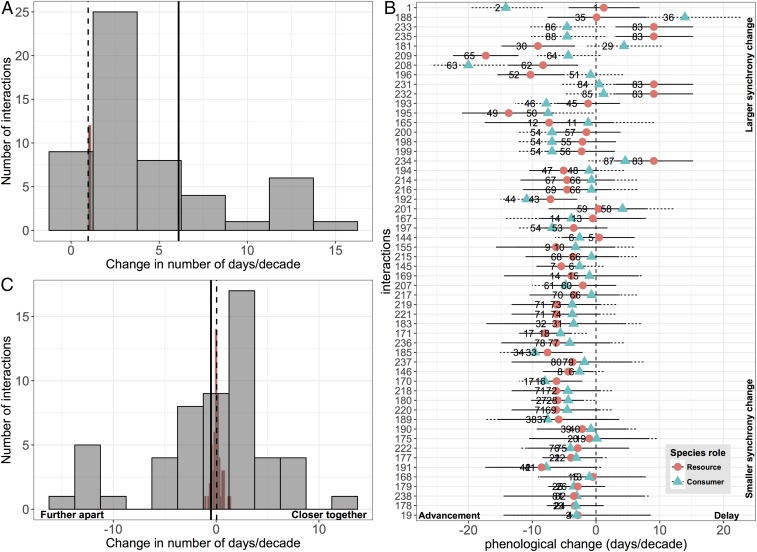
We found that the relative timing of interacting species has substantially changed compared with 35 years ago. Synchrony shifted in magnitude by 6.1 days/decade (with zero indicating no change in synchrony; 95% CI: 5.2, 7.0; n = 54; Fig. 1A), which was substantially greater than the magnitude of synchrony shifts estimated from our null model based on shifts before 1981 (0.97 days/decade; 95% CI: 0.96, 0.98; null model details provided in SI Appendix, section 2). There was no consistency in the direction of shifts (−0.50 days/decade, 95% CI: −2.1, 1.1; n = 54; Fig. 1C): while timing for the majority of interacting species is shifting closer together (57% of interactions; 31 of 54) (Fig. 1C), the phenologies of many interacting species are shifting further apart (43% of interactions; 23 of 54). Most interacting species advanced their phenology by similar magnitudes, leading to smaller overall changes (Fig. 1B). Nevertheless, overall shifts were 10 times greater in magnitude than expected based on our null model estimating synchrony shifts before 1981 (−0.50 days/decade vs. 0.048 days/decade; 95% CI: −0.08, 0.2; Fig. 1C) and five times greater than the overall synchrony change observed until 1981 (−0.50 days/decade vs. 0.097 days/decade; 95% CI: −1.7, 1.9; n = 16).
Fig. 1.
Changes in the relative timing of interacting species (n = 54). (A) The magnitude of synchrony change (days/decade) across interactions, with zero indicating no change in synchrony. Presented are the species-level intercepts (αs) from the intercept-only model. The solid vertical line denotes the mean synchrony change (μα = 6.1 days/decade; 95% CI: 5.2, 7.0). The distribution in red represents the null model, with the dashed line as the mean synchrony change (μα = 0.97 days/decade; 95% CI: 0.96, 0.98). (B) Phenological change (days/decade) across interacting species. Interactions are ordered top to bottom by the magnitude of synchrony change. Numbers on the y axis represent their unique identifier in the analysis. Means with 95% CIs are shown. To improve viewing, overlapping labels have been removed. (C) The direction and magnitude of synchrony change (days/decade) across interactions, where positive values indicate that the timing of interacting species is getting closer together in the season (days) and negative values mean that the timing of interacting species is getting further apart in the season (days). Presented are the species-level intercepts (αs) from the intercept-only model. The solid vertical line denotes the mean synchrony change (μα = −0.50 days/decade; 95% CI: −2.1, 1.1). The distribution in red represents the null model, with the dashed line as the mean synchrony change (μα = 0.048 days/decade; 95% CI: −0.08, 0.2).
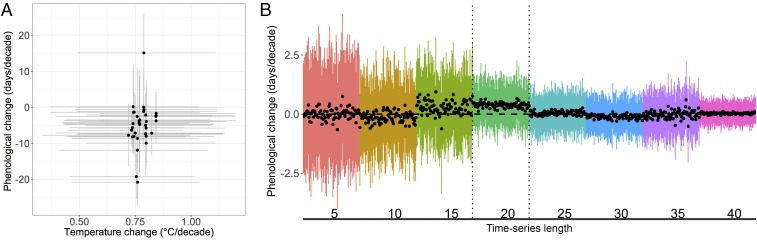
The recent changes in phenology and synchrony we detected are consistent but could not be directly linked with warming since the early 1980s. As expected, the phenologies of species in this dataset were sensitive to temperature (−4.8 d/°C; 95% CI: −6.6, −3.0; n = 37; SI Appendix, Fig. S3A): warmer temperatures led to earlier phenology. Also as expected, temperatures increased over the past 35 years (0.8 °C/decade; 95% CI: 0.5, 1.0; n = 18; SI Appendix, Fig. S3B). Despite these relationships, neither the observed shifts in phenology nor synchrony were significantly related to differences in temperature change since the early 1980s [phenology: −0.02 d/°C; 95% CI: −0.3, 0.3; n = 37 (Fig. 2A); synchrony: −0.1 d/°C; 95% CI: −0.5, 0.2; n = 22].
Fig. 2.
Uncertainty in estimates of phenological and temperature change. (A) Relationship between phenological change (days/decade) and temperature change (°C/decade) across species (n = 37). Means with 95% CIs for both axes are shown. (B) Effect of time-series length (5–40 years, in increments of 5, assessed using a null model) on estimates of species’ phenological change (days/year) represented as black dots (n = 31), with 95% CIs represented by colored lines. Dotted lines bracket 20 years of data, corresponding to the average length of time series (mean = 21.7 years, SD = 8.4).
Our study is relatively rare among synthetic studies in attempting to attribute phenological changes directly to long-term trends in temperature, rather than interannual variations in temperature (2, 35, 37); the few previous efforts have found variable results (15, 36, 38, 39). More commonly, recent changes in biological traits (e.g., range shifts) are inferred to be due in part to climate change, without rigorous attribution (40).
The difficulty we found in attributing changes in phenology or synchrony to temperature change could be a function of both methodology and biology. In our synthesis, there is a great deal of uncertainty in the estimates of temperature and of phenological change over time (Fig. 2A). Many methodological challenges related to estimating phenological shifts over time (e.g., sampling design, spatial scale, changes in population size) can add uncertainty to estimates (40). Given that our estimated responses and interannual variation in the data were similar to previous studies, we suspect that the high uncertainty was primarily related to the short length of many of our time series. The mean dataset length was 21.7 years (SD = 8.4, range = 6–37), with aquatic datasets being longer on average than terrestrial ones (24 vs. 20.3 years, respectively) and datasets at lower latitudes being longer than higher latitudes (SI Appendix, Fig. S4). The null model we built in which we varied the length of the time series (SI Appendix, section 2) supports this hypothesis (Fig. 2B): we were more likely to retrieve the true value for phenological change and with less uncertainty with longer time series than currently available. We also likely had low power due to the small variation in temperature change among species (0.7–0.8 °C/decade) relative to the uncertainty of the estimates of temperature change. We note, however, that our hierarchical model structure will generally reduce variation in the study-level estimates of temperature change when most studies’ time series are short and noisy (as is the case here); this makes it difficult to identify whether the actual temperature change across studies was small or whether the combination of low sample size and high uncertainty made it difficult to robustly estimate differences across studies in temperature change. Finally, it could also be difficult to predict phenological shifts from temperature changes when temperature change is measured as a trend. While we detected species-specific sensitivities to temperature on an annual scale (SI Appendix, Fig. S3A), these responses may be weakened when change is estimated (and thus averaged) across years.
Biologically, it is challenging to link phenological shifts directly to temperature changes because temperature is a complex phenological cue. Temperature can influence phenology differently depending on when (41) or how (42–44) temperature changes, or the importance of other environmental factors (45, 46). While all studies in our temperature attribution analysis indicated that temperature was a phenological cue for at least one of the species, temperature alone might be too simple an indicator for these events. For example, in marine ecosystems, nutrient availability, mixing, solar irradiance, stratification, and grazing all regulate the timing and magnitude of phenological shifts (36). Improved mechanistic understanding of phenological cues would greatly help in understanding and predicting synchrony changes (14).
While the timing of interacting species has shifted in recent decades, predicting which interactions are likely to experience greater shifts in synchrony, how the interaction will change, and the biological importance of these shifts remain uncertain. First, the interactions included in this review differ in many respects that could influence the magnitude and direction of synchrony change in response to climate change. For example, the phenology of species in more specialized interactions may rely to a greater extent on similar cues than those in less specialized interactions, which could lead to smaller overall climate-driven changes in synchrony for more specialized interactions (but see ref. 47). Changes in synchrony can have fitness consequences (7, 48, 49) and influence ecosystem-level properties like primary productivity (6) and pollination (50), but such consequences are not always found (51, 52) or they are scale dependent (53, 54). This variation in responses is driving a growing discussion about how common ecological consequences of shifts in synchrony are likely to be (8, 9, 55). It is unclear the extent to which species will be able to adapt evolutionarily to restore synchrony (10, 56). Moreover, in systems where asynchrony may be the baseline state (i.e., without climate change) (9) or for less specialized interactions (57), climate change-driven shifts in synchrony may not lead to substantial effects on fitness in those interactions. The question of determining how synchronous a population and its food resources, or a resource and its consumer, should be—both theoretically and empirically—remains an ongoing challenge. Understanding the baseline level of synchrony, as well as the factors determining this level, will be important for evaluating the consequences of synchrony changes for an interaction due to climate change.
Our findings provide a step toward understanding how climate change is influencing the timing of interacting species. While the relative timing of key life cycle events between interacting species is significantly different from what it was 35 years ago, there has been no consistent trend in the direction of these changes: the seasonal timing of some interacting species are now closer together, while others are further apart. However, any change in the relative timing of interacting species could represent a disruption in that interaction leading to fitness consequences for one or both. Small overall changes in synchrony are likely a function of interacting species shifting their phenologies at similar rates. Although we only found a weak relationship between temperature change and species’ phenological shifts, the estimated changes in phenology and synchrony we detected were greater than predicted based on the estimated levels of synchrony before recent climate change began, suggesting that climate change could have caused these shifts. Our findings suggest that there will be widespread climate change-related shifts in the synchrony of species interactions in the future; the next challenges are to improve our ability to predict the direction of change and to understand the full consequences for communities and ecosystems.
Methods
Literature Search.
To build the database, we located papers that recorded phenology for >4 years for interacting species by conducting keyword searches in Web of Science up to August 2015. Authors had to be explicit that the two species interacted (e.g., specifying type of interaction). However, the interpretation of this definition by authors and the degree to which two species interacted (i.e., interaction strength) likely varied across studies. We were not able to quantify the strength of interactions or account for this potential source of variation in our analyses given that this level of detail was not reported in most studies (SI Appendix, section 2). Phenology was typically measured as the first date of occurrence, which can be sensitive to changes in population size and sampling frequency (57). However, additional analyses suggest that the key results are unaffected by this data limitation (SI Appendix, section 3). In total, there were 54 unique pairwise species interactions among 27 studies (SI Appendix, Table S8) with time-series phenological data that spanned the years 1951–2013. Our dataset included 88 species that spanned a wide range of taxonomic groups from aquatic and terrestrial ecosystems across four continents (SI Appendix, Fig. S1).
Pre-Recent Climate Change Data.
To test for evidence that phenology and synchrony shifted alongside major climatic change, we defined a “pre-recent climate change” dataset. To do this, we subset the phenological data for years before and including 1981 (see SI Appendix, section 2 for more discussion on choosing 1981). We excluded time series for species with <5 years of data before 1981. The pre-recent climate change dataset included 31 species from 11 studies and the mean time-series length was 12 years. More information on how these data were used is provided in the Statistical Modeling section below and in SI Appendix, section 2.
Temperature Data.
For those studies that considered temperature as a main phenological cue for at least one of the interacting species, we extracted temperature data for all papers when possible; otherwise, we contacted authors to request the data underlying their analyses. We included temperature data for those years with phenological data for both species. In total, there were 37 species with temperature data from 22 interactions and 13 studies (SI Appendix, Table S1).
Statistical Modeling.
To account for variation in variance and sample size across species, thus accounting for key methodological differences across studies, we used Bayesian hierarchical modeling for all analyses. All statistical analyses were conducted in the R 3.3.2 environment (58). The models were fit using the programming language Stan (59) (www.mc-stan.org), accessed via the rstan package (version 2.14.1). All estimated model parameters, as well as variance parameters, were assigned weakly noninformative priors (see individual models for details in SI Appendix, section 5). Estimates in the text refer to the overall response (e.g., see μβ below) across 3,000 iterations with 95% CIs unless otherwise noted.
To estimate changes in phenology, temperature, and synchrony over time, as well as to evaluate phenological sensitivity to temperature, a two-level hierarchical model with partially pooled slopes (also commonly referred to as random slopes) was used (SI Appendix, section 5, model 1). All models followed the same basic equation:
where y is temperature or phenology (depending on the model), x is year, i represents a single observation, s represents species, and ŷ is the predicted value of y. When we fit a linear trend (i.e., nonhinge model), xi = (yeari − 1981). When we fit a hinge model (for studies that met our criteria, see below) years before and equal to 1981 were set to 0 (xi = 0), whereas xi = (yeari − 1981) for years after 1981. This partial-pooling slope model estimates both species-level responses [yielding an estimate for each species (i.e., βs)] and the distribution from which the species-level estimates are drawn, yielding a higher-level estimate of the overall response across species (i.e., μβ). We partially pooled slopes given the documented directional changes in phenology and temperature over time (35, 36). By pooling slopes, we also accounted for variation in variance and sample size across observations (e.g., species, datasets). We did not pool intercepts because our goal was to model past changes in the best possible way and our posterior predictive checks showed that unpooled intercepts improved the model.
Given significant warming trends in recent decades and the detection of nonstationarities in both temperature data and recent ecological responses to climate change (60–62), we used a hinge model (SI Appendix, Fig. S2). We used 1981 as the inflection point to reflect the major change in temperature observed in the early 1980s. We only included a hinge for those species with >4 years of data before 1981. For species with <4 years of data, a linear trend was fit [i.e., xi = (yeari − 1981)]. (Additional methods are provided in SI Appendix, section 2.)
Phenological and Synchrony Models.
To estimate changes in phenology, we estimated phenological change (days/decade) as specified above for each individual species. To put those changes into context, we estimated phenological change on the pre-recent climate change dataset using a nonhinge model. Then, to estimate the mean change and 95% CI, we used an intercept-only simple linear model based on the difference in posterior distributions of μβ between the two models (the hinge model using all data and a nonhinge model using data before 1981).
To estimate synchrony, we first estimated phenological change (days/decade) as specified above for each individual species and then took the difference in slope between interacting species (days/decade). We estimated synchrony change over the same years for both species. Therefore, some of the time series for some interacting species were shortened to match their partner. The difference in slope was taken for each model iteration. To standardize direction of change across interactions (e.g., increase or decrease in the number of days/decade), we needed to determine the seasonal order of the interaction (i.e., consumer emerges before resource or resource emerges before consumer). To calculate this seasonal order, we took the difference in the average day of year across all years for each species’ phenological event. We used this order to establish which interactions were effectively moving together or apart. We corrected for any instances where the interaction order switches (i.e., the slopes cross over the years of observation; n = 3) and assigned such instances as moving further apart.
To calculate the magnitude of change in synchrony, we used an intercept-only hierarchical model based on the posterior distribution of the absolute value of synchrony change (days/decade), partially pooling intercepts following this equation:
where y is synchrony change, i represents a single iteration, s represents an interaction, and ŷ is the predicted value of y. Because of the skewed distribution (due to taking the absolute value of changes), we used a truncated normal distribution for the overall (μα) and species-level intercept (αs) parameters defined in the Stan environment (SI Appendix, section 5, model 2). To calculate the overall change in synchrony, we took the same approach but did not use a truncated normal distribution. We report the overall response (μα) and 95% CIs from both of these models.
Temperature Models.
We constructed two models of temperature change, one that estimated temperature change per interaction (n = 22) and one that estimated temperature change per unique dataset, to get an estimate of overall temperature change (n = 18; SI Appendix, Fig. S3). To obtain a more comprehensive estimate of overall temperature change, we included studies where nutrients have been shown to explain phenology of one of the interacting species (63–65) in this model. The model estimating the relationship between phenological sensitivity and temperature (days/°C) was fit for each individual species (SI Appendix, Fig. S3A). Both temperature sensitivity and temperature change models were fit as hierarchical models as described above (SI Appendix, section 5, model 1).
Covariate Models.
To estimate the relationship between temperature change and phenological change, we fit phenological change as a function of temperature change. To incorporate uncertainty of the posterior distribution at the species level, we took two steps. First, on the smaller dataset (which relied on temperature data), we fit two models: temperature and phenological change (as described above). From these hierarchical models, we used the lower-level estimates [i.e., the species’ slopes from the posterior distribution from both models (βs)] from the last 3,000 iterations as observations in a new two-level hierarchical model (iterations are nested within species). In this model, we partially pooled intercepts (also commonly referred to as random intercepts) following this equation (SI Appendix, section 5, model 3):
where y is phenological or synchrony change (depending on the model), x is temperature change, i represents a single iteration, s represents species, and ŷ is the predicted value of y. This model allows variation across species in their phenological change, but estimates a single slope (i.e., change in phenology per change in temperature) across all species (β). Slopes were not allowed to vary across species because there was not enough variation within species (i.e., most species experienced only one amount of temperature change). We assumed species within studies had similar variance. In the final model, there were 13 studies and 37 species.
To estimate the relationship between temperature change and synchrony change, we fit synchrony change as a function of temperature change. To do so, we took the same steps as the covariate model for phenology but with one modification. To obtain one estimate of temperature change for interactions where different temperature metrics were provided for each species in an interaction (n = 8), we used the temperature data for the resource. Synchrony change was estimated as described above but for this dataset (n = 22 interactions). We took the absolute value of synchrony change because it is unclear whether long-term temperature change should lead to interacting species getting further apart or closer together in seasonal timing. Given that none of the synchrony change estimates could be less than zero (given that we worked with the absolute value of synchrony change), we used a truncated normal distribution for the overall (μα) and species-level (αs) intercept parameters defined in the Stan environment (SI Appendix, section 5, model 4). To evaluate the strength of the relationship for the covariate models, the estimated slope (β) (with the 95% CIs) is reported in the text.
Null Modeling.
We constructed three different null models (for details, SI Appendix, section 2). The first two were used to test the degree to which synchrony has changed since recent climate change began in the early 1980s. We first estimated the amount of natural variation either in magnitude or overall change in synchrony among interacting species before recent climate change began using simulated or raw data, which we then compared with changes after recent climate change started. This approach is an important step toward testing the hypothesis that recent shifts in synchrony have diverged from some baseline level of synchrony that existed before large changes in climate occurred in the early 1980s (16). To define this hypothesis of baseline synchrony rigorously, one would need long time series encompassing periods of natural climate variability and anthropogenic climate change. These criteria were met by only a subset of the datasets. Our approach allowed us to use existing data to quantify the degree to which synchrony has changed since recent climate change began. Finally, the third null model was used to explore the effect of time-series length on estimates of phenological change.
Supplementary Material
Acknowledgments
We thank P. Adamik, A. Iler, G. Kudo, H. Lynch, E. Vatka, the Finnish Meteorological Institute, D. Schindler, and J. Costello for providing temperature data; B. I. Cook, the Harvard Stanleyi discussion group, D. Flynn, and M. Kosmala for providing statistical advice; and Elsa Cleland for help with the initial stages of database construction. We are grateful to A. Ettinger, J. Forrest, and three anonymous reviewers for their valuable feedback on the manuscript. This project was supported in part by the Forecasting Phenology Working Group supported by the National Center for Ecological Analysis and Synthesis (Grant EF-0553768), and the Center for Population Biology at the University of California, Davis (H.M.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5057.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714511115/-/DCSupplemental.
References
- 1.Ovaskainen O, et al. Community-level phenological response to climate change. Proc Natl Acad Sci USA. 2013;110:13434–13439. doi: 10.1073/pnas.1305533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thackeray SJ, et al. Phenological sensitivity to climate across taxa and trophic levels. Nature. 2016;535:241–245. doi: 10.1038/nature18608. [DOI] [PubMed] [Google Scholar]
- 3.Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC. Rapid advancement of spring in the high Arctic. Curr Biol. 2007;17:R449–R451. doi: 10.1016/j.cub.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. Climate change and unequal phenological changes across four trophic levels: Constraints or adaptations? J Anim Ecol. 2009;78:73–83. doi: 10.1111/j.1365-2656.2008.01458.x. [DOI] [PubMed] [Google Scholar]
- 5.CaraDonna PJ, Iler AM, Inouye DW. Shifts in flowering phenology reshape a subalpine plant community. Proc Natl Acad Sci USA. 2014;111:4916–4921. doi: 10.1073/pnas.1323073111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 7.Post E, Forchhammer MC. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos Trans R Soc Lond B Biol Sci. 2008;363:2369–2375. doi: 10.1098/rstb.2007.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolmgren K, Eriksson O. Are mismatches the norm? Timing of flowering, fruiting, dispersal and germination and their fitness effects in Frangula alnus (Rhamnaceae) Oikos. 2015;124:639–648. [Google Scholar]
- 9.Singer MC, Parmesan C. Phenological asynchrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Philos Trans R Soc Lond B Biol Sci. 2010;365:3161–3176. doi: 10.1098/rstb.2010.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson J, Jonzén N. Game theory sheds new light on ecological responses to current climate change when phenology is historically mismatched. Ecol Lett. 2012;15:881–888. doi: 10.1111/j.1461-0248.2012.01812.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington R, Woiwod I, Sparks T. Climate change and trophic interactions. Trends Ecol Evol. 1999;14:146–150. doi: 10.1016/s0169-5347(99)01604-3. [DOI] [PubMed] [Google Scholar]
- 12.Stenseth NC, Mysterud A. Climate, changing phenology, and other life history traits: Nonlinearity and match-mismatch to the environment. Proc Natl Acad Sci USA. 2002;99:13379–13381. doi: 10.1073/pnas.212519399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser ME, Both C, Lambrechts MM. Global climate change leads to mistimed avian reproduction. Adv Ecol Res. 2004;35:89–110. [Google Scholar]
- 14.Pau S, et al. Predicting phenology by integrating ecology, evolution and climate science. Glob Chang Biol. 2011;17:3633–3643. [Google Scholar]
- 15.Kharouba HM, Vellend M. Flowering time of butterfly nectar food plants is more sensitive to temperature than the timing of butterfly adult flight. J Anim Ecol. 2015;84:1311–1321. doi: 10.1111/1365-2656.12373. [DOI] [PubMed] [Google Scholar]
- 16.Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Cambridge Univ Press; New York: 2013. [Google Scholar]
- 17.Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proc Biol Sci. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly A, Caffarra A, O’Neill BF. A review of climate-driven mismatches between interdependent phenophases in terrestrial and aquatic ecosystems. Int J Biometeorol. 2011;55:805–817. doi: 10.1007/s00484-011-0426-5. [DOI] [PubMed] [Google Scholar]
- 19.Mayor SJ, et al. Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Sci Rep. 2017;7:1902. doi: 10.1038/s41598-017-02045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinney AM, et al. Asynchronous changes in phenology of migrating broad-tailed Hummingbirds and their early-season nectar resources. Ecology. 2012;93:1987–1993. doi: 10.1890/12-0255.1. [DOI] [PubMed] [Google Scholar]
- 21.Ross MV, Alisauskas RT, Douglas DC, Kellett DK. Decadal declines in avian herbivore reproduction: Density-dependent nutrition and phenological mismatch in the Arctic. Ecology. 2017;98:1869–1883. doi: 10.1002/ecy.1856. [DOI] [PubMed] [Google Scholar]
- 22.Aebischer NJ, Coulson JC, Colebrookl JM. Parallel long-term trends across four marine trophic levels and weather. Nature. 1990;347:753–755. [Google Scholar]
- 23.Seebens H, Einsle U, Straile D. Copepod life cycle adaptations and success in response to phytoplankton spring bloom phenology. Glob Chang Biol. 2009;15:1394–1404. [Google Scholar]
- 24.Bartomeus I, et al. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci USA. 2011;108:20645–20649. doi: 10.1073/pnas.1115559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafferty NE, Ives AR. Effects of experimental shifts in flowering phenology on plant-pollinator interactions. Ecol Lett. 2011;14:69–74. doi: 10.1111/j.1461-0248.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- 26.Johansson J, Kristensen NP, Nilsson JÅ, Jonzén N. The eco‐evolutionary consequences of interspecific phenological asynchrony—A theoretical perspective. Oikos. 2015;124:102–112. [Google Scholar]
- 27.Iler AM, et al. Maintenance of temporal synchrony between syrphid flies and floral resources despite differential phenological responses to climate. Glob Chang Biol. 2013;19:2348–2359. doi: 10.1111/gcb.12246. [DOI] [PubMed] [Google Scholar]
- 28.Gustine D, et al. Advancing the match-mismatch framework for large herbivores in the Arctic: Evaluating the evidence for a trophic mismatch in caribou. PLoS One. 2017;12:e0171807. doi: 10.1371/journal.pone.0171807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser ME, Van Noordwijk AJ, Tinbergen JM, Lessells CM. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc R Soc B. 1998;265:1867–1870. [Google Scholar]
- 30.Bauer Z, et al. Changing climate and the phenological response of great tit and collared flycatcher populations in floodplain forest ecosystems in Central Europe. Int J Biometeorol. 2010;54:99–111. doi: 10.1007/s00484-009-0259-7. [DOI] [PubMed] [Google Scholar]
- 31.Charmantier A, et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320:800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- 32.Hua F, et al. Community-wide changes in intertaxonomic temporal co-occurrence resulting from phenological shifts. Glob Chang Biol. 2016;22:1746–1754. doi: 10.1111/gcb.13199. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen LO, et al. Analysis of trophic interactions reveals highly plastic response to climate change in a tri-trophic high-Arctic ecosystem. Polar Biol. 2016;39:1467–1478. [Google Scholar]
- 34.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Chang Biol. 2006;12:1969–1976. [Google Scholar]
- 35.Parmesan C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Chang Biol. 2007;13:1860–1872. [Google Scholar]
- 36.Poloczanska ES, et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;3:919–925. [Google Scholar]
- 37.Thackeray SJ, et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Chang Biol. 2010;16:3304–3313. [Google Scholar]
- 38.Root TL, MacMynowski DP, Mastrandrea MD, Schneider SH. Human-modified temperatures induce species changes: Joint attribution. Proc Natl Acad Sci USA. 2005;102:7465–7469. doi: 10.1073/pnas.0502286102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenzweig C, et al. Attributing physical and biological impacts to anthropogenic climate change. Nature. 2008;453:353–357. doi: 10.1038/nature06937. [DOI] [PubMed] [Google Scholar]
- 40.Parmesan C, Duarte C, Poloczanska E, Richardson AJ, Singer MC. Overstretching attribution. Nat Clim Chang. 2011;1:2–4. [Google Scholar]
- 41.Cook BI, Wolkovich EM, Parmesan C. Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci USA. 2012;109:9000–9005. doi: 10.1073/pnas.1118364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karl TR, et al. Asymmetric trends of daily maximum and minimum temperature. Bull Am Meteorol Soc. 1993;74:1007–1023. [Google Scholar]
- 43.Schaper SV, et al. Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. Am Nat. 2012;179:E55–E69. doi: 10.1086/663675. [DOI] [PubMed] [Google Scholar]
- 44.Rossi S, Isabel N. Bud break responds more strongly to daytime than night-time temperature under asymmetric experimental warming. Glob Chang Biol. 2017;23:446–454. doi: 10.1111/gcb.13360. [DOI] [PubMed] [Google Scholar]
- 45.Wingfield JC, Hahn TP, Wada M, Schoech SJ. Effects of day length and temperature on gonadal development, body mass, and fat depots in white-crowned sparrows, Zonotrichia leucophrys pugetensis. Gen Comp Endocrinol. 1997;107:44–62. doi: 10.1006/gcen.1997.6894. [DOI] [PubMed] [Google Scholar]
- 46.Keller F, Körner C. The role of photoperiodism in alpine plant development. Arct Antarct Alp Res. 2003;35:361–368. [Google Scholar]
- 47.Deacy WW, et al. Phenological synchronization disrupts trophic interactions between Kodiak brown bears and salmon. Proc Natl Acad Sci USA. 2017;114:10432–10437. doi: 10.1073/pnas.1705248114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plard F, et al. Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biol. 2014;12:e1001828. doi: 10.1371/journal.pbio.1001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doiron M, Gauthier G, Lévesque E. Trophic mismatch and its effects on the growth of young in an Arctic herbivore. Glob Chang Biol. 2015;21:4364–4376. doi: 10.1111/gcb.13057. [DOI] [PubMed] [Google Scholar]
- 50.Burkle LA, Marlin JC, Knight TM. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science. 2013;339:1611–1615. doi: 10.1126/science.1232728. [DOI] [PubMed] [Google Scholar]
- 51.Burthe S, et al. Phenological trends and trophic mismatch across multiple levels of a North Sea pelagic food web. Mar Ecol Prog Ser. 2012;454:119–133. [Google Scholar]
- 52.Vatka E, Orell M, RytkÖnen S. Warming climate advances breeding and improves synchrony of food demand and food availability in a boreal passerine. Glob Chang Biol. 2011;17:3002–3009. [Google Scholar]
- 53.Reed TE, Jenouvrier S, Visser ME. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J Anim Ecol. 2013;82:131–144. doi: 10.1111/j.1365-2656.2012.02020.x. [DOI] [PubMed] [Google Scholar]
- 54.Senner NR, Stager M, Sandercock BK. Ecological mismatches are moderated by local conditions for two populations of a long‐distance migratory bird. Oikos. 2017;126:61–72. [Google Scholar]
- 55.Bewick S, Cantrell RS, Cosner C, Fagan WF. How resource phenology affects consumer population dynamics. Am Nat. 2016;187:151–166. doi: 10.1086/684432. [DOI] [PubMed] [Google Scholar]
- 56.Visser ME. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc Biol Sci. 2008;275:649–659. doi: 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philos Trans R Soc Lond B Biol Sci. 2010;365:3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Development Core Team 2016. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.3.2.
- 59.Carpenter B, et al. Stan: A probabilistic programming language. J Stat Softw. 2017;76 doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolkovich EM, Cook BI, McLauchlan KK, Davies TJ. Temporal ecology in the Anthropocene. Ecol Lett. 2014;17:1365–1379. doi: 10.1111/ele.12353. [DOI] [PubMed] [Google Scholar]
- 61.Medhaug I, Stolpe MB, Fischer EM, Knutti R. Reconciling controversies about the “global warming hiatus”. Nature. 2017;545:41–47. doi: 10.1038/nature22315. [DOI] [PubMed] [Google Scholar]
- 62.Risbey JS, Lewandowsky S. Climate science: The “pause” unpacked. Nature. 2017;545:37–39. doi: 10.1038/545037a. [DOI] [PubMed] [Google Scholar]
- 63.Winder M, Schindler DE. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85:2100–2106. [Google Scholar]
- 64.Winder M, Schindler D. Climate effects on the phenology of lake processes. Glob Chang Biol. 2004;10:1844–1856. [Google Scholar]
- 65.George D. The effect of nutrient enrichment and changes in the weather on the abundance of Daphnia in Esthwaite Water, Cumbria. Freshw Biol. 2012;57:360–372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.