Significance
Tissue antigen-specific Tregs are critical for the prevention of organ-specific autoimmune diseases, yet very little is known about their specificity in a natural repertoire. Here, we show an accumulation of antigen-experienced Tregs in the inflamed islets using a mouse model of autoimmune diabetes. MHC tetramer staining and single-cell T cell receptor (TCR) analyses of islet Tregs reveal their specificity for insulin and other islet-derived antigens. Tregs from inflamed islets prevent diabetes when transferred to diabetes-susceptible recipients. The identification of functional islet antigen-specific Tregs with distinct TCR usage paves the way for future investigations of their development and homeostasis to better understand pathogenesis and treatment of autoimmune diabetes.
Keywords: regulatory T cells, type 1 diabetes, antigen specificity, T cell receptor, TCR sequencing
Abstract
Regulatory T cells (Tregs) control organ-specific autoimmunity in a tissue antigen-specific manner, yet little is known about their specificity in a natural repertoire. In this study, we used the nonobese diabetic (NOD) mouse model of autoimmune diabetes to investigate the antigen specificity of Tregs present in the inflamed tissue, the islets of Langerhans. Compared with Tregs present in spleen and lymph node, Tregs in the islets showed evidence of antigen stimulation that correlated with higher proliferation and expression of activation markers CD103, ICOS, and TIGIT. T cell receptor (TCR) repertoire profiling demonstrated that islet Treg clonotypes are expanded in the islets, suggesting localized antigen-driven expansion in inflamed islets. To determine their specificity, we captured TCRαβ pairs from islet Tregs using single-cell TCR sequencing and found direct evidence that some of these TCRs were specific for islet-derived antigens including insulin B:9–23 and proinsulin. Consistently, insulin B:9–23 tetramers readily detected insulin-specific Tregs in the islets of NOD mice. Lastly, islet Tregs from prediabetic NOD mice were effective at preventing diabetes in Treg-deficient NOD.CD28−/− recipients. These results provide a glimpse into the specificities of Tregs in a natural repertoire that are crucial for opposing the progression of autoimmune diabetes.
Among the multiple mechanisms involved in peripheral immune tolerance, CD4+ Foxp3+ regulatory T cells (Tregs) are essential in preventing autoimmunity in mice and humans (1, 2). T cell receptor (TCR) signaling is essential for Treg development, homeostasis, and function. Tregs are thought to be predominantly specific for self-antigens (3). Exposure to self-antigens in the thymus during T cell development can drive the development of thymic Tregs (4–8), whereas noninflammatory exposure to self-antigens in the periphery can induce mature conventional T (Tconv) cells to differentiate into peripheral Tregs (9–11). In the periphery, Tregs are continuously stimulated by antigens, as evidenced by their dependence on CD28 costimulation to maintain peripheral homeostasis and proliferation in the steady state (12, 13). Further supporting this notion, Tregs have high basal expression of the orphan nuclear receptor and immediate-early gene, Nur77, largely as a consequence of TCR signaling (14, 15). TCR stimulation activates Tregs to exert their suppressive effects in vitro (16). Similarly, Tregs specific for antigens expressed in target organs are more effective at controlling organ-specific autoimmune diseases in vivo (17–20). Ablation of TCR from Tregs leads to changes in Treg gene signature and impairment of Treg function (21, 22). Collectively, these findings demonstrate the importance of antigen stimulation of Tregs in maintaining normal immune homeostasis. However, the exact antigen specificities of Tregs in most settings have not been defined.
The nonobese diabetic (NOD) mouse spontaneously develops an autoimmune attack against the islets of Langerhans (23). The destructive inflammation is primarily driven by autoreactive T cells that are specific for a diverse array of islet beta cell antigens (24). Antigen specificities of these diabetogenic T cells include insulin, islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), glutamic acid decarboxylase (GAD), zinc transporter 8 (ZnT8), insulinoma antigen 2 (IA-2), phogrin (IA-2β), chromogranin, and islet amyloid polypeptide (IAPP) (25, 26). These specificities likely arise from inefficient deletion of autoreactive T cells during thymic development and creation of neoantigen in the islets from posttranslational modifications such as hybrid insulin peptide (HIP) formation (27–30).
Despite progress in mapping the specificity of diabetogenic T cells, specificities of Tregs that control the progression of diabetes have not been determined. The challenge in determining Treg specificity is partly due to the rarity of islet antigen-specific Tregs in a natural highly diverse Treg repertoire. This is compounded by the difficulty in measuring Treg function in diabetes protection in vivo. Several published Treg repertoire studies used mice with a transgenic TCRβ chain to experimentally restrict T cell repertoire for easier identification of Treg antigen specificity. This practical approach is effective in tracking Treg clonotypes enriched in the colons and in prostate tumors (31–33). However, a normal T cell repertoire of a mouse uses more than 5 × 105 distinct TCRβ chains (34). Thus, the T cell repertoire in a mouse with fixed TCRβ chain is severely skewed, and Treg clonotypes found in these mice may not reflect those in a normal immune repertoire.
In this study, we set out to map the natural TCR repertoire and specificities of Tregs that protect against diabetes in NOD mice. Our investigation is based on the premise that diabetes develops with delayed kinetics and reduced penetrance in NOD mice compared with Treg-deficient NOD.CD28−/− mice. This suggests a presence of functional Tregs in NOD mice. We hypothesize that these Tregs are islet antigen specific and can be identified by their responsiveness to islet antigens.
Results
TCR Stimulation of Tregs in Inflamed Islets.
To investigate TCR activation of Tregs in NOD mice, we backcrossed a strain of Nur77GFP reporter mice (15) more than 10 generations onto the NOD background. The NOD.Nur77GFP mice developed diabetes at comparable kinetics and overall incidence to standard NOD mice (SI Appendix, Fig. S1A) and exhibited normal cellularity and proportions of T cells in peripheral lymphoid organs (SI Appendix, Fig. S1B). We then further crossed the NOD.Nur77GFP mice with NOD.Foxp3RFP mice (35, 36) to aid the isolation and analysis of Tregs. Tregs in the NOD.Nur77GFP.Foxp3RFP mice showed constitutively high GFP expression indicative of antigen stimulation of the TCR as previously reported (14, 15). To further define the correlation between GFP expression and the strength and immediacy of TCR stimulation, we analyzed lymph node (LN) cells from NOD.Nur77GFP.Foxp3RFP mice that had received varying doses of TCR stimulation in vitro. CD4+Foxp3− Tconv and CD8+ T cells showed dose-dependent increases in the percentages of Nur77GFP+ cells and in GFP mean fluorescence intensity (MFI) (SI Appendix, Fig. S1 C and D). Tregs were nearly all GFP positive directly ex vivo before stimulation. Increasing TCR stimulation also led to a dose-dependent increase in GFP MFI in Tregs (SI Appendix, Fig. S1 C and D). To determine the persistence of GFP expression after the cessation of TCR stimulation, we stimulated purified Tconv cells and Tregs with plate-bound anti-CD3 and anti-CD28 in vitro for 24 h, removed the cells from stimulation, and monitored GFP levels over time. GFP MFI in both Tconv cells and Tregs decayed with a half-life of ∼3 d (SI Appendix, Fig. S1E). Unstimulated Tregs showed a rate of Nur77-GFP decay similar to that seen in in vitro stimulated Tregs and Tconv cells. Together, these data demonstrate that the intensity of GFP in T cells of Nur77GFP reporter mice integrates both the strength of the TCR signal received and the recency of antigen exposure.
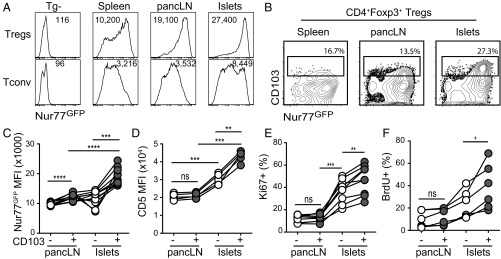
We then used the NOD.Nur77GFP.Foxp3RFP mice to identify Tregs with more recent islet antigen exposure. We compared GFP expression in Tregs from pancreatic LN (pancLN) and inflamed islets to those from spleen. Tregs from islets had the highest expression of Nur77GFP compared with those from pancLN and spleen (Fig. 1A). A subset of Tregs in the inflamed islets coexpressed previously reported TCR-dependent activation markers CD103, TIGIT, and ICOS (21) (Fig. 1B and SI Appendix, Fig. S2 A and B) and had higher expression of Foxp3 and CTLA4 than CD103− islet Tregs (SI Appendix, Fig. S2C). These CD103+ islet Tregs had the highest Nur77GFP expression compared with CD103− islet Tregs and Tregs from lymphoid organs (Fig. 1 B and C). The expression of CD5, another marker of recent TCR stimulation (37, 38), was also higher on islet Tregs, especially on the CD103+ subset (Fig. 1D). Moreover, islet Tregs were more proliferative than those in the pancLN by Ki67 staining (Fig. 1E), and islet CD103+ Tregs were the most proliferative subset by both Ki67 staining and in vivo BrdU labeling (Fig. 1 E and F). These results indicate that Tregs in islets, especially those coexpressing CD103, ICOS, and TIGIT, have been more recently and/or strongly activated by antigens.
Fig. 1.
Evidence of antigen exposure at the site of inflammation. (A) Representative histograms of flow cytometric analysis of Nur77GFP expression in CD4+Foxp3+ Tregs and CD4+Foxp3− Tconv cells from prediabetic NOD mice. Results represent at least 80 mice analyzed in more than 20 independent experiments. (B) Representative contour plots of CD103 and Nur77GFP expression by Tregs from prediabetic NOD mice. Results represent at least 80 mice analyzed in more than 20 independent experiments. (C) Quantification of Nur77GFP in CD103+ or CD103− Tregs in B. Results shown are a summary of three independent experiments. Statistical significance was determined using a repeated measures one-way ANOVA followed by Tukey’s multiple comparison test; ***P < 0.001; ****P < 0.0001. (D) CD5 MFI in CD103+ or CD103− Tregs from prediabetic NOD mice. Results shown are a summary of two independent experiments. Statistical significance was determined using a repeated measures one-way ANOVA followed by Tukey’s multiple comparison test; **P < 0.01; ***P < 0.001; ns, not significant. (E) Ki67 staining in CD103+ or CD103− Tregs from prediabetic NOD mice. Results shown are a summary of three independent experiments. Statistical significance was determined using a repeated measures one-way ANOVA followed by Tukey’s multiple comparison test; **P < 0.01; ***P < 0.001; ns, not significant. (F) BrdU staining in CD103+ or CD103− Tregs from prediabetic NOD mice. BrdU was administered continuously for 1 wk before analysis. Results shown are a summary of two independent experiments. Statistical significance was determined using a paired t test; *P < 0.05; ns, not significant.
Islet Tregs Have a Distinct and Restricted TCR Repertoire.
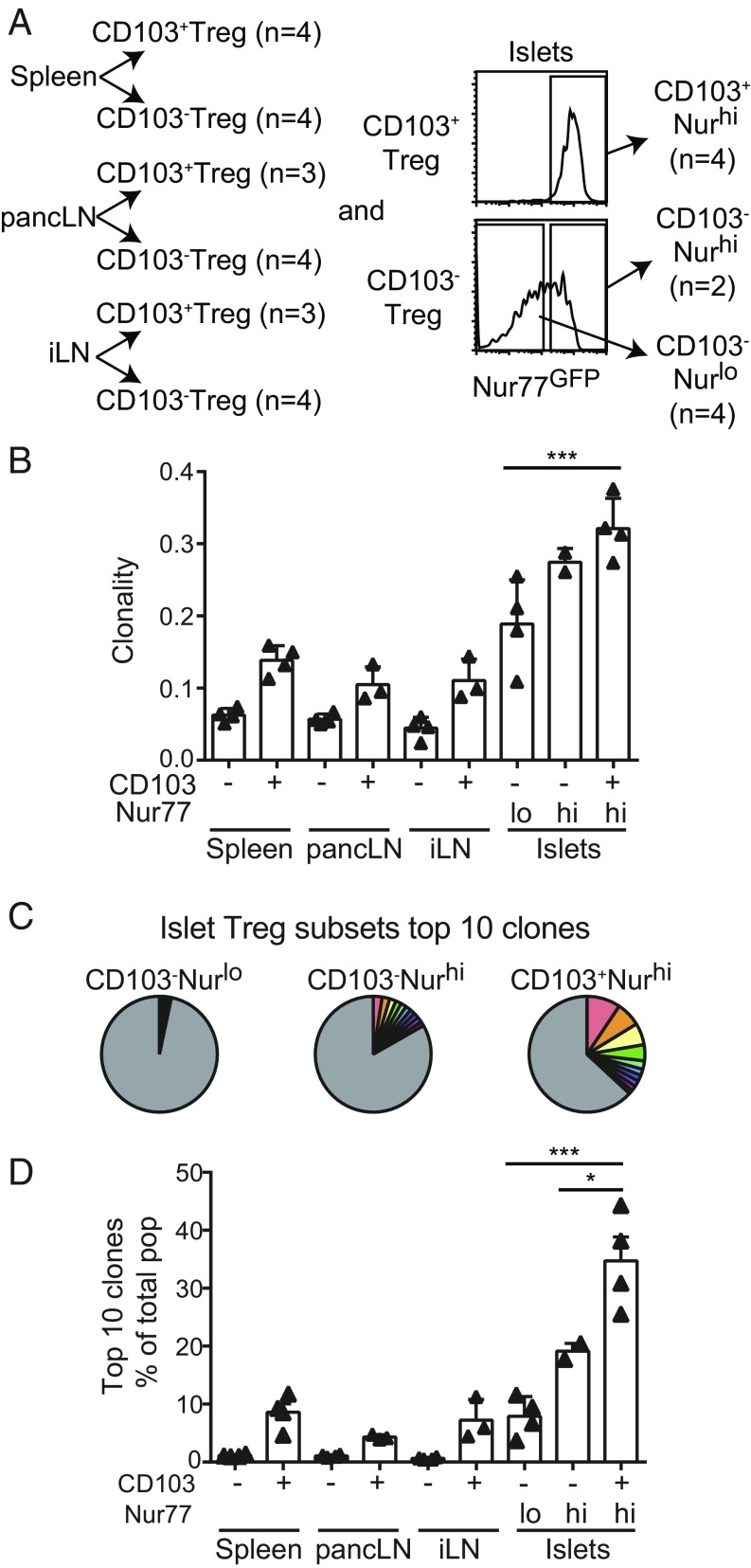
The activation and proliferation of Tregs in inflamed islets may result in local enrichment of islet antigen-specific clonotypes and formation of distinct TCR repertoires. We therefore compared islet Treg TCRβ sequences with those used by Tregs from pancLN, spleen, and nondraining inguinal-popliteal-sciatic LN (iLN) using high-throughput TCRβ sequencing analysis. In each organ, we divided Tregs into CD103− and CD103+ subsets, and the CD103− islet Tregs were further divided into Nur77GFP high and low fractions (Fig. 2A). Between 1,600 and 600,000 Tregs were analyzed from different compartments, and 1,144 to 62,604 unique TCRβ sequences were identified (SI Appendix, Table S1). We used the clonality index—the inverse of normalized Shannon’s entropy—to quantify the clonal dominance in each population, with 0 indicating that each clone appears only once and 1 representing a monoclonal population. While all Treg populations showed polyclonality, with a diversity index of less than 0.4, the CD103+ islet Tregs were the least diverse (Fig. 2B). Clonal dominance can also be visualized by the contribution of the top 10 clones to the repertoire they reside in, which was 30% for CD103+ islet Tregs, markedly higher than the CD103− islet Treg subsets (Fig. 2 C and D). CD103− Tregs in spleen, pancLN, and iLN were highly diverse, with the 10 most abundant clones representing less than 2% of the population (Fig. 2D). These results further support local clonal expansion of activated Tregs in the inflamed islets.
Fig. 2.
Treg TCR repertoires in prediabetic NOD mice. (A) A summary of Treg populations used for TCRβ chain sequencing analysis. CD103+ and CD103− Treg populations were sorted from spleen, pancLN, and iLN. Islet Tregs were additionally sorted on Nur77GFP expression from islets: CD103+Nurhi, CD103−Nurhi, and CD103−Nurlo. A total of 32 populations from four mice were analyzed individually (SI Appendix, Table S1). (B) Clonality of each Treg population’s TCR repertoire. Each triangle represents a single mouse, and the columns represent the mean and SD of the group. Statistical significance was determined using a one-way ANOVA followed by Tukey’s multiple comparison test; ***P < 0.001. (C) Frequency of the 10 most abundant clones in different islet Treg populations in a representative mouse. (D) Summary graph of the sum frequencies of the 10 most abundant clones in different Treg populations in each mouse. Each triangle represents a single mouse, and the columns represent the mean and SD of the group. Statistical significance determined as described in B; *P < 0.01.
Islet Antigen Specificity of Islet Tregs.
Our results thus far suggest that Tregs specific to islet autoantigens are locally activated, clonally expand, and accumulate in the islets. However, distinct TCRs may recognize the same antigen. To further determine the specificity of islet Tregs, we performed single-cell sequencing of paired TCRαβ chains and then further interrogated for their specificity using an in vitro reporter assay (39, 40). FACS-purified ICOS+TIGIT+ islet Tregs, which also express high levels of CD103 and Nur77GFP (SI Appendix, Fig. S2A), were sorted into single wells for barcoding and sequencing. Among the 71 paired TCRαβ sequences identified, six TCRs were found more than once in a single mouse, indicating clonal expansion, and two other TCRs were found in multiple mice (SI Appendix, Table S2). We thus selected these eight TCRs for further analysis of their reactivity to islet antigens.
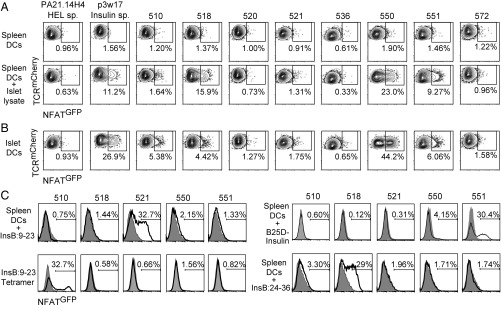
We reconstituted the paired TCRs using retroviral transduction in a hybridoma cell line that expresses an NFATGFP reporter construct so that TCR triggering can be measured by GFP expression. A hen egg lysosome (HEL)-specific TCR (PA21.14H4) was used as a negative control, and a TCR for insulin (p3w17) was used as a positive control in the screening. We stimulated all TCR-transduced hybridomas with splenic CD11c+ dendritic cells (DCs) pulsed with lysate from NOD.Rag2−/− islets. Five of eight clones (i.e., 510, 518, 521, 550, and 551) showed measurable reactivity to islet lysates (Fig. 3A). To determine whether these TCRs respond to islet antigen naturally processed and presented by DCs in the inflamed islets, we stimulated the hybridomas with freshly isolated islet-derived CD11c+ DCs. In this assay, the same five clones responded to islet DCs, but not to control DCs from spleen or colon (Fig. 3 A and B and SI Appendix, Fig. S3B). To further map antigen specificity of these TCRs, we screened the hybridomas against 19 peptides previously reported to be stimulatory to islet antigen-specific CD4+ T cells in NOD mice (SI Appendix, Table S3). None of the TCRs responded to BDC mimotopes, HIPs, or peptides from ZnT8, GAD, IGRP, IAPP, or IA-2β (SI Appendix, Table S3).
Fig. 3.
Islet antigen reactivity of activated islet Tregs. (A) Hybridomas expressing NFATGFP reporter and TCRs derived from activated islet Tregs were stimulated for 20 to 24 h with splenic DCs or splenic DCs with islet lysates. GFP expression was determined using flow cytometry, and contour plots for all of the hybridomas analyzed are shown. (B) As described in A, except the hybridomas were stimulated with islet DCs. (C) As described in A, except the hybridomas were stimulated with splenic DCs with insulin B:9–23 peptide, plate-bound insulin tetramers p8E and p8G, human B25D-insulin, or proinsulin B:24–36 peptide. Filled histograms are the HEL-specific hybridoma; open histograms are the TCR hybridoma listed at the top of the column. In A–C, representative flow plots for at least two independent experiments are shown.
Insulin is a dominant autoantigen in type 1 diabetes in both NOD mice and human patients (41, 42). Therefore, we tested Treg TCRs against insulin. One TCR, 521, responded to splenic DCs loaded with insulin B:9–23 peptide (Fig. 3C and Table 1). Another TCR, 510, responded to immobilized insulin B:9–23 p8E and p8G tetramers (29) (Fig. 3C and Table 1). To determine reactivity to other insulin epitopes, we stimulated the hybridomas with splenic DCs pulsed with metabolically inactive human insulin, B25D-insulin (43). The use of B25D-insulin avoided the hormonal activation of the hybridomas that altered the assay background. TCR 551 responded to B25D-insulin (Fig. 3C and Table 1). Additionally, we found that TCR 518 responded to a previously described proinsulin peptide, insulin B:24–36 (Fig. 3C and Table 1) (44). The remaining islet antigen-specific TCR, 550, did not respond to any of the above insulin stimulations (Fig. 3C, Table 1, and SI Appendix, Table S3). In summary, five of eight islet Treg-derived TCRs were stimulated by islet antigens, and four of the five were specific for insulin (Table 1).
Table 1.
Summary of islet Treg reactivity
| Sequence ID | Reactivity to: | Islet antigen specific? | Islet antigen | ||
| Insulin* | Islet DC | Islet lysate | |||
| 510 | + | + | + | Yes | Insulin B:9–23 |
| 518 | + | + | + | Yes | Proinsulin |
| 520 | − | − | − | Unknown | Unknown |
| 521 | + | ± | ± | Yes | Insulin B:9–23 |
| 536 | − | − | − | Unknown | Unknown |
| 550 | − | + | + | Yes | Unknown |
| 551 | + | + | + | Yes | Mature insulin |
| 572 | − | − | − | Unknown | Unknown |
Definition of insulin reactivity includes specificity to mature insulin, proinsulin, and preproinsulin.
Insulin Specificity Among Islet Tregs.
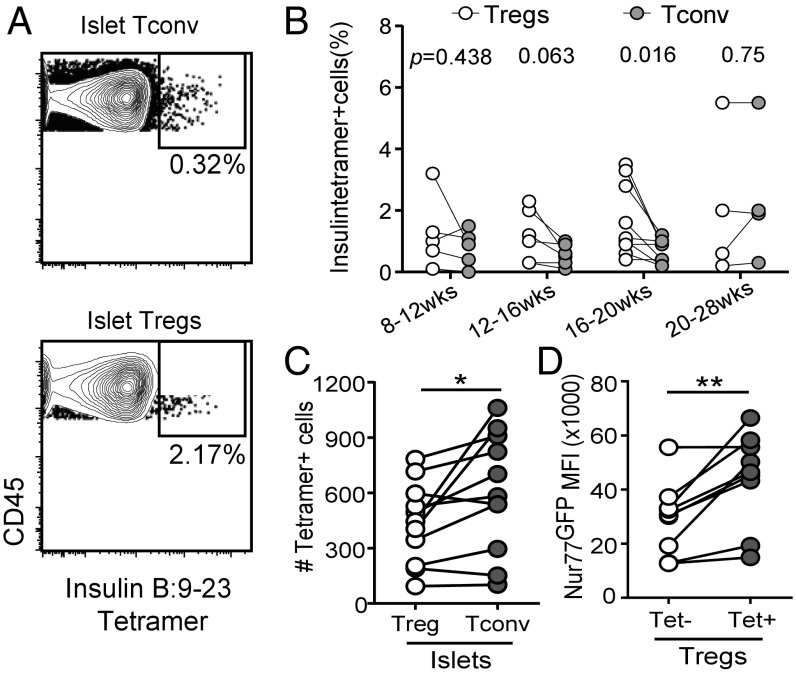
Our results suggest that insulin specificity may be common among islet-infiltrating Tregs. To further test this notion, we analyzed Tregs and Tconv cells in islets and pancLN of NOD mice (8 to 28 wk old) using insulin I-Ag7 tetramer containing modified insulin B:9–23 peptides p8E and p8G (29). Insulin B:9–23-specific cells were readily detectable among islet Treg and Tconv cells but were not consistently found in the pancLN (SI Appendix, Fig. S4). Insulin B:9–23-specific islet Tregs were often present at higher frequencies than insulin B:9–23-specific Tconv cells in prediabetic mice (Fig. 4B). Overall, the total numbers of insulin-specific Tconv cells per islet were slightly higher than the total number of insulin-specific Tregs (Fig. 4C). Moreover, insulin tetramer-positive Tregs had higher Nur77GFP expression than insulin tetramer-negative Tregs, indicating stronger TCR activation (Fig. 4D). Thus, the insulin tetramer stain result is consistent with single-cell TCR sequencing analysis and shows that a demonstrable proportion of islet Tregs is insulin specific.
Fig. 4.
Insulin-specific Tregs in NOD mice. (A) Representative contour plots of pooled insulin B:9–23 tetramers p8G and p8E staining versus control HEL tetramer staining in islet Tconv cells (Upper) and Tregs (Lower). Cells were gated as live CD45+Thy1.1+CD8− and Foxp3+ (Tregs) or Foxp3− (Tconv cells). Results are representative of at least eight mice analyzed in three independent experiments. (B) Quantification of insulin tetramer staining in islet Tconv cells and Tregs. Each pair of connected circles represents one mouse, and the mice (n = 24) are grouped according to age. Results shown are a summary of six independent experiments. Statistical significance was determined using a paired Student’s t test, and P values are listed at the top of the groups. (C) Total number of insulin tetramer-positive Tregs and Tconv cells from islets. Results shown are a summary of three independent experiments. Statistical significance was determined using a paired Student’s t test; *P < 0.05. (D) Nur77GFP MFI of insulin tetramer-positive and -negative Tregs in islets. Results shown are a summary of three independent experiments. Statistical significance was determined using a paired Student’s t test; **P < 0.001.
Function of Islet Tregs.
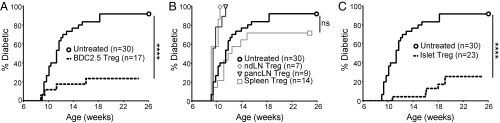
Islet antigen-specific Tregs are more effective at protecting against diabetes (17, 18). Given their islet antigen specificity and propensity to be activated in the islets, we hypothesized that islet Tregs in the NOD mice would be effective at suppressing diabetes. We assessed the in vivo capacity of islet Tregs to prevent diabetes in an adoptive transfer model using NOD.CD28−/− mice as recipients. These mice develop diabetes at a younger age and at higher penetrance due to Treg defects (12, 13). More than 80% of NOD.CD28−/− mice become diabetic between 8 and 12 wk of age, which can be largely prevented by adoptive transfer of 50,000 islet antigen-specific Tregs from BDC-2.5 TCR transgenic mice (Fig. 5A). The same dose of Tregs isolated from spleen, pancLN, or nonpancreas draining LNs (mesenteric LN and inguinal LN) of prediabetic NOD mice were unable to prevent diabetes (Fig. 5B). Transfer of 50,000 islet Tregs prevented diabetes as efficiently as BDC-2.5 Tregs (Fig. 5C). Taken together, this study demonstrates that inflamed islets contain an enrichment of islet antigen-specific Tregs that exhibit potent antidiabetes activity.
Fig. 5.
In vivo function of islet Tregs. (A) CD28−/− mice between 2 and 3 wk of age were treated with 50,000 BDC-2.5 TCR transgenic Tregs sorted from nonpancreas draining LN (dashed line, n = 17) or untreated (bold solid line, n = 30). Diabetes development was monitored until the mice were 25 wk of age or until diabetes development. Statistical significance was determined using the log-rank (Mantel–Cox) test; ****P < 0.0001. (B) Same as in A, except polyclonal Tregs sorted from nonpancreas draining LN (ndLN, n = 7), pancLN (n = 9), and spleen (n = 14) of NOD mice were used to treat CD28−/− mice and compared with nontreated controls (bold solid line, n = 30). Statistical significance was determined using the log-rank (Mantel–Cox) test; ns, not significant. (C) Same as in A, except polyclonal Tregs sorted from inflamed islets of NOD mice (dashed line, n = 23) were used to treat CD28−/− mice and compared with nontreated controls (bold solid line, n = 30). Statistical significance was determined using the log-rank (Mantel–Cox) test; ****P < 0.0001. Results in A–C are summaries of more than 10 independent experiments performed over a 24-mo period.
Discussion
Collectively, results from this study show that islet antigen-specific (particularly insulin-specific) Tregs are enriched in the inflamed islets of NOD mice. These Tregs are functional in curbing the progression of islet destruction. It is worth noting that most NOD mice eventually succumb to diabetes, likely because islet antigen-specific Tregs die due to IL-2 deficiency in inflamed islets and exhaustion of the islet antigen-specific Treg pool from chronic attrition (45).
Insulin is the most abundant protein in beta cells and a key autoantigen for driving the initiation and progression of autoimmune beta cell destruction (42, 46, 47). In this study, we found a prominence of insulin-specific Tregs in inflamed islets. A recent study has reported that human subjects with long-standing antiislet autoimmunity without progression to overt diabetes have increased frequency of insulin-specific Tregs compared to healthy subjects and those with diabetes onset at a young age (48). These results suggest an important role for insulin-specific Tregs in controlling diabetes progression. To our surprise, none of the TCRs responded to any of the 19 peptides previously reported to stimulate islet antigen-specific CD4+ T cells. Previous analyses used CD4+ T cells and likely did not include many Treg clones. Moreover, the Treg clone analysis in this report is limited and aimed only at identifying dominant clones. Nonetheless, the two insulin epitopes we are able to identify in this study have been previously reported for Tconv cells. Overall, our mapping of Treg specificities in the inflamed islets suggest insulin as a dominant specificity and that insulin-specific Tregs and Tconv cells can recognize the same epitopes.
The prominent and highly localized presence of insulin-specific Tregs in inflamed islets is somewhat unexpected. Insulin is a systemic hormone that can potentially be presented by DCs throughout the body. However, insulin-specific T cells are hard to detect outside islets, and insulin-specific TCRs were not activated by splenic DCs in vitro without addition of islet antigens or insulin peptides. These results suggest that insulin-specific Tregs likely require high abundance of insulin to be activated, which limits their suppressive activity to the islets at the source of insulin production. This observation has important implications for developing islet antigen-specific Treg therapies. Polyclonal Treg therapy is being evaluated in patients with type 1 diabetes in early-phase clinical trials (49, 50). Genetic engineering of Tregs with TCR-specific islet antigens has the potential to increase therapeutic efficacy and minimize systemic immunosuppression. A key issue in engineering TCRs for islet antigen-specific Tregs is to ensure that the targeted antigens are naturally presented at adequate levels in the islets and draining LN to activate the engineered Tregs. Our results suggest that insulin-specific TCRs are viable candidates for engineering Treg specificity for the protection of beta cells.
Materials and Methods
Bulk TCRβ Sequencing.
Total RNA was extracted from FACS-purified Tregs using ARCTURUS PicoPure RNA Isolation Kit (Life Technologies) for <100,000 cells or a microRNA extraction kit (QIAGEN) for >100,000 cells. TCRβ repertoires were amplified and sequenced using Illumina MiSeq by iRepertoire, Inc. Data analysis was performed using iRepertoire, Inc. web tools (https://www.irepertoire.com). Clonality was calculated using the inverse of normalized Shannon’s diversity index.
Single-Cell TCRαβ Sequencing.
Individual activated islet Tregs were flow sorted into single wells of a 96-well plate, and TCR sequences from single cells were obtained using three rounds of PCR to amplify and barcode before sequencing on an Illumina MiSeq as previously described (39, 40).
Hybridoma Assays.
Splenic and islet DCs were enriched using a CD11c+ selection kit (STEMCELL Technologies). DCs and hybridomas, 5 × 105 each per well, were cocultured 20 to 24 h in round-bottom 96-well plates with various forms of islet antigens as indicated, before flow cytometric analysis of GFP expression.
Insulin Tetramer Staining.
The APC-conjugated tetramer of I-Ag7 bound to insulin B:9–23 mimotopes p8E (HLVERLYLVCGEEG) and p8G (HLVERLYLVCGGEG) (29) and HEL (AMKRHGLDNYRGYSL) were obtained from the National Institutes of Health Tetramer Core Facility at Emory University and stained for 2 h at 37 °C for pancLN and islet T cell analysis.
Mice.
All experiments involving mice have been approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Statistical Analysis.
Analyses were performed with GraphPad Prism (GraphPad Software) as detailed in the figure legends.
Additional information can be found in SI Appendix, Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank J. E. Klementowicz, V. Nguyen, J. Wang, L. Lee, V. Panchumarthi, R. Guerrero-Moreno, V. Dang, and N. Lescano for technical assistance; S. Cheng and L. Bates for laboratory management; J. Greenland, M. Rosenblum, and M. Hermiston for advice; and A. Weiss and J. Zikherman for Nur77GFP mice, L. Wen for NOD.Foxp3RFP mice, and D. Vignali for the PA21.14H4 TCR. This work was supported by the National Institutes of Health Grants R01DK08231 (to Q.T.), P30DK063720 (to Michael German to support research cores at the University of California, San Francisco Diabetes Center), P01AI118688 (to M.S.A.), R01AR06367603 (to W.H.R.), U19AI11049103 (to W.H.R.), and T32DK74-18-31 (to A.S.); a National Science Foundation Graduate Research Fellowship (to A.S.); and The Larry L. Hillblom Foundation (W.P. and M.S.A.).
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715590115/-/DCSupplemental.
References
- 1.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 5.Kawahata K, et al. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 6.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cozzo Picca C, et al. CD4+CD25+Foxp3+ regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci USA. 2011;108:14890–14895. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paiva RS, et al. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc Natl Acad Sci USA. 2013;110:6494–6499. doi: 10.1073/pnas.1221955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 12.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 13.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 14.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KS. Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol. 2008;180:4366–4370. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler KM, Samy ET, Tung KS. Cutting edge: Normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183:7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vahl JC, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 24.Graham KL, et al. Pathogenic mechanisms in type 1 diabetes: The islet is both target and driver of disease. Rev Diabet Stud. 2012;9:148–168. doi: 10.1900/RDS.2012.9.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2:a007781. doi: 10.1101/cshperspect.a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Donelan W, Wang H, Reeves W, Yang LJ. Novel autoantigens in type 1 diabetes. Am J Transl Res. 2013;5:379–392. [PMC free article] [PubMed] [Google Scholar]
- 27.Stadinski BD, et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford F, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delong T, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–714. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leventhal DS, et al. Dendritic cells coordinate the development and homeostasis of organ-specific regulatory T cells. Immunity. 2016;44:847–859. doi: 10.1016/j.immuni.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casrouge A, et al. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 35.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang Y, et al. The dual effects of B cell depletion on antigen-specific T cells in BDC2.5NOD mice. J Immunol. 2012;188:4747–4758. doi: 10.4049/jimmunol.1103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzam HS, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 38.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han A, Glanville J, Hansmann L, Davis MM. Corrigendum: Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2015;33:210. doi: 10.1038/nbt0215-210c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grönholm J, et al. Metabolically inactive insulin analogue does not prevent autoimmune diabetes in NOD mice. Diabetologia. 2017;60:1475–1482. doi: 10.1007/s00125-017-4276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, et al. Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol. 2001;167:4926–4935. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 45.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5:1028–1035. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 48.Serr I, et al. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat Commun. 2016;7:10991. doi: 10.1038/ncomms10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bluestone JA, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marek-Trzonkowska N, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.