Significance
Symbiotic relationships benefit organisms in utilization of new niches. In parasitoid wasps, symbiotic viruses and venom that are injected together with wasp eggs into the host caterpillar suppress immune responses of the host and enhance parasitoid survival. We found that the virus also has negative effects on offspring survival when placing these interactions in a community context. The virus and venom drive a chain of interactions that includes the herbivore and its food plant and attracts the hyperparasitoid enemies of the parasitoid. Our results shed new light on the importance of symbionts associated with their host in driving ecological interactions and highlight the intricacy of how multispecies interactions are reflected in adaptations of individual species such as the host-finding behavior of hyperparasitoids.
Keywords: multitrophic interactions, plant-mediated interaction network, herbivore saliva, herbivore-induced plant volatiles, parasitic wasp
Abstract
Symbiotic relationships may provide organisms with key innovations that aid in the establishment of new niches. For example, during oviposition, some species of parasitoid wasps, whose larvae develop inside the bodies of other insects, inject polydnaviruses into their hosts. These symbiotic viruses disrupt host immune responses, allowing the parasitoid’s progeny to survive. Here we show that symbiotic polydnaviruses also have a downside to the parasitoid’s progeny by initiating a multitrophic chain of interactions that reveals the parasitoid larvae to their enemies. These enemies are hyperparasitoids that use the parasitoid progeny as host for their own offspring. We found that the virus and venom injected by the parasitoid during oviposition, but not the parasitoid progeny itself, affected hyperparasitoid attraction toward plant volatiles induced by feeding of parasitized caterpillars. We identified activity of virus-related genes in the caterpillar salivary gland. Moreover, the virus affected the activity of elicitors of salivary origin that induce plant responses to caterpillar feeding. The changes in caterpillar saliva were critical in inducing plant volatiles that are used by hyperparasitoids to locate parasitized caterpillars. Our results show that symbiotic organisms may be key drivers of multitrophic ecological interactions. We anticipate that this phenomenon is widespread in nature, because of the abundance of symbiotic microorganisms across trophic levels in ecological communities. Their role should be more prominently integrated in community ecology to understand organization of natural and managed ecosystems, as well as adaptations of individual organisms that are part of these communities.
Across trophic levels in ecological communities, individual macroorganisms often carry a suite of microorganisms (1–3). In some organisms, these associations have evolved in symbiotic relationships that provide the host organism with novel traits (4, 5). These symbiotic relationships may drive species interactions and ecosystem processes, for example, when endophytes provide plants with new resistance traits against their herbivorous enemies or when aphids carry symbionts providing traits that allow them to exploit new food plants or providing immunity to attack by parasitoid wasps (2, 6).
Some clades of endoparasitoid wasps, which lay their eggs inside the bodies of other insects, have acquired symbiosis with viruses. The viruses enable the parasitoid larvae to develop inside other organisms and maximize the success of their parasitic lifestyle (7, 8). The symbiosis has evolved into the integration of the full virus genome into the genome of the parasitoid. These so-called polydnaviruses (PDVs) originated more than 100 Mya and are now obligatorily associated with parasitoids in several subfamilies of the Ichneumonoidea, including the Microgastrinae and Campopleginae (4, 7, 9). Each polydnavirus is a species in its own right associated with and named after its own parasitoid species. The polydnavirus benefits from the mutualism by replicating in the calyx of a female parasitoid’s ovaries without expressing virulence. In return, the parasitoid benefits from the virus when it is injected along with the wasp eggs into the insect that is host for the parasitoid larvae. The polydnavirus interferes with the host’s immune response to the eggs of the parasitoid. It benefits by regulating the host’s growth and physiology and thereby allows the parasitoid offspring to develop optimally within the host (4, 10).
Here we show that these symbiotic polydnaviruses also have a major disadvantage for the parasitoid larvae by driving a chain of interactions used by the enemies of the parasitoid, so-called “hyperparasitoids,” to locate their victims. Hyperparasitoids lay their eggs in the larvae or pupae of parasitoids and, as fourth trophic-level organisms, complete their development at the expense of the parasitoid. In natural and agricultural ecological communities, hyperparasitoids are abundant and may cause up to 55% of mortality to parasitoid progeny (11). To locate its victims, the hyperparasitoid Lysibia nana uses plant volatiles that are produced in response to feeding by caterpillars parasitized by larvae of the parasitoid Cotesia glomerata (11, 12). Herbivore-induced plant volatiles (HIPVs) of wild cabbage plants (Brassica oleracea) emitted, in response to feeding damage by parasitized or by unparasitized caterpillars of the large cabbage white butterfly (Pieris brassicae) differ in composition (11). The plant volatiles induced by feeding of a parasitized caterpillar thus indirectly reveal the presence of the parasitoid larvae that live concealed inside the caterpillar body. This implies that hyperparasitoids use information derived from an interaction chain involving several trophic levels to locate their hosts. The mechanism triggering this interaction chain is unknown. It has been suggested that the parasitoid larvae manipulate their herbivore host, including its physiology that affects induction of plant volatiles (11, 12). However, in addition to eggs, the parasitoid also injects PDVs and venom into the host. Because PDVs are known to affect host physiology (4, 5, 7, 8), the PDVs may also trigger the interaction chain that hyperparasitoids use to locate the parasitoid larvae.
To test the hypothesis that the PDV of the parasitoid Cotesia glomerata (CgPDV) is the key driver of the interaction chain that allows hyperparasitoids to locate the parasitoid progeny, we collected each of three components of parasitism events—CgPDV and venom from the adult female parasitoid and its eggs—and separated the components in PBS. These components were injected separately or in combination into anesthetized P. brassicae caterpillars, the main host of C. glomerata. We tested their effect on HIPV emission and hyperparasitoid attraction in comparison with HIPV induction by caterpillars treated with a mock injection of PBS solution (SI Appendix, SI Methods).
Previous studies have shown that parasitism affects the composition of oral secretions regurgitated from the midgut during feeding and causes differential plant responses compared with regurgitate of unparasitized caterpillars (13). Regurgitate is a complex mix of saliva, midgut contents, and microorganisms. Because elicitors in caterpillar saliva are known to play key roles in induction of plant volatiles (14–16) and PDVs have been identified to target salivary glands (17), we tested the hypothesis that the caterpillar salivary gland is crucial in the interaction chain. We surgically removed the labial salivary gland in anesthetized parasitized and unparasitized caterpillars and investigated whether this altered the differential attraction of hyperparasitoids to HIPVs. We used RNA sequencing (RNA-seq) to compare gene transcript levels in the salivary glands of parasitized and unparasitized caterpillars, identified differential expression of genes regulating elicitors of plant defense responses to caterpillar feeding, and investigated whether injection of CgPDV into caterpillars leads to altered activity of these elicitors in caterpillar saliva (SI Appendix, SI Methods).
Results and Discussion
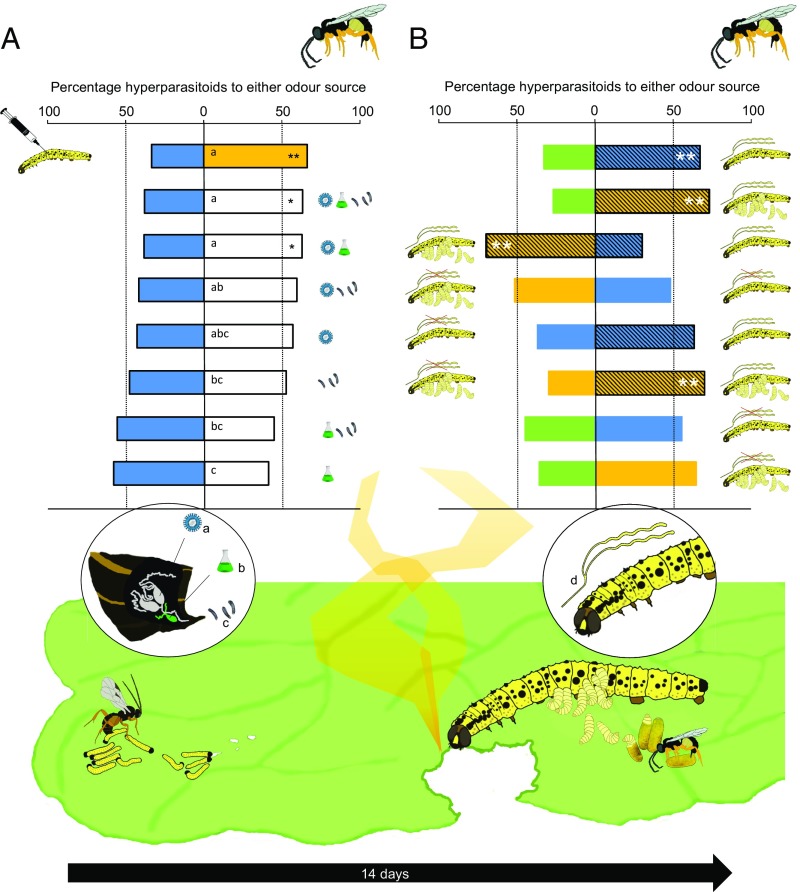
In two-choice Y-tube olfactometer tests, the hyperparasitoid L. nana preferred plant volatiles induced by parasitized caterpillars over those emitted by plants induced by unparasitized caterpillars when both were injected with a mock PBS solution (binomial test, P = 0.006; Fig. 1A). These results confirm that the microinjection technique does not affect the hyperparasitoid preference for HIPVs of plants induced by parasitized caterpillars over unparasitized caterpillars previously established for the hyperparasitoid species (11, 12). Hyperparasitoids also preferred volatiles of plants damaged by caterpillars that had a full event of parasitism mimicked by injection of a solution containing eggs, venom, and CgPDV over plant volatiles induced by PBS-injected unparasitized caterpillars (binomial test, P = 0.038; Fig. 1A). Moreover, injection of the combination of venom and CgPDV into caterpillars in the absence of parasitoid eggs produced similar results (binomial test, P = 0.031; Fig. 1A). Preference distributions of all treatments in which CgPDV was injected into the caterpillars, both alone and in addition with eggs or venom, were similar and resulted in more hyperparasitoids choosing for plant volatiles induced by CgPDV-injected caterpillars over those induced by PBS-injected unparasitized caterpillars [generalized linear model (GLM); Wald χ2 = 15.753; df = 7; P = 0.027; Fig. 1A]. Injection of venom alone as well as simultaneous injection of eggs and venom resulted in HIPVs that were not preferred over those induced by PBS-injected caterpillars (binomial test, P = 0.154 and P = 0.400, respectively; Fig. 1A) and these choice distributions differed from the preference of hyperparasitoids for treatments in which CgPDV was injected into the caterpillars (GLM; Wald χ2 = 15.753; df = 7; P = 0.027; Fig. 1A).
Fig. 1.
Preference of the hyperparasitoid L. nana for plant volatiles induced by P. brassicae caterpillars that are parasitized by C. glomerata. In the event of parasitism, parasitoids inject PDV (a), venom (b), and eggs (c) into the caterpillar. These components alter the physiology of the caterpillar and its interaction with the plant through its saliva (d). The parasitized caterpillar continues feeding, and within approximately 14 d, the larvae of the parasitoid have fully developed. They leave the caterpillar, spin their silk cocoons, and pupate. Hyperparasitoids that in turn lay their eggs inside the pupae of the parasitoid find these pupae by using HIPVs emitted by feeding of the parasitized caterpillar. (A) In two-choice preference tests, hyperparasitoids were tested for their preference for HIPVs induced by caterpillars that had received microinjections with a PBS solution with one or multiple components injected by parasitic wasps into the caterpillar: polydnavirus (a), venom (b), and eggs (c). HIPVs induced by the microinjected caterpillars were tested against HIPVs induced by unparasitized caterpillars injected with PBS (blue bars). In a control experiment testing the effect of the microinjection event, we found that microinjection with PBS did not affect the preference of hyperparasitoids for parasitized caterpillars (orange bar) over unparasitized caterpillars (blue bar). In the figure, treatment comparisons are organized in order of significance of hyperparasitoid preference for HIPVs induced by caterpillars that were injection with components of parasitism. Letters in the bars represent post hoc groups based on GLM comparisons of preference distributions among the two-choice tests. (B) In similar two-choice preference tests, we tested the role of the salivary gland in inducing plant volatiles that attract hyperparasitoids. HIPVs of plants damaged by intact unparasitized (striped blue bars) or parasitized caterpillars (striped orange bars) were tested against plants damaged by caterpillars that had their salivary gland surgically removed (blue bars for unparasitized and orange bars for parasitized caterpillars). The intact caterpillars were mock-treated without removal of the salivary glands. The first three pairwise comparisons between undamaged control plants (green bars), P. brassicae-damaged plants (blue), and plants damaged by C. glomerata-parasitized P. brassicae caterpillars (orange) are from Zhu et al. (12) and presented for clarity of the phenomenon that hyperparasitoids prefer HIPVs induced by parasitized caterpillars. Here we show that removal of the salivary glands abolished the preference of hyperparasitoids for HIPVs induced by parasitized caterpillars. In all experiments, >70% of the hyperparasitoids made a choice for one of the treatments within 10 min from the start of the experiment. *P < 0.05; **P < 0.01.
These findings indicate that CgPDV is the main initiator of the interaction chain, which is supported by similar findings for McPDV as the driver of interactions between the parasitoid Microplitis croceipes and the host caterpillar Helicoverpa zea feeding on tomato plants (18). However, for CgPDV, injection of venom may be an important catalyst. Although injection of venom alone did not result in attraction of hyperparasitoids, addition of venom to CgPDV injection enhanced the effect of CgPDV (Fig. 1A). The venom of parasitoids may facilitate the expression of the PDV genes in the caterpillar (19) and is known to strengthen physiological regulation by PDV (20). Thus, the injection of a combination of CgPDV and venom into the caterpillar, but not the parasitoid progeny, is critical for the hyperparasitoid L. nana to locate parasitized caterpillars.
Once PDVs have triggered the interaction chain by altering the physiology of the caterpillar, feeding by the parasitized caterpillar on the food plant induces changes in the plant’s volatile blend compared with feeding by unparasitized caterpillars. Although parasitism may affect the amount and pattern of feeding by the caterpillar (13, 21) and could result in quantitative differences in HIPVs, previous experiments have shown that regurgitate of parasitized caterpillars applied to plant damage inflicted by a pin or pattern wheel induces similar plant responses that attract the hyperparasitoid independent of quantitative effects of variation in leaf damage (11, 13, 22). Unparasitized caterpillars and parasitized caterpillars have distinctly different-colored regurgitate (13). Although regurgitate of parasitized caterpillars has been identified to elicit plant responses that attract hyperparasitoids (11), caterpillars regurgitate only small volumes when feeding and predominantly use saliva from their labial glands to aid digestion of plant material (23). Elicitors in herbivore saliva have been identified as the main inducers of plant responses, including release of specific HIPVs (13–16). In addition to silk production, in Lepidoptera, the labial glands secrete compounds involved in digestion of plant tissue as well as compounds that elicit plant defense responses active against the caterpillars (13–16). Through surgical removal of the labial glands in anesthetized caterpillars (24), we discovered that these glands play a major role in the interaction of parasitized caterpillars and their food plant. Parasitized and unparasitized caterpillars that had their labial glands surgically removed induced very similar plant volatile blends (Fig. 2 and SI Appendix, SI Text and Table S1). Hyperparasitoids lost their odor-based preference for parasitized caterpillar-induced plant volatiles over those induced by unparasitized caterpillars when both caterpillars were feeding without producing saliva (Fig. 1B). Volatiles induced by feeding of parasitized caterpillars were also preferred over plant volatiles induced by caterpillars whose salivary glands had been ablated. In similar choice tests involving unparasitized caterpillars, hyperparasitoids did not discriminate between volatiles from plants induced by ablated and intact caterpillars (Fig. 1B). Thus, the changes in the labial gland after parasitism are crucial for the interaction chain that allows hyperparasitoids to locate the parasitoid larvae.
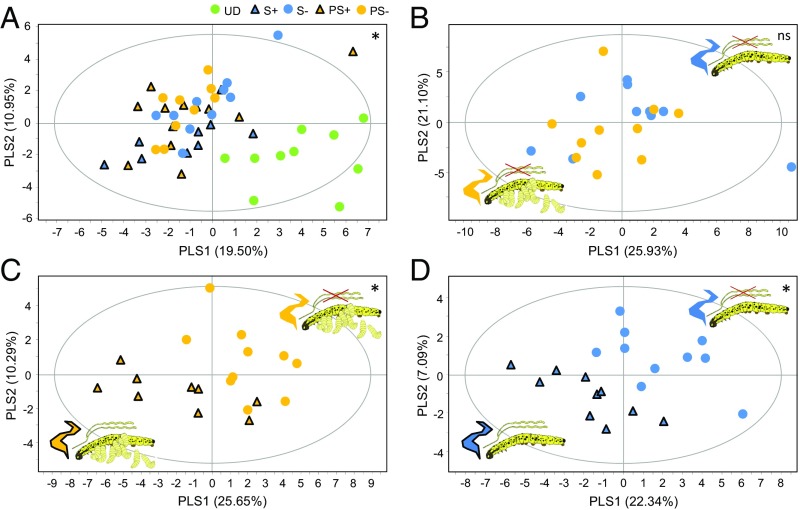
Fig. 2.
Herbivore-induced plant volatile composition for plants damaged by parasitized and unparasitized P. brassicae caterpillars and those with salivary glands removed. (A) In a partial least squares discriminant analysis, the volatile blend of undamaged plants (green circle; UD) differs from plants damaged by parasitized (orange triangle, PS+) and unparasitized caterpillar feeding (blue triangle, S+) as well as plants damaged by parasitized (PS−) or unparasitized caterpillars (S−) that had their salivary glands removed (orange and blue circle, respectively). (B) In pair wise comparisons among these treatments, surgical removal of the salivary glands in parasitized (orange circle, PS−) and unparasitized caterpillars (blue circle, S−) was found to knock down differences in plant volatile emission after herbivory. (C) Plant volatile emission induced by intact parasitized caterpillars (orange triangle, PS+) differs from volatiles induced by parasitized caterpillars that had their salivary glands removed (orange circle, PS−). (D) Similarly, plant volatile blends differ for plants induced by intact (blue triangle, S+) or ablated (blue circle, S−) unparasitized caterpillars (SI Appendix, Table S1). *P < 0.05; ns, not significantly different.
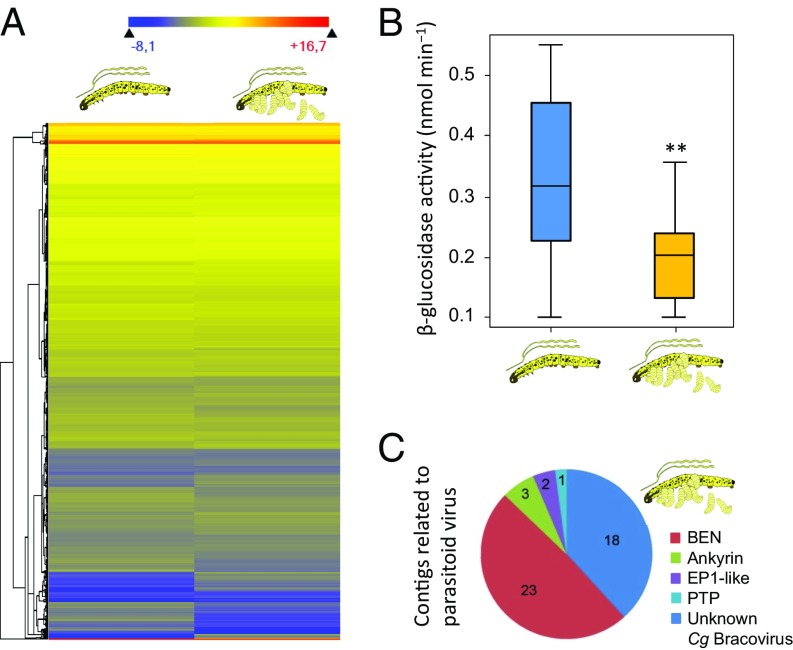
Full transcriptome analysis using RNA-seq on the salivary gland content of parasitized caterpillars in which the parasitoid larvae have fully developed revealed that out of 24,054 contigs generated by de novo transcriptome assembly, a total of 347 contigs were differentially expressed in labial salivary glands between unparasitized and parasitized caterpillars (false discovery rate, P < 0.05; fold change > 2) (SI Appendix, SI Text). There were 237 contigs with higher expression in salivary glands extracted from parasitized caterpillars, whereas 110 contigs were expressed more strongly in salivary glands of unparasitized caterpillars (Fig. 3A and SI Appendix, Table S2). Contigs of two elicitors, β-glucosidase and glucose dehydrogenase, were differentially expressed in labial glands of parasitized P. brassicae caterpillars (SI Appendix, Figs. S1 and S2 and Tables S1 and S2). These elicitors have been previously identified as key players in induction of plant responses to caterpillar feeding, including the emission of HIPVs (16). Direct quantification of β-glucosidase enzyme activity revealed that indeed parasitism reduces enzymatic activity (Fig. 3B). Moreover, concerted microinjection of venom and virus into the caterpillars reduced β-glucosidase activity similar to that in parasitized caterpillars (SI Appendix, Fig. S3). Thus, parasitism may indirectly affect plant responses by changing the composition of caterpillar-derived elicitors in the saliva. However, the causal role for the specific elicitors studied here remains to be confirmed by, for example, targeted modification of elicitor activity in the caterpillars.
Fig. 3.
Gene expression differences in salivary glands derived from unparasitized and parasitized caterpillars. (A) Heat map illustrating the differences in gene expression measured with RNA-seq among salivary glands of parasitized and unparasitized caterpillars. Log2-transformed RPKM values are plotted, with warmer colors representing higher relative gene expression levels. (B) Enzymatic activity [4-nitrophenyl β-d-glucopyranoside converted at pH 6, 30 °C/time (nmol min−1)] of the elicitor β-glucosidase in the saliva of unparasitized and parasitized caterpillars. Genes encoding for β-glucosidase were found to be differentially regulated in the RNA-seq analysis (SI Appendix, Table S2). (C) Summary of gene families that identify parasitoid-related virus activity in the salivary gland of parasitized caterpillars (SI Appendix, Table S2). **P < 0.01.
An alternative explanation for the observed effects of caterpillar saliva is that the PDV particles of the parasitoid that end up in the salivary gland directly affect induction of plant volatiles. The identification of a number of BEN domain proteins and other proteins associated with the specific symbiotic virus (CgPDV) of the parasitoid C. glomerata in our RNA-seq analysis of the labial glands of parasitized caterpillars suggests the potential for direct virus-induced plant responses in our study system (Fig. 3C and SI Appendix, Table S3). Nevertheless, we provide evidence that hyperparasitoids locate the presence of parasitoid larvae by symbiotic PDVs and venom that the parasitoids inject into the host. At the same time, this raises many new questions regarding the reliability of initiation of the interaction network by PDVs in hyperparasitoid host location and the costs of attracting hyperparasitoids compared with the benefits of the parasitoid’s symbiosis with PDVs. Data for another parasitoid–host system demonstrate that the PDVs start to affect elicitors in caterpillar oral secretions already a few days after parasitism (18). We speculate that a few days after parasitism, the CgPDVs in our study system may start to affect HIPV profiles of plants in response to feeding by parasitized caterpillars. Hyperparasitoids parasitize late instar larvae or early stages of parasitoid pupae and may arrive too early to plants when they cannot discriminate between HIPVs induced by young and old parasitized caterpillars. Therefore, it would be interesting to identify when HIPV profiles of plants are affected by caterpillars injected with CgPDV and at which time point onward this results in attraction of hyperparasitoids. When hyperparasitoids would arrive early to plants infested with parasitized caterpillars, the hyperparasitoids may use spatial memory to monitor when the parasitoid larvae become suitable for parasitism (25). Body odors of parasitized caterpillars may allow hyperparasitoids to monitor at close range whether parasitoid larvae have fully developed in the caterpillar body (26).
The results of this study highlight how intimately multispecies interactions are reflected in adaptations of individual species, such as the host-finding behavior of hyperparasitoids. Carrying mutualistic symbionts on which parasitoids critically depend for offspring fitness at the same time incurs fitness costs by enhancing the ability of hyperparasitoids to locate parasitoid offspring. The study by Tan et al. (18) that parallels our work on the role of PDVs has identified that the effect of PDVs on caterpillar saliva also enhances the food plant quality, such that it benefits the parasitoid larvae developing in the herbivore host. These benefits, as well as the suppression of host immune responses, may outweigh the costs of attraction of hyperparasitoids. Nevertheless, placing mutualistic interactions in a community context not only reveals potential costs to mutualisms, but also demonstrates the importance of symbionts associated with their host in driving ecological interactions across multispecies interactions at multiple trophic levels (27, 28).
The extended phenotype of the polydnavirus in ecological interactions may also be highly relevant for agro-ecosystems. Our findings identify both challenges and opportunities for optimization of biological control of these agro-ecosystems in which parasitoids are released to control herbivore pests but the populations of parasitoids suffer from high rates of hyperparasitism. Microorganisms associated with parasitoids not only may be used to influence the performance of these biocontrol agents (29–31), they also should be evaluated for opportunities to reduce the negative effects of hyperparasitoids.
Materials and Methods
Experimental Organisms.
Plants.
The wild B. oleracea population “Kimmeridge” was used in our study (seeds were collected in Dorset, UK, 50°360N, 2°070W). This Brassica population has been shown to differentially respond to feeding by healthy and parasitized Pieris caterpillars (12). For all experiments, plants were grown in 2-L pots containing peat soil (Lentse potgrond no. 4; Lent, The Netherlands). When plants were 4 wk old, they were fertilized by applying 100 mL of nutrient solution of 2.5 mg/L Kristalon Blauw (N-P-K-Mg 19-6-20-3; Hydro Agri Rotterdam) to the soil. The plants were grown in a glasshouse compartment (18–26 °C, 50–70% relative humidity) and provided with SON-T light (500 μmol·m-2·s-1; L16:D8; Philips) in addition to natural daylight. Five-week-old plants were used in the experiments.
Caterpillars.
Caterpillars of the large cabbage white P. brassicae L. (Lepidoptera: Pieridae) were routinely reared on cultivated cabbage plants (B. oleracea var. gemmifera cv. Cyrus) in a glasshouse compartment (22 ± 1 °C, 50–70% relative humidity, and a 16:8 h L:D photoperiod) at the Laboratory of Entomology, Wageningen University. Second instar caterpillars (L2) were used in preparation of microinjected or naturally parasitized caterpillars.
Parasitic wasps.
The larval endoparasitoid wasp Cotesia glomerata L. (Hymenoptera: Braconidae), the most common parasitoid found to parasitize P. brassicae caterpillars in The Netherlands, was used in all treatments that used parasitized or microinjected caterpillars. To obtain parasitized caterpillars for plant induction treatments, individual second-instar P. brassicae larvae were exposed to a single female C. glomerata, which was allowed to parasitize the larva in a glass vial. The caterpillar was considered parasitized when the wasp had inserted her ovipositor in the caterpillar for at least 5 s. The parasitoid is gregarious and lays up to 35 eggs per parasitism event. To avoid effects caused by depletion of the parasitoid’s egg load, no more than 10 caterpillars were offered to a single female parasitoid. The parasitized caterpillars were reared on B. oleracea plants until the fifth instar that contains fully developed larvae of the parasitoid before they were used for plant induction treatments.
Hyperparasitoids.
The hyperparasitoid Lysibia nana Gravenhorst (Hymenoptera: Ichneumonidae) used in this study was originally retrieved from field-collected C. glomerata cocoons found in field sites near Wageningen University, The Netherlands. It was reared on C. glomerata cocoons in the absence of plant- and herbivore-derived cues. Adults were provided with water and honey ad libitum. Lysibia nana is a solitary hyperparasitoid that parasitizes the pupae of parasitoids in the genus Cotesia and is the most common hyperparasitoid of C. glomerata in The Netherlands. It locates the cocoons of C. glomerata by using plant volatiles induced by late instar parasitized caterpillars (11, 12). It is found waiting next to parasitized caterpillars until the parasitoid larvae leave the caterpillar to spin their silk cocoon and pupate. The full brood of C. glomerata larvae that egresses from a parasitized P. brassicae caterpillar stays together in a cluster of silk cocoons that can be parasitized by L. nana until 2 d after the cocoons have formed. The hyperparasitoids use plant volatiles to locate the parasitized caterpillar, likely because the silk cocoons of C. glomerata emit low quantities of volatiles that are not strongly attractive to L. nana and because of the limited time frame in which the pupae of the parasitoid can be parasitized (11). Some hyperparasitoids can discriminate between body odors of parasitized and unparasitized caterpillars during host location at close range (26). In all preference experiments testing the attraction to herbivore-induced plant volatiles, 2- to 10-d-old females without oviposition experience were used. The age of the hyperparasitoids did not affect their response to plant volatiles.
Experimental Approach.
Microinjection and hyperparasitoid preference to HIPVs.
We prepared seven different caterpillar treatments to test the effect of each of three component of parasitism individually (eggs, PDVs, venom) and their synergistic effects in a full factorial design: (i) eggs; (ii) PDVs; (iii) venom; (iv) eggs + PDVs; (v) eggs + venom; (vi) PDVs + venom; (vii) eggs + PDVs + venom (SI Appendix, SI Methods). The last treatment represents a microinjection of the full restoration of a parasitism event. Two additional treatments were used as controls to test whether the microinjection treatment per se affected the interaction of the caterpillars with the food plant: (viii) unparasitized caterpillars injected with 100 nL of PBS representing a treatment assumed to be less attractive to hyperparasitoids and (ix) C. glomerata parasitized caterpillars injected with PBS of which feeding-induced plant volatiles should be preferred over those by unparasitized PBS-injected caterpillars. After microinjections, the caterpillars that recovered within 2 h were introduced to and allowed to feed on new fresh B. oleracea var. gemmifera cv. Cyrus plants for 7–10 d until they reached the fifth instar. At this point, the nine different caterpillar treatments were used to induce B. oleracea “Kimmeridge” plants to obtain the nine corresponding plant treatments. Two caterpillars were inoculated on each individual plant and allowed to feed for 24 h, after which they were used in two-choice Y-tube experiments for hyperparasitoid preference of HIPVs.
In previous work, we have shown that L. nana prefers plant volatiles induced by unparasitized or parasitized caterpillars over undamaged plants, and that volatiles from plants damaged by parasitized caterpillars are preferred over those from plants damaged by unparasitized caterpillars in the laboratory as well as in the field (11, 12). Here we tested hyperparasitoid preference for plants induced by each of eight treatments in which caterpillars were microinjected with a component of parasitism against a plant damaged by unparasitized caterpillars injected with PBS. We addressed which component of parasitism or combination of components was needed to reach preference for the parasitized caterpillar-induced plant volatiles over volatiles induced by unparasitized control caterpillars. The Y-tube olfactometer assays followed the procedures described by Zhu et al. (12). We removed caterpillars and their feces from the plants and placed the plants in one of two glass jars (30 L each) that were connected to the two olfactometer arms. A charcoal-filtered airflow (4 L/min) was led through each arm of the Y-tube olfactometer system and a single wasp was released at the base of the stem section (3.5 cm diameter, 22 cm length) in each test (32). Wasps that reached the end of one of the olfactometer arms within 10 min and stayed there for at least 10 s were considered to have chosen the odor source connected to that olfactometer arm. We swapped the jars containing the plants after testing five wasps, to compensate for unforeseen asymmetry in the setup. Each set of plants was tested for 10 wasps, and nine sets of plants for each treatment combination were tested. After each set of plants was tested, the glass jars were cleaned using distilled water and dried with tissue paper. The Y-tube olfactometer setup was placed in a climatized room, and in addition to daylight, it was illuminated with four fluorescent tubes (FTD 32 W/84 HF; Pope) (SI Appendix, SI Methods).
Surgical removal of caterpillar salivary glands and hyperparasitoid preference to HIPVs.
Ablation of labial salivary glands was performed on both unparasitized and C. glomerata-parasitized P. brassicae caterpillars when they reached the second day of their fifth larval instar, following methods described by Musser et al. (24) (SI Appendix, SI Methods). Caterpillars that started feeding on the plant leaf within 3 h after surgery were selected for subsequent plant induction. Mock-treated unparasitized and parasitized caterpillars were subjected to the same protocol, including the incision, but the labial salivary glands were not removed from the body cavities. To ensure that ablated caterpillars fed similar amounts of leaf tissue as mock-treated caterpillars, we quantified the amount of leaf damage for 10 plants for each herbivore induction treatment, using a transparent plastic sheet with a 1-mm2 grid. We did not find an apparent reduction in food consumption of ablated caterpillars compared with mock-treated caterpillars (Student’s t tests; for unparasitized caterpillars, t = 1.197, df = 18, P = 0.471; for parasitized caterpillars, t = 1.202, df = 18, P = 0.118). After the experiments, the ablated unparasitized caterpillars successfully pupated and eclosed as adult butterflies. For ablated parasitized caterpillars, fully grown parasitoid larvae eventually emerged and pupated.
We offered female hyperparasitoids (L. nana) two-choice tests for combinations of five plant induction treatments in a Y-tube olfactometer setup as described by Takabayashi and Dicke (32). The wild B. oleracea plants were treated with two fifth-instar caterpillars for 24 h: (i) P. brassicae caterpillars with intact labial salivary glands (S+); (ii) P. brassicae caterpillars with ablated labial salivary glands (S−); (iii) C. glomerata parasitized P. brassicae caterpillars with intact labial salivary glands (PS+); (iv) C. glomerata parasitized P. brassicae caterpillars with ablated labial salivary glands (PS−); or (v) plants were left untreated serving as the undamaged control (UD). In our previous work, we have shown that L. nana prefers plant volatiles induced by unparasitized and parasitized caterpillars over undamaged plants, and that volatiles from plants damaged by parasitized caterpillars are preferred over those from plants damaged by unparasitized caterpillars (12). For clarity of the results obtained in the current study, we included these results as a reference in Fig. 1B.
In the present study, we tested whether the labial salivary gland plays a crucial role in differential induction of plant responses and whether ablation of the glands eliminates the hyperparasitoid preference for plant volatiles induced by parasitized caterpillars over unparasitized caterpillars. We first offered L. nana plant volatiles induced by either unparasitized or parasitized P. brassicae, both ablated of labial salivary glands to test whether this hyperparasitoid could still discriminate volatile blends resulting from these treatments. Subsequently, we tested L. nana attraction to plant volatiles induced by mock-treated caterpillars vs. volatiles induced by caterpillars from which the labial salivary glands had been ablated within the same category (unparasitized or parasitized). Finally, we tested preferences of L. nana for plant volatiles released by undamaged control plants vs. plant volatiles induced by unparasitized or parasitized P. brassicae caterpillars with the labial salivary glands ablated, to test whether hyperparasitoids respond to plant volatiles induced by caterpillars without labial salivary glands. For each pairwise comparison, 70 L. nana females were tested. The Y-tube olfactometer assays followed the procedures described in the choice tests with microinjected caterpillars (SI Appendix, SI Methods).
Identification of Underlying Mechanisms.
To characterize the B. oleracea plant volatiles induced by parasitized and unparasitized caterpillars, as well as the effect of labial saliva of P. brassicae on emission of HIPVs, we collected headspace samples of 10 replicate plants for each of five plant treatments. In each of these treatments, herbivores were allowed to feed for 24 h following the methods of the Y-tube hyperparasitoid preference tests: (i) P. brassicae caterpillars with intact labial salivary glands (S+); (ii) P. brassicae caterpillars ablated of labial salivary glands (S−); (iii) C. glomerata-parasitized P. brassicae caterpillars with intact labial salivary glands (PS+); (iv) C. glomerata-parasitized P. brassicae caterpillars ablated of labial salivary glands (PS−); or (v) plants were left untreated serving as the undamaged control (UD). The subsequent plant volatile collections followed procedures described by Zhu et al. (12) (SI Appendix, SI Methods).
To study the labial salivary gland tissue-specific transcriptional differences of genes in unparasitized and C. glomerata parasitized caterpillars, labial salivary glands of the two types of caterpillars were extracted following the ablation procedure described above. We pooled 15 pairs of labial salivary glands per sample, collecting four biological replicates of the two treatments. After extraction, samples were immediately flash-frozen in liquid nitrogen. Total RNA was extracted from each of the labial salivary gland samples (four samples from unparasitized P. brassicae and four samples from C. glomerata parasitized P. brassicae larvae) using the innuPREP RNA Mini Isolation Kit (Analytik Jena) following the manufacturers’ guidelines. The integrity of the RNA was verified using an Agilent 2100 Bioanalyzer and a RNA 6000 Nano Kit (Agilent Technologies). The quantity as well as OD260/280 and OD260/230 ratios of the isolated RNA samples were determined using a Nanodrop ND-1000 spectrophotometer. RNA-seq and data analyses followed protocols described by Vogel et al. (33) and Conesa et al. (34) (SI Appendix, SI Methods).
To measure the β-glucosidase activity in labial salivary glands of parasitized and unparasitized caterpillars, labial salivary glands were extracted following the ablation procedure described above. Other caterpillar treatments included microinjection of parasitoid eggs, venom, calyx fluid containing PDVs, and combinations of these in PBS solution (prepared from tablets; Oxoid). In 1.5-mL Safe-Lock tubes (Biosphere SafeSeal; Sartstedt), labial salivary glands of 3 or 15 caterpillars (unmanipulated caterpillars or microinjected caterpillars respectively) were pooled into a single sample. We prepared 25 samples for the comparison of unparasitized and parasitized caterpillars, along with 10 replicates for each of the microinjection treatments. Samples were kept first on ice and then stored at −80 °C. To resume sample preparation, samples were sonicated for cell disruption using a Digital Sonifier (102C; Branson) in two intervals of 10 s, with the intensity set to 5%. Samples were kept on ice during sonication to reduce damage to proteins by overheating. The sonication step was followed by centrifugation at 10,000 × g for 10 min (Centrifuge 5430; Eppendorf). Supernatants were transferred to clean-1.5 mL Safe Lock tubes and stored at −80 °C until use. The protocol for measuring β-glucosidase activity was based on work by Mattiacci et al. (16), Pankoke et al. (35), and Reed et al. (36) (SI Appendix, SI Methods).
Data Availability.
Data have been deposited in the Dryad repository (doi: 10.5061/dryad.ss5r686).
Supplementary Material
Acknowledgments
Funding was provided by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement 677139, to E.H.P.), a Marie Skłodowska-Curie Individual Fellowship within the Horizon 2020 Framework Programme (H2020-MSCA-IF-2014; Grant Agreement 655178, to A.C.), and the Max Planck Gesellschaft and the Earth and Life Sciences Council of the Netherlands Organisation for Scientific Research (Ecogenomics Grant 844.10.005, to M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been deposited in the Dryad Repository (doi: 10.5061/dryad.ss5r686).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717904115/-/DCSupplemental.
References
- 1.Douglas AE. Multiorganismal insects: Diversity and function of resident microorganisms. Annu Rev Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert SF, Sapp J, Tauber AI. A symbiotic view of life: We have never been individuals. Q Rev Biol. 2012;87:325–341. doi: 10.1086/668166. [DOI] [PubMed] [Google Scholar]
- 3.Zhu F, Poelman EH, Dicke M. Insect herbivore-associated organisms affect plant responses to herbivory. New Phytol. 2014;204:315–321. [Google Scholar]
- 4.Strand MR, Burke GR. Polydnavirus-wasp associations: Evolution, genome organization, and function. Curr Opin Virol. 2013;3:587–594. doi: 10.1016/j.coviro.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Espagne E, et al. Genome sequence of a polydnavirus: Insights into symbiotic virus evolution. Science. 2004;306:286–289. doi: 10.1126/science.1103066. [DOI] [PubMed] [Google Scholar]
- 6.Frago E, Dicke M, Godfray HCJ. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol Evol. 2012;27:705–711. doi: 10.1016/j.tree.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Drezen JM, Chevignon G, Louis F, Huguet E. Origin and evolution of symbiotic viruses associated with parasitoid wasps. Curr Opin Insect Sci. 2014;6:35–43. doi: 10.1016/j.cois.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton Univ Press; Princeton, NJ: 1994. [Google Scholar]
- 9.Dorémus T, et al. Venom gland extract is not required for successful parasitism in the polydnavirus-associated endoparasitoid Hyposoter didymator (Hym. Ichneumonidae) despite the presence of numerous novel and conserved venom proteins. Insect Biochem Mol Biol. 2013;43:292–307. doi: 10.1016/j.ibmb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Webb BA, et al. Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology. 2006;347:160–174. doi: 10.1016/j.virol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Poelman EH, et al. Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol. 2012;10:e1001435. doi: 10.1371/journal.pbio.1001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F, et al. Parasitism overrides herbivore identity allowing hyperparasitoids to locate their parasitoid host using herbivore-induced plant volatiles. Mol Ecol. 2015;24:2886–2899. doi: 10.1111/mec.13164. [DOI] [PubMed] [Google Scholar]
- 13.Poelman EH, et al. Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc Natl Acad Sci USA. 2011;108:19647–19652. doi: 10.1073/pnas.1110748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuman MC, Baldwin IT. The layers of plant responses to insect herbivores. Annu Rev Entomol. 2016;61:373–394. doi: 10.1146/annurev-ento-010715-023851. [DOI] [PubMed] [Google Scholar]
- 15.Rivera-Vega LJ, Acevedo FE, Felton GW. Genomics of Lepidoptera saliva reveals function in herbivory. Curr Opin Insect Sci. 2017;19:61–69. doi: 10.1016/j.cois.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Mattiacci L, Dicke M, Posthumus MA. beta-glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitra K, Zhang S, Strand MR. Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage-specific patterns of activity. J Gen Virol. 2011;92:2060–2071. doi: 10.1099/vir.0.032680-0. [DOI] [PubMed] [Google Scholar]
- 18.Tan C-W, et al. Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc Natl Acad Sci. 2018;115:5199–5204. doi: 10.1073/pnas.1717934115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Schmidt O, Asgari S. A novel venom peptide from an endoparasitoid wasp is required for expression of polydnavirus genes in host hemocytes. J Biol Chem. 2004;279:41580–41585. doi: 10.1074/jbc.M406865200. [DOI] [PubMed] [Google Scholar]
- 20.Asgari S. Venoms from endoparasitoids. In: Beckage NE, Drezen J-M, editors. Parasitoid Viruses: Symbionts and Pathogens. Academic Press; London: 2012. pp. 217–231. [Google Scholar]
- 21.Poelman EH, et al. Indirect plant-mediated interactions among parasitoid larvae. Ecol Lett. 2011;14:670–676. doi: 10.1111/j.1461-0248.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 22.Cusumano A, et al. Parasitic wasp-associated symbiont affects plant-mediated species interactions between herbivores. Ecol Lett. 2018 doi: 10.1111/ele.12952. [DOI] [PubMed] [Google Scholar]
- 23.Vadassery J, Reichelt M, Mithöfer A. Direct proof of ingested food regurgitation by Spodoptera littoralis caterpillars during feeding on Arabidopsis. J Chem Ecol. 2012;38:865–872. doi: 10.1007/s10886-012-0143-5. [DOI] [PubMed] [Google Scholar]
- 24.Musser RO, Farmer E, Peiffer M, Williams SA, Felton GW. Ablation of caterpillar labial salivary glands: Technique for determining the role of saliva in insect-plant interactions. J Chem Ecol. 2006;32:981–992. doi: 10.1007/s10886-006-9049-4. [DOI] [PubMed] [Google Scholar]
- 25.van Nouhuys S, Kaartinen R. A parasitoid wasp uses landmarks while monitoring potential resources. Proc Biol Sci. 2008;275:377–385. doi: 10.1098/rspb.2007.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu F, et al. Body odors of parasitized caterpillars give away the presence of parasitoid larvae to their primary hyperparasitoid enemies. J Chem Ecol. 2014;40:986–995. doi: 10.1007/s10886-014-0500-7. [DOI] [PubMed] [Google Scholar]
- 27.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Microbiology. Animal behavior and the microbiome. Science. 2012;338:198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- 28.Shikano I, Rosa C, Tan C-W, Felton GW. Tritrophic interactions: Microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol. 2017;55:313–331. doi: 10.1146/annurev-phyto-080516-035319. [DOI] [PubMed] [Google Scholar]
- 29.Beckage NE, Gelman DB. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu Rev Entomol. 2004;49:299–330. doi: 10.1146/annurev.ento.49.061802.123324. [DOI] [PubMed] [Google Scholar]
- 30.Pennacchio F, Giordana B, Rao R. Applications of parasitoid virus and venom research in agriculture. In: Beckage NE, Drezen J-M, editors. Parasitoid Viruses: Symbionts and Pathogens. Academic Press; London: 2012. pp. 269–283. [Google Scholar]
- 31.Beckage NE, Tan FF, Schleifer KW, Lane RD, Cherubin LL. Characterization and biological effects of Cotesia congregata polydnavirus on host larvae of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol. 1994;26:165–195. [Google Scholar]
- 32.Takabayashi J, Dicke M. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl. 1992;64:187–193. [Google Scholar]
- 33.Vogel H, Badapanda C, Knorr E, Vilcinskas A. RNA-sequencing analysis reveals abundant developmental stage-specific and immunity-related genes in the pollen beetle Meligethes aeneus. Insect Mol Biol. 2014;23:98–112. doi: 10.1111/imb.12067. [DOI] [PubMed] [Google Scholar]
- 34.Conesa A, et al. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 35.Pankoke H, Bowers MD, Dobler S. The interplay between toxin-releasing β-glucosidase and plant iridoid glycosides impairs larval development in a generalist caterpillar, Grammia incorrupta (Arctiidae) Insect Biochem Mol Biol. 2012;42:426–434. doi: 10.1016/j.ibmb.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Reed R, Holmes D, Weyers J, Jones A. Practical Skills in Biomolecular Sciences. 2nd Ed. Prentice-Hall; Englewood Cliffs, NJ: 2003. pp. 381–383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in the Dryad repository (doi: 10.5061/dryad.ss5r686).